Abstract
IMPORTANCE
Geographic atrophy (GA) is the major cause of blind registration in Western communities, although, with few exceptions, it is less common than choroidal neovascular disease. The variation of phenotype implies that age-related macular degeneration (AMD) does not follow the same course from one case to another and that phenotyping may be important before initiating a therapeutic trial.
OBJECTIVE
To document photoreceptor and retinal pigment epithelium (RPE) cell loss and other changes at the RPE-choroid interface in donated human eyes in which visual loss was deemed to be due to GA.
DESIGN, SETTING, AND PARTICIPANTS
Histological study of a consecutive series of eyes donated by individuals previously diagnosed clinically as having GA. Donors were chosen on the basis of available clinical records (from MidAmerica Transplant Services, St Louis, Missouri; the Iowa Lions Eye Bank, Iowa City; and the Utah Lions Eye Bank, Salt Lake City) and selected were those considered to have GA due to AMD. Tissues in the regions of atrophy were examined with light, electron, and autofluorescence microscopy.
RESULTS
In most of the 37 donors examined, there was marked loss of photoreceptor cells for variable distances distal from the edge of the GA. Rod loss was greater than cone loss. An inverse relationship existed between the quantity of autofluorescent inclusions in the RPE and the thickness of sub-RPE basal laminar deposit. Integrity of the choroid varied from one eye to another and was not related strictly to photoreceptor survival. In some eyes, photoreceptor loss existed in the absence of obvious morphological changes in the Bruch membrane or RPE.
CONCLUSIONS AND RELEVANCE
The findings support the view that photoreceptor loss occurs early in AMD in a proportion of cases and imply that photoreceptor-cell loss may contribute to the functional loss recorded in early stages of AMD at least in part. The variation of changes from one eye to another implies that patients selected for a specific prophylactic therapy for early AMD should be chosen on the basis of the characteristics of their disease.
Geographic atrophy (GA) of the retinal pigment epithelium (RPE) is a term used originally by Gass1 to designate one form of late age-related macular degeneration (AMD). It is the major cause of blind registration in Western communities,2,3 although, with few exceptions, it is less common than choroidal neovascular disease.4–6 Concepts as to the pathological processes leading to GA are incompletely understood, and the sequence of events leading to visual loss has been debated for some years. On the basis of histological studies, Hogan7 took the view that photoreceptor cells were lost early in the disease and that cell loss was possibly consequent on RPE dysfunction. Sarks and colleagues8,9 undertook meticulous morphological studies on donor eyes and supported this view. They observed accumulation of phagosomal debris in the RPE accompanied by enlargement and displacement of the RPE cells. The overlying photoreceptor cells were fewer in number and lost outer segments prior to GA becoming apparent. In addition, it was considered that thickening of the Bruch membrane (BM) undoubtedly played a role on the genesis of disease,8,9 although the significance of the BM change to photoreceptor-cell loss was not clear. They also concluded that closure of the choriocapillaris followed atrophy of the RPE and outer retina rather than causing it. However, these conclusions were drawn on a small number of donor eyes.
Clinical studies of a larger number of eyes using autofluorescence have supported these general conclusions. The observation that GA is preceded by excess autofluorescence implies that that accumulation of lipofuscin in the RPE is intrinsic to the evolution of GA at least in some cases.10,11
Two studies were published in 2008 concerning the state of the photoreceptor layer in cases with GA examined in vivo with high-quality imaging using ophthalmic coherence tomography (OCT), and both came to the same conclusion.12,13 In the area of GA, there was complete or almost complete loss of photoreceptor cells. The alternatives that RPE may be absent or thinned and depigmented could not be distinguished one from the other. Beyond the edge of the GA, 2 patterns of transition were reported. In the first, there was an abrupt transition to a normal outer nuclear thickness with integrity of the line considered to be derived from the junction of the inner and outer segments of the photoreceptor cells. In the second, the photoreceptor-cell layer was thought to be thin for some distance beyond the edge of GA. In the second case, there appeared to be substantial photoreceptor loss in the retina that would have been unremarkable clinically. In neither article was the relative frequency of the 2 patterns reported.12,13 A further study was published the following year that showed similar variation in the pattern of loss and reported that both patterns of loss may occur in the same eye.14
Psychophysical testing in early AMD consistently shows sensitivity losses in up to 25% of cases of early AMD with normal visual acuity.15–19 All studies showed that scotopic loss is greater than photopic loss, with sensitivity loss of up to 3.4 log units.15 Determining whether these losses are due to cell death or cell dysfunction was not sought.
