Abstract
Purpose
To investigate the association of the complement factor H gene (CFH) Y402H polymorphism and age-related macular degeneration (AMD) in the Austrian population (Caucasoid descent), and to determine whether there is an association between exposure to Chlamydia pneumoniae—responsible for up to 20% of community-acquired pneumoniae—and the AMD-associated CFH risk polymorphism.
Methods
Genotypes were determined by polymerase chain reaction-restriction fragment length polymorphism analysis in 75 unrelated AMD patients and compared with 75 healthy, age-matched control subjects. C. pneumoniae serum IgG was tested by ELISA (R&D) in both groups. The association between the CFH Y402H genetic polymorphism and the disease was examined by χ2-test and logistic regression.
Results
CFH Y402H genotype frequencies differed significantly between AMD patients and healthy controls (1277 TT, 22.7%; 1277 TC, 53.3%; and 1277 CC, 22.7% in the AMD group; 1277 TT, 48.0%; 1277 TC, 38.7%; and 1277 CC, 13.3% in the control group) showing a P-value <0.005 (OR:2.920/3.811).
No association was found between a positive C. pneumoniae titre and AMD (P = 0.192), nor was any association found between C. pneumoniae and the CFH Y402H polymorphism.
Conclusions
Our data confirm that the CFH Y402H polymorphism is a risk factor for AMD in the Austrian population with a higher frequency of the Y402 polymorphism in AMD patients. No association between preceding C. pneumoniae infection and diagnosed AMD was found.
Keywords: age-related macular degeneration, complement factor H, polymorphism, chlamydia pneumoniae, infection, aetiology
Introduction
Age-related macular degeneration (AMD) is a progressive degenerative disorder of the central macular region of the retina. It is the leading cause of legal blindness in people greater than 55 years at age in developed countries.1
The aetiology of AMD is complex. Besides genetic factors, ischaemia, oxidative stress, aging, and inflammation are the proposed aetiologic factors.2 Candidate gene screening, positional cloning, and full genome-wide scans have defined the association of some gene variants with AMD, major associations including complement factor H (CFH),3–9 LOC387715,10 and small associations including ABCA4,11 APOE,12 FBLN5,13 ELOVL4,14 and TLR4.15 In contrast, variants in the complement factor B (CFB) gene and in the complement component 2 (C2) gene have been shown to confer a significantly reduced risk of AMD.16
The CFH is an inhibitor of the alternative complement activation pathway. The alternative complement pathway is activated by a trigger, which is often of a microbial nature. Different infectious agents are currently implicated in AMD aetiology models.17,18 One of them is Chlamydia pneumoniae, responsible for up to 20% of community-acquired pneumoniae.19–20 This potential activator has previously been linked to AMD in a few studies, although other studies where unable to identify an association.21–26
In this study we determined:
The frequency of the CFH Y402H polymorphism in patients with AMD in the Austrian population of Caucasian descent; and
The association between exposure to C. pneumoniae and risk of AMD in patients with the CFH polymorphism.
Materials and methods
Study design: case–control study
A total of 150 patients were recruited from the Department of Ophthalmology at our Rudolf Foundation Hospital (75 unrelated patients with AMD and 75 control subjects (patients assigning for cataract surgery with a good visible fundus) aged 55 years and above). All participants were of Caucasian origin. They lived in the same geographical area of Austria and were seen at the local Department of Ophthalmology. Written informed consent was obtained before enrolment. The study was performed in accordance with the Austrian Gene Technology Act, the tenets of the Declaration of Helsinki, and the guidelines of the local ethics committee.
All AMD patients enrolled in this study were classified according to the Age-Related Eye Disease Study system (drusen, geographic atrophy, predominantly classic choroidal neovascularisation, minimal classic choroidal neovascularisation, occult choroidal neovascularisation, and retinal angiomatous proliferation).27 AMD was diagnosed by ophthalmoscopic fundus examination, optical coherence tomography, and fluorescein/indocyanine angiography. Excluded from the study were patients with hereditary diseases, polypoidal choroidal vasculopathy, or secondary CNV due to pathologic myopia (>−2 D, spherical equivalent), angioid streaks, inflammatory or infectious chorioretinal disease, trauma, or diabetic retinopathy.
The age- and sex-matched control group had a thorough eye examination with a detailed fundus examination. Exclusion criteria for controls were evidence of any stage of age-related maculopathy, macular haemorrhages of any cause, or media opacities resulting in impaired visualisation of the macula—cataract grades 3 and 4 according to the Lens Opacities Classification System III (LOCS III).28
Genotyping
Genotyping was carried out in a random order by an experienced technician who was masked to the disease status of the samples. An aliquot of 5 ml venous blood from each subject was withdrawn and collected in an EDTA-containing tube. Genomic DNA was isolated from whole blood using a commercial kit (QIA-AMP DNA blood mini kit, Qiagen, Vienna, Austria). The single-nucleotide polymorphism (Y402H; rs 1061170) located in exon 9 of CFH was genotyped by PCR-directed sequencing using the following primer sequences: 5′-CTT TAG TTC GTC TTC AGT TAT AC-3′ (forward) and 5′-GTC ATC TAT GTT ACT TAG AAA GT-3′ (reverse). PCR products were then purified and sequenced by Single-Read Sequencing reaction (VBC-Biotech, Vienna/Austria).
C. pneumoniae
Peripheral venous blood samples were collected according to the standard hospital procedure. Red-topped tubes were centrifuged at 3000 r/min for 10 min at 4°C, and then the serum was separated and stored at − 70°C.
Plasma samples were analysed with an enzyme-linked immunosorbent assay to determine IgG antibody titres to the elementary bodies from C. pneumoniae using a quantitative sandwich enzyme immunoassay technique according to the manufacturer’s guidelines (R&D Systems Inc., 614 McKinley Place, Minneapolis, MN 55413, USA).
Statistics
The statistical analysis was performed using R 2.4.0 and SAS 9.1. All P-values <0.008333 were considered statistically significant. The critical boundary of 0.008333 results from the correction for multiplicity according to Bonferroni assessment due to the number of tests (six tests were performed, 0.05/6 = 0.008333).
Continuous variables were analysed by t-test and presented as mean±SD. Categorial variables were presented as percentages and were compared by χ2-test. A stepwise binary logistic regression analysis was performed to assess the influence of C. pneumoniae and CFH genotypes on AMD risk. Odds ratios (ORs) estimated from logistic regression were reported with corresponding 95% confidence intervals (95% CI). Where there was no statistical significant effect, a t-test was performed.
Results
A total of 150 patients were included in this study (75 unrelated AMD patients compared with 75 healthy controls). Age and gender were similar in both groups. The AMD group consisted of 41 (54.6%) female and 34 (45.3%) male patients, and the mean age was 77.0±7.3 years (range: 56–92). The control group consisted of 37 (49.3%) female and 38 (50.6%) male subjects, and the mean age was 76.5±6.5 years (range: 62–94).
Genotypes were successfully determined in all participants. The prevalence of the CFH polymorphism was significantly higher in patients with AMD than in the control group (P<0.05/P = 0.0054).
Table 1 shows the genotype distribution in both groups (AMD and healthy controls).
Table 1.
Genotype distribution in both groups
| Complement factor H Y402H genotype | Patients with AMD (n = 75) | Controls (n =75) | P-value |
|---|---|---|---|
| TT | 22.7% (n = 17) | 48.0% (n =36) | — |
| TC | 53.3% (n = 40) | 38.7% (n =29) | 0.005 |
| CC | 24.0% (n = 18) | 13.3% (n =10) | 0.0066 |
AMD = age-related macular degeneration.