In an attempt to establish the extent of photoreceptor-cell loss associated with GA and the topographical relationship between photoreceptor and RPE cells, we examined donor eyes in which GA had been documented clinically and we related the loss to other manifestations of AMD.
Methods
The clinical records of the human eyes used in this study were obtained from MidAmerica Transplant Services (St Louis, Missouri), the Iowa Lions Eye Bank (Iowa City), and the Utah Lions Eye Bank (Salt Lake City) following written informed consent from patients. Institutional review board committee approval from the University of Utah, University of Iowa, and St Louis University for the use of human donor tissues was obtained. Most of the eyes were collected and fixed within 4 hours of death. All fundi were photographed before processing. Fundi were classified according to a modified version of the International Classification and Grading System for Age-Related Macular Degeneration.20 The gross pathologic features and the corresponding clinical imaging data were reviewed and graded by retinal specialists.
All donors from the repository were identified in which clinical records implied that a diagnosis of GA had been made in 1 or both eyes. The diagnosis was verified by examination of fundus photographs and histology, and eyes in which it was thought that visual loss may not have been due to GA were excluded from the initial survey.
Structural changes of the outer retina, BM, and choroid were documented using light, autofluorescence, and electron microscopy. For light microscopy, posterior poles were fixed in 4% paraformaldehyde in 100mM sodium cacodylate, pH 7.4, as described previously.21,22 After 2 to 4 hours of fixation, eyes were transferred to 100mM sodium cacodylate and rinsed (3 × 10 minutes), infiltrated, and embedded in acrylamide. These tissues were subsequently embedded in optimal cutting temperature medium, snap frozen in liquid nitrogen, and stored at −80°C. A few 4% paraformaldehyde-fixed eyes were embedded in paraffin and stained with hemotoxylin and eosin. Tissues were sectioned to a thickness of 8 to 10 µm using a cryostat or paraffin microtome. Tissues used for transmission electron microscopic studies were fixed by immersion fixation in one-half strength Karnovsky fixative for a minimum of 24 hours. Trephine-punched specimens of the posterior pole were fixed, transferred to 100mM sodium cacodylate buffer, pH 7.4, and subsequently dehydrated, embedded in epoxy resin, sectioned, and photographed, as described previously.23,24 Tissue for confocal microscopy was prepared, as previously published.25
Between 6 and 12 sections of each donor eye were examined by light and autofluorescence imaging. Special attention was paid to photoreceptor numbers, the presence of inner and outer segments, state of the RPE, BM thickness, and loss of choriocapillaris, particularly in regions distal and proximal to the edge of atrophic regions. The area (square micrometer) of basal laminar deposit (BLD) per micron of RPE/BM and the area (square micrometer) of RPE lipofuscin granules per micron of RPE basal lamina were quantified from electron micrographs. Measurements were made at 2 defined locations–1 to 2 mm and 12 to 13 mm from the foveal center–in the inferotemporal quadrant. Oriented, 4-mm diameter, full-thickness punches of RPE-choroid-sclera were taken using a trephine punch and prepared for electron microscopy, as previously described. Four random photographic images were taken from each punched specimen using a JEOL JEM 1220 microscope (JEOL USA Inc). Light microscopical images were collected at ×2500 actual magnification and electron microscopical images at ×500 actual magnification.
Results
Eyes from 80 donors were selected on the basis of clinical records. Twenty-eight were rejected after examination of fundus or gross photographs because it was judged that there was evidence of choroidal neovascularization, such as patent sub-RPE vessels, subretinal fibrosis, and/or hemorrhage, which might have preceded atrophy. In 3 donors, alternative diagnoses, such as pattern dystrophy, were considered more likely. A further 8 donors were rejected when evidence of choroidal neovascularization within the GA was found on histopathology. In 37 donors, it was thought likely that visual loss was caused by GA and these formed the basis of this study. Of the 37 donors, 21 were female. The age range at donation was 70 to 94 years (median, 86 years). Twenty-five had bilateral GA. Of the 12 with unilateral GA, 10 had early AMD and 2 had a disciform lesion in the other eye. The remaining 4 donors lacked a clear edge of depigmentation of the fundus and it was considered that the clinical diagnosis was made on the basis of choroidal depigmentation. These eyes were also examined to test the conclusions derived from examination of the GA cases.