An OR of 2.92 (95% CI: 1.381–6.177) for AMD patients was found among carriers of the CFH 402YH genotype, whereas homozygosity for the CFH polymorphism showed an OR of 3.811 (95% CI: 1.453–9.997).
Neither age (P = 0.9140) nor sex (P = 0.1724) showed any statistically significant difference on the polymorphism. Men had a higher tendency of having a risk polymorphism than women.
The genotype distribution according to the form of AMD (dry/wet) showed no statistically significant difference (P = 0.5075; Table 2).
Table 2.
Genotype distribution in non-exudative and exudative AMD patients
| Hetero | Homo | Normal | |
|---|---|---|---|
| Dry | 5 | 1 | 3 |
| Wet | 35 | 17 | 14 |
AMD = age-related macular degeneration.
Genotype distribution according to the angiographic type of CNV is shown in Table 3.
Table 3.
Genotype distribution in different subtypes of AMD
| AMD | CFH polymorphism
|
||
|---|---|---|---|
| Normal | Hetero | Homo | |
| Drusen | 2 (28.6%YY) | 4 (57.1%YH) | 1 (14.3%HH) |
| GA | 1 (50.0%YY) | 1 (50.0%YH) | 0 (0%HH) |
| Predominantely classic CNV | 2 (12.5%YY) | 10 (62.5%YH) | 4 (25.0%HH) |
| Minimally classic CNV | 3 (27.3%YY) | 7 (63.3%YH) | 1 (9.0%HH) |
| Occult CNV | 6 (19.4%YY) | 13 (41.9%YH) | 12 (38.7%HH) |
| RAP | 3 (37.5%YY) | 5 (62.5%YH) | 0 (0%HH) |
CNV = choroidal neovascularisation; GA = geographic atrophy; RAP = retinal angiomatous proliferation.
No statistically significant difference (P = 0.3781) between the individual groups of AMD could be shown. In eyes with occult CNV, homozygosity and heterozygosity were found to be nearly equally. Homozygosity was more prevalent in eyes with occult CNV compared to those with RAP and predominantly classic and minimal classic CNVs.
C. pneumoniae serology
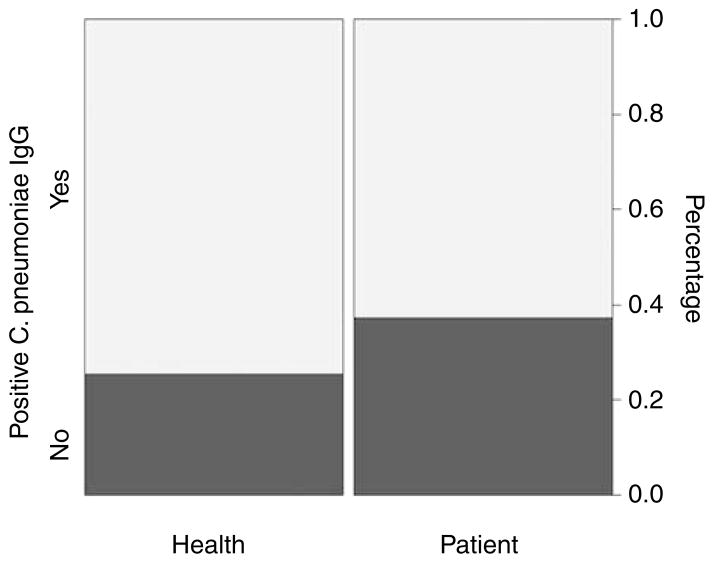
Serological data were available from all 75 AMD patients and 75 healthy controls. Regarding positive C. pneumoniae-specific IgG, there was no statistical significance between the two groups (P = 0.192), Figure 1. The control group even showed slightly higher incidence of C. pneumoniae IgG-positive samples.
Figure 1.
Chlamydia pneumoniae IgG in AMD patients and healthy controls.
Distribution of C. pneumoniae-specific IgG
Out of 69 patients and controls with heterozygosity 53 were positive and 16 were negative. Out of 28 patients and controls with homozygosity 22 were positive for C. pneumoniae-specific IgG and 6 negative. Out of 53 patients and controls with normal genotype 43 were positive for C. pneumoniae-specific IgG and 10 were negative.
Looking only on patients and controls with a C. pneumoniae-specific IgG, following distribution was registered: in AMD patients, 32 were heterozygote, 13 were homozygote, and 14 had a normal genotype, whereas in the healthy control group, 21 were heterozygote, 9 were homozygote, and 29 had a normal genotype.
The study population was too small to make serious conclusions, and therefore, further statistical analysis for the CFH polymorphism and C. pneumoniae were not performed because of the lack of significance.
Discussion
The CFH Y402 gene polymorphism is a major risk factor for AMD. Several studies have confirmed an association of the CFH gene, an inhibitor of the alternative complement activation pathway to the risk of AMD.
In CFH, the non-synonymous variant of exon 9 c. 1279 (T→C) results in a tyrosine-to-histidine change at codon 402.3–9
This study was set up to investigate two major aspects in the aetiology of developing AMD.
The primary aim was to investigate the prevalence of the CFH Y402 polymorphism in patients with AMD compared with a healthy control group in the Austrian population.
Here, we confirm the higher frequency of the Y402 polymorphism of CFH in AMD patients. The observed ORs were similar to those previously reported for North American and European populations.
The analysis of the genotype distribution among different subtypes of AMD showed no statistical difference. Interestingly, in eyes with occult CNV, homozygosity and heterozygosity were found nearly equally. Homozygosity was more prevalent in eyes with occult CNV in comparison to those with RAP, predominantly classic and minimal classic CNVs. Subgroup analysis by Wegscheider and colleagues29 showed similar results, suggesting that the CFH Y402 polymorphism to be a major risk factor for all types of exudative AMD, but homozygosity was more prevalent in predominantly classic CNV than in other forms of CNV.
The secondary aim of this study was to examine an association between exposure to C. pneumoniae and risk of AMD in patients with CFH polymorphism.
CFH is an inhibitor of the alternative pathway of the complement activation. Some epidemiological studies have found associations between the risk of AMD and serum levels of C-reactive protein (CRP), a systemic marker of inflammation.30
The complement system must be activated. By far, the most common triggers of the alternative pathway are microbes. Therefore, certain microbial infections have been postulated to be triggers for AMD.7
C. pneumoniae has been considered to be a trigger for the complement activation in AMD.17,18
C. pneumoniae is an obligate intracellular pathogen responsible for up to 20 percent of cases of community-aquired pneumoniae. Most infections are mild or asymptomatic. The organism is thought to be disseminated through the bloodstream and has a particular attraction for vascular tissue, where it may establish a chronic infection. As there is evidence that chronic inflammation may be involved in the development of AMD,19,20 a few studies to date have linked C. pneumoniae to AMD; however, the outcomes have been very differing. Two studies showed serological association between C. pneumoniae infection and AMD,26,31 but consisted only of a small number of patients. C. pneumoniae has also been identified in surgically excised subretinal neovascular membranes,24 and AMD progression has been linked to exposure to C. pneumoniae.25 In contrast, two other studies showed no association between C. pneumoniae and AMD, neither could C. pneumoniae be identified in surgically excised subretinal neovascular membranes23 nor in a recent serological study.21
Regarding a positive C. pneumoniae-specific IgG, our study shows no statistical significance between AMD patients and healthy controls (P = 0.192), Figure 1.
Our data do not support the hypothesis of the involvement of C. pneumoniae in the pathogenesis of AMD.
In conclusion, we confirm the increased risk for developing AMD in individuals carrying the His402 allele in the Austrian population. An association between C. pneumoniae and the risk of developing AMD could not be confirmed.