The edge of GA was recognized as the termination of continuous RPE. Internal to the edge there was absence of RPE cells over wide areas, although a few RPE cells were scattered over the area of GA in some specimens (Figure 1). A small number of cone nuclei were present in all cases, which were more numerous at the site of surviving RPE cells. None had inner or outer segments. In most eyes, the BM was thickened and the choriocapillaris was sparse in the region of GA, although this was quite variable from eye to eye.
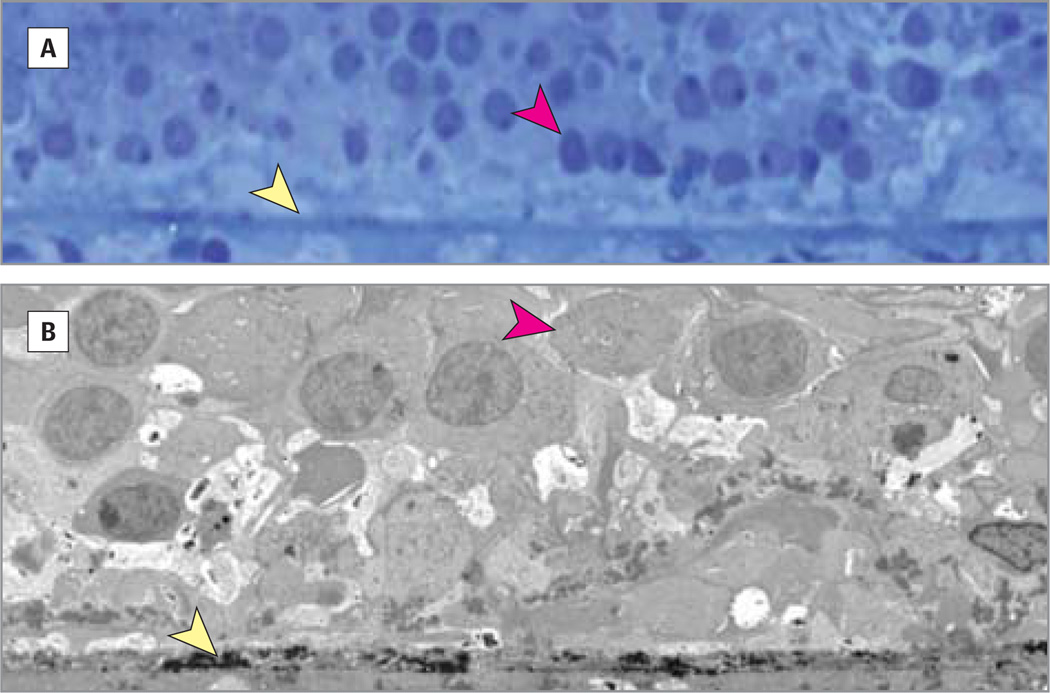
Figure 1. Micrographs.
In geographic atrophy photoreceptors and retinal pigment epithelium are largely absent. Light (A) and electron (B) micrographs of an 83-year-old female donor showing Bruch membrane (yellow arrowhead) loss of retinal pigment epithelium, and a few remaining cone nuclei (red arrowhead) in the area of geographic atrophy. The Bruch membrane is only moderately thickened and the choriocapillaris is perfused. Original magnifications are ×2500 (A) and ×500 (B).
In 5 donors, there was a sharp transition at the edge of the GA from a few cone nuclei to 4 to 5 rows of photoreceptor cells, both rods and cones, with inner and outer segments (Figure 2A and B). In the remaining 32 donors, there was a large transition zone in which there were only cone photoreceptors that may or may not have inner segments but no outer segments (Figures 3, 4, and 5). These were not more than 2 rows of nuclei thick. The zone extended as far as 1400 µm from the edge of the GA. Further from the edge, the outer nuclear layer became progressively thicker, cones had outer segments, and rods with outer segments appeared. The nature of the transition at the edge of the GA may vary within an eye (Figure 2). In areas lacking rods and cone outer segments, the RPE was well pigmented and contained as many autofluorescent phagolysosomes as more peripheral retina (Figures 2 and 3).
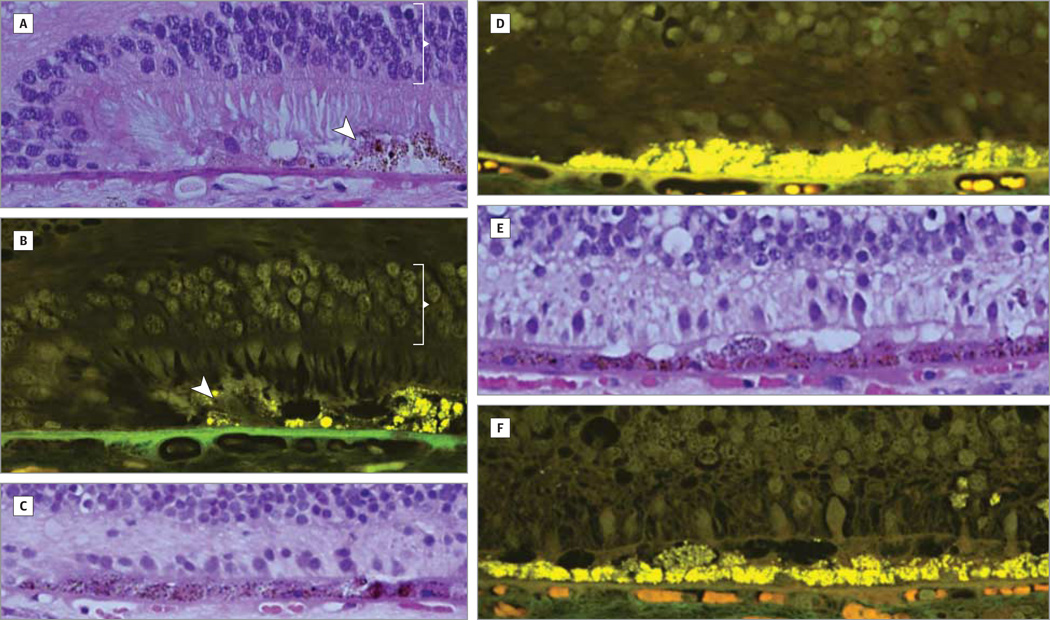
Figure 2. Micrograph and Autofluorescent Images.
There is variable loss of photoreceptor cells beyond the edge of geographic atrophy. Light micrographs and autofluorescent images of a 70-year-old male donor with bilateral geographic atrophy showing the edge of continuous retinal pigment epithelium (arrowhead) and a well-defined transition from geographic atrophy to retina with intact retinal pigment epithelium and rods and cones with outer segments (bracket; A and B). At another edge, the photoreceptor cells are restricted to a few cones with inner segments only (C and D). Over a distance of 1400 µm, only cones with few inner segments are seen (E and F). The Bruch membrane is irregularly thickened. The choriocapillaris is perfused throughout. Original magnification ×2500.