Acknowledgments
This study was supported by the Bürgermeisterfond, Vienna, Austria
References
- 1.Ambatti J. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–293. doi: 10.1016/s0039-6257(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 2.Spaide RF, Armstrong D, Browne R. Continuing medical education review: choroidal neovascularisation in age-related macular degeneration—what is the cause? Retina. 2003;2003:595–614. doi: 10.1097/00006982-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards AO, Ritter R, III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 5.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 6.Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci USA. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zareparsi S, Branham KE, Li M, Shah S, Klein RJ, Ott J, et al. Strong association of the Y402H variant in complement factor H at 1q32 with susceptibility to age-related macular degeneration. Am J Hum Genet. 2005;77:149–153. doi: 10.1086/431426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magnusson KP, Duan S, Sigurdsson H, Petursson H, Yang Z, Zhao Y, et al. CFH Y402H confers similar risk of soft drusen and both forms of advanced AMD. PLoS Med. 2006;3:e5. doi: 10.1371/journal.pmed.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sepp T, Khan JC, Thurlby DA, Shahid H, Clayton DG, Moore AT, et al. Complement factor H variant Y402H is a major risk determinant for geographic atrophy and choroidal neovascularization in smokers and nonsmokers. Invest Ophthalmol Vis Sci. 2006;47:536–540. doi: 10.1167/iovs.05-1143. [DOI] [PubMed] [Google Scholar]
- 10.Rivera A, Fisher SA, Fritsche LG, Keilhauer CN, Lichtner P, Meitinger T, et al. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet. 2005;14:3227–3236. doi: 10.1093/hmg/ddi353. [DOI] [PubMed] [Google Scholar]
- 11.Allikmets R, Shroyer NF, Singh N, Seddon JM, Lewis RA, Bernstein PS, et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997;277:1805–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- 12.Klaver CC, Kliffen M, van Duijn CM, Hofman A, Cruts M, Grobbee DE, et al. Genetic association of apolipoprotein E with age-related macular degeneration. Am J Hum Genet. 1998;63:200–206. doi: 10.1086/301901. Erratum in: Am J Hum Genet 1998; 63:1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone EM, Braun TA, Russell SR, Kuehn MH, Lotery AJ, Moore PA, et al. Missense variations in the fibulin 5 gene and age-related macular degeneration. N Engl J Med. 2004;351:346–353. doi: 10.1056/NEJMoa040833. [DOI] [PubMed] [Google Scholar]
- 14.Conley YP, Thalamuthu A, Jakobsdottir J, Weeks DE, Mah T, Ferrell RE, et al. Candidate gene analysis suggests a role for fatty acid biosynthesis and regulation of the complement system in the etiology of age-related maculopathy. Hum Mol Genet. 2005;14:1991–2002. doi: 10.1093/hmg/ddi204. [DOI] [PubMed] [Google Scholar]
- 15.Zareparsi S, Buraczynska M, Branham KE, Shah S, Eng D, Li M, et al. Toll-like receptor 4 variant D299G is associated with susceptibility to age-related macular degeneration. Hum Mol Genet. 2005;14:1449–1455. doi: 10.1093/hmg/ddi154. [DOI] [PubMed] [Google Scholar]
- 16.Spencer KL, Hauser MA, Olson LM, Schmidt S, Scott WK, Gallins P, et al. Protective effect of complement factor B and complement component 2 variants in age-related macular degeneration. Hum Mol Genet. 2007;16(16):1986–1992. doi: 10.1093/hmg/ddm146. [DOI] [PubMed] [Google Scholar]