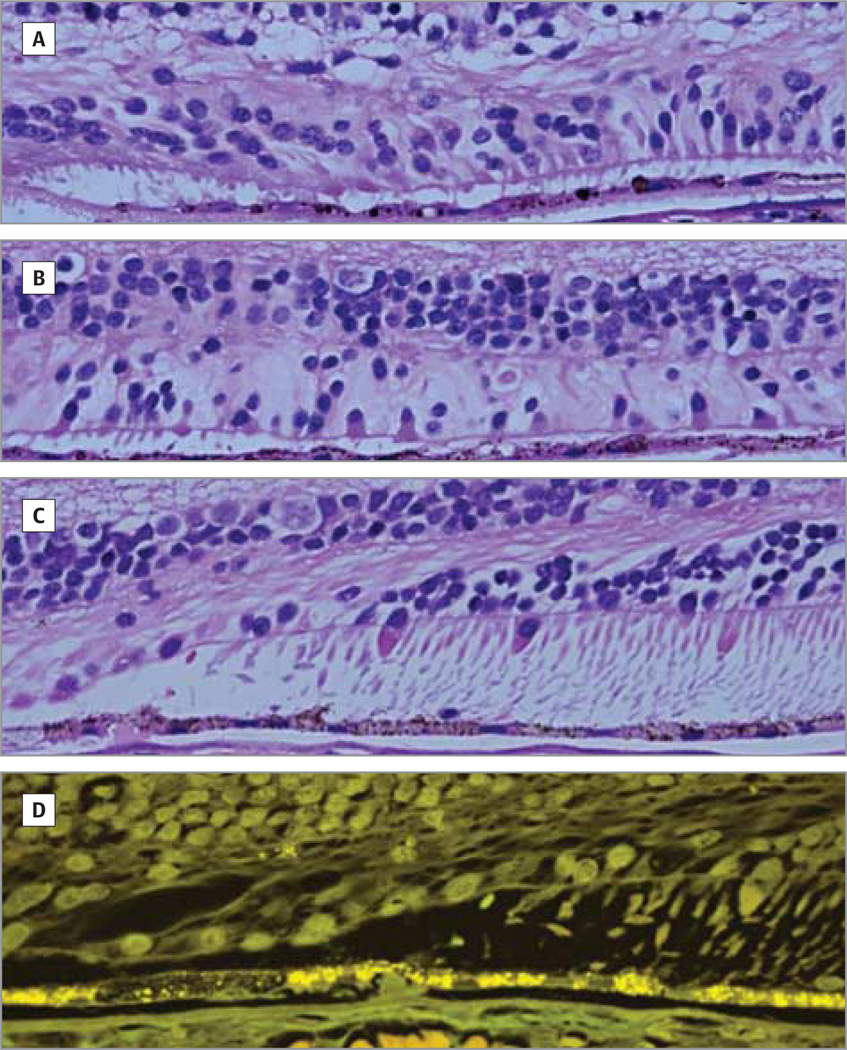
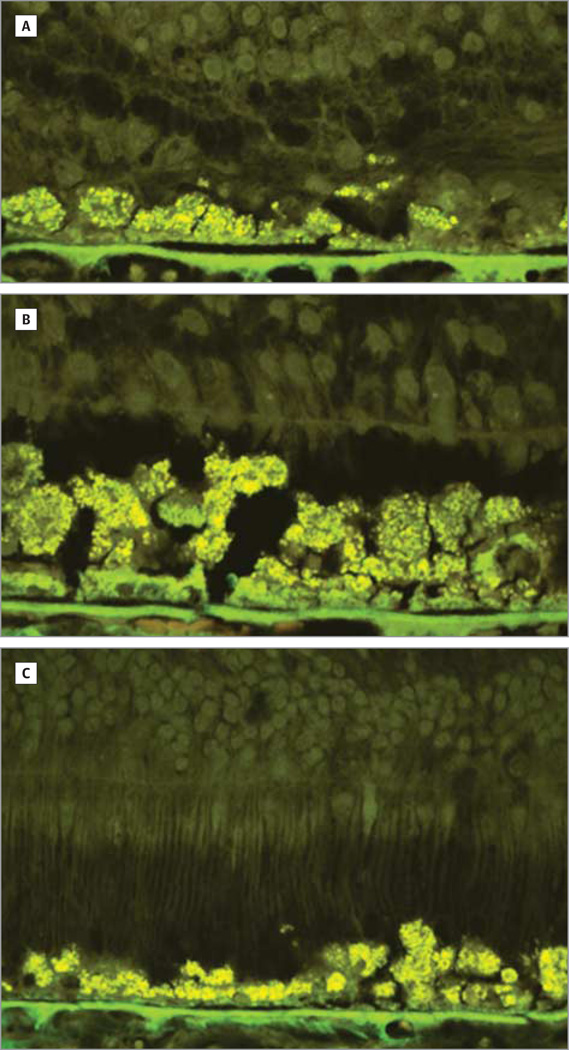
Figure 3. Light and Autofluorescence Micrographs.
There is variable loss of photoreceptor cells beyond the edge of geographic atrophy. Light (A–C) and autofluorescence (D) micrographs of an 84-year-old female donor in whom geographic atrophy had been diagnosed 3 years before death. A, At the edge of geographic atrophy, 1 to 2 layers of photoreceptor nuclei are seen. B, For up to 1200 µm from the edge, there are few cones with inner segments. Beyond 1200 µm, rods and cones with outer segments are seen (C and D), without there being a difference in the retinal pigment epithelium between that near the edge of geographic atrophy and that at 1200 µm away from the edge. The Bruch membrane is modestly thickened and the choriocapillaris is perfused. Original magnification ×2500.
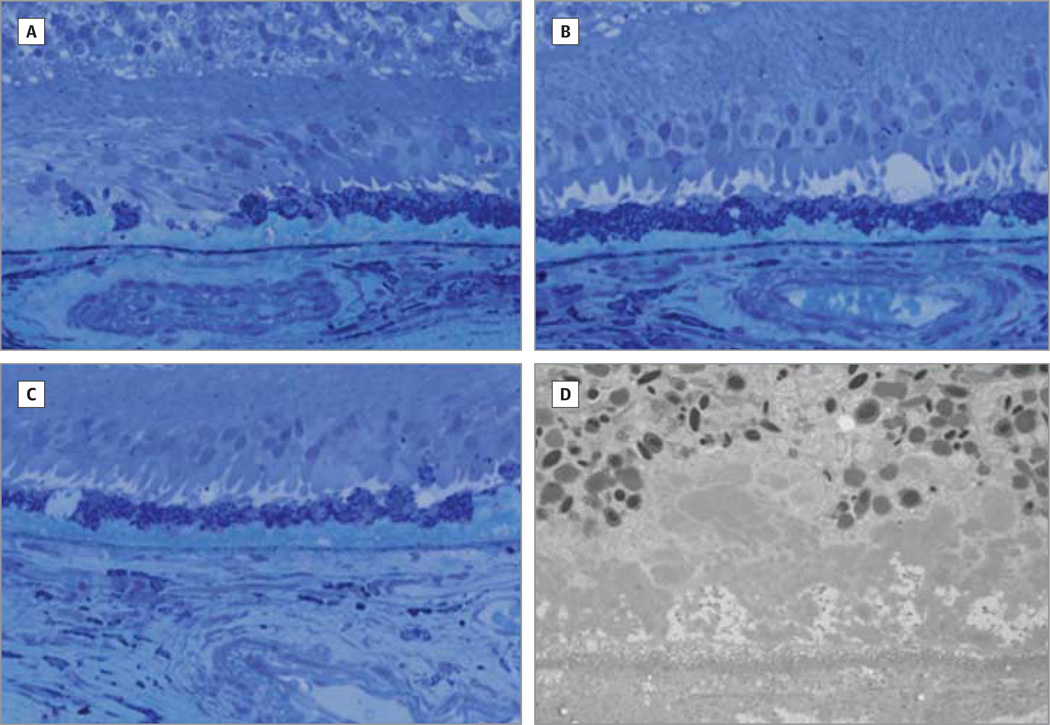
Figure 4. Light and Electron Microscopy.
Geographic atrophy associated with thickened Bruch membrane and absence of perfused choriocapillaris. Light (A–C) and electron (D) microscopy of a 78-year-old female donor in whom geographic atrophy had been diagnosed. Beyond the edge of geographic atrophy, the photoreceptor cell layer is 1 to 3 layers thick. The Bruch membrane is very thick owing largely to the presence basal laminar deposits. The choriocapillaris is not perfused. Original magnifications are ×2500 (A–C) and ×500(D).
Figure 5. Autofluorescent Imaging.
Photoreceptor-cell population is variable over multilayered retinal pigment epithelium. Autofluorescent imaging of a 96-year-old female donor showing areas of the retina with no (A), few (B), and normal (C) photoreceptors. In each case, the retinal pigment epithelium is more than 1-cell thick in places with considerable autofluorescence. In all, the Bruch membrane is moderately thickened and the choriocapillaris is perfused.
The thickness of the BM varied greatly from one specimen to another (Figures 2–5). In many, the BM was only modestly thickened, whereas in others, it was markedly thickened largely owing to the presence of large quantities of BLDs. There was no obvious correlation between BM thickness and photoreceptor survival, although in none was the photoreceptor thickness normal. In those with a very thick BM, the choriocapillaris was often absent (Figure 4), whereas in the remainder, the choroidal capillaries appeared to be perfused (Figure 2). In many specimens, the RPE was hyperplastic, with 2 or 3 layers of cells (Figure 5). This was most prominent in areas of reduced photoreceptor cells but was also seen where the outer nuclear layer was of normal thickness (Figure 5C).
The area of BLD per unit length of BM (a surrogate measure of BM thickness) was plotted against the area occupied by autofluorescent residual bodies over the same distance (Figure 6). There was an inverse relationship between the 2 features with a low coefficient of determination (R2 = 0.34; P = .03), implying a weak but undoubted inverse relationship between the 2 measures. In a proportion, there was neither great thickening of BM nor excess accumulation of autofluorescence. Thus, there was variability of physical changes in choroid, BM, RPE, and photoreceptor cells relative one to another and from one eye to another. In some, there was major photoreceptor loss in the absence of morphological changes in other tissues (Figure 2E).
Figure 6. Inverse Relationship Between Bruch Membrane Thickness and Retinal Pigment Epithelium (RPE) Autofluorescence.
The plot shows the cross-sectional area of the Bruch membrane plotted against the area of autofluorescent material in the RPE. There is a negative relationship between the 2 measures (P = .03, R2 = 0.34). BLD indicates basal laminar deposit.
The eyes considered to have choroidal depigmentation all had continuous RPE. The photoreceptor layer varied from 4 to 1 nuclei in thickness, but unlike the GA cases, they contained both rods and cones (Figure 7). Also unlike GA specimens, the photoreceptor cells had outer and inner segments.
Figure 7. Photoreceptor Loss in an Eye With No Geographic Atrophy But Choroidal Depigmentation.
Gross photograph (A) and light (B) and electron (C) microscopy of an 86-year-old woman who had depigmentation of the choroid showing reduction of photoreceptor cells with inner but few outer segments. The Bruch membrane is moderately thickened and the choriocapillaris is perfused. Original magnifications are ×2500 (B) and ×500 (C).
Discussion
The patterns of photoreceptor loss associated with GA in the assessed specimens are similar to those seen with OCT.12–14 We found that large numbers of photoreceptors close to the edge of GA existed in only a minority of specimens, although without serial sections of each eye it is impossible to give an accurate figure of the relative prevalence of the 2 patterns. The relative frequency of the different patterns differs from one clinical study in which an abrupt change was reported as the most common.14 The prevalence of different patterns is not stated in the other clinical studies.12,13 Differences could be explained on the basis of age. The average age of our donors was 85 years, which is greater than in the OCT studies (77.8, 72.4, and 57–80 years12–14). However, 8 of our donors were 76 years old or younger and the changes in them did not appear to be radically different from the cohort as a whole (Figure 2).
If the spatial pattern of change can be equated with the temporal course of the disease, it is evident that the demise of photoreceptor cells occurs earlier than the loss of RPE cells in most cases. This conclusion is supported by the observation that there was considerable photoreceptor loss in the eyes with choroidal depigmentation but without GA. The concept is also in accord with the conclusions drawn from previous morphological studies.7–9 It is notable that it is the rods that are more vulnerable and it appears that cones can survive for long periods without outer or inner segments. The observation of early rod loss would explain the functional loss in early AMD,15–19 implying that the functional loss in early AMD is likely to be due to photoreceptor cell death at least in part. In light of our findings, elevation of scotopic thresholds in early AMD by several thousand-fold is not surprising.
These observations are clearly important to the assessment of benefit in therapeutic trials. In patients with GA, the area of photoreceptor loss may be much greater than the area of RPE atrophy. Assuming the autofluorescence seen histopathologically is indicative of autofluorescence seen by clinical imaging, it is evident that fundus autofluorescence is not a reliable indicator of the extent or presence of photoreceptor loss. Thus, treatment trials that use visual acuity or autofluorescence delineation of RPE loss alone as a measure of therapeutic effect are not recording reliably the state of the photoreceptor cells.26–28 If the objective of treatment is directed at preservation of photoreceptors, autofluorescence imaging alone gives an incomplete record of the main therapeutic target . Other imaging techniques or functional testing would give a more complete index of therapeutic effect. This could be achieved by psychophysical measurement of rod and cone function or structural analysis using OCT, as has been advocated,14 or adaptive