- 17.Miller DM, Espinosa-Heidmann DG, Legra J, Dubovy SR, Sner IJ, Sedmak DD, et al. The association of prior cytomegalovirus infection with neovascular age-relatedvmacular degeneration. Am J Ophthalmol. 2004;138(3):323–328. doi: 10.1016/j.ajo.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Kijlstra A, La Heij E, Hendrikse F. Immunological factors in the pathogenesis and treatment of age-related macular degeneration. Ocul Immunol Inflamm. 2005;13(1):3–11. doi: 10.1080/09273940590909185. [DOI] [PubMed] [Google Scholar]
- 19.Kuo CC, Jackson LA, Campbell LA, Greystone JT. Chlamydia pneumoniae (TWAR) Clin Microbiol Rev. 1995;8:451–461. doi: 10.1128/cmr.8.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verkooyen R, Willemse D, Hiervan Casteren S, Joulandan SA, Snijder RJ, van den Bosch JM, et al. Evaluation of PCR, culture, and serology for diagnosis of Chlamydia pneumoniae respiratory infections. J Clin Microbiol. 1998;36:2301–2307. doi: 10.1128/jcm.36.8.2301-2307.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robman L, Mahdi OS, Wang JJ, Burlutsky G, Mitchell P, Byrne G, et al. Exposure to Chlamydia pneumoniae infection and age-related macular degeneration: the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci. 2007;48(9):4007–4011. doi: 10.1167/iovs.06-1434. [DOI] [PubMed] [Google Scholar]
- 22.Guymer R, Robman L. Chlamydia pneumoniae and age-related macular degeneration: a role in pathogenesis or merely a chance association? Clin Experiment Ophthalmol. 2007;35(1):89–93. doi: 10.1111/j.1442-9071.2006.01392.x. [DOI] [PubMed] [Google Scholar]
- 23.Kessler W, Jantos CA, Dreier J, Pavlovic S. Chlamydia pneumoniae is not detectable in subretinal neovascular membranes in the exudative stage of age-related macular degeneration. Acta Ophthalmol Scand. 2006;84(3):333–337. doi: 10.1111/j.1600-0420.2005.00591.x. [DOI] [PubMed] [Google Scholar]
- 24.Kalayoglu MV, Bula D, Arroyo J, Gragoudas ES, D’Amico D, Miller JW. Identification of Chlamydia pneumoniae within human choroidal neovascular membranes secondary to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2005;243(11):1080–1090. doi: 10.1007/s00417-005-1169-y. [DOI] [PubMed] [Google Scholar]
- 25.Robman L, Mahdi O, McCarty C, Dimitrov P, Tikellis G, McNeil J, et al. Exposure to Chlamydia pneumoniae infection and progression of age-related macular degeneration. Am J Epidemiol. 2005;161(11):1013–1019. doi: 10.1093/aje/kwi130. [DOI] [PubMed] [Google Scholar]
- 26.Ishida O, Oku H, Ikeda T, Nishimura M, Kawagoe K, Nakamura K. Is Chlamydia pneumoniae infection a risk factor for age-related macular degeneration? Br J Ophthalmol. 2003;87 (5):523–524. doi: 10.1136/bjo.87.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study Report Number 6. Am J Ophthalmol. 2001;132(5):668–681. doi: 10.1016/s0002-9394(01)01218-1. [DOI] [PubMed] [Google Scholar]
- 28.Chylack LT, Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, et al. The lens opacities classification system III. The longitudinal study of Cataract study group. Arch Ophthalmol. 1993;111(6):831–836. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 29.Wegscheider BJ, Weger M, Renner W, Steinbrugger I, März W, Mossböck G, et al. Association of complement factor H Y402H gene polymorphism with different subtypes of exudative age-related macular degeneration. Ophthalmology. 2007;114(4):738–742. doi: 10.1016/j.ophtha.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 30.Seddon JM, Georg S, Rosner B, Rifai N. Progression of age-related macular degeneration: prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers. Arch Ophthalmol. 2005;123:774–782. doi: 10.1001/archopht.123.6.774. [DOI] [PubMed] [Google Scholar]
- 31.Kalayoglu MV, Galvan C, Mahdi OS, Byrne GI, Mansour S. Serological association between Chlamydia pneumoniae infection and age-related macular degeneration. Arch Ophthalmol. 2003;121(4):478–482. doi: 10.1001/archopht.121.4.478. [DOI] [PubMed] [Google Scholar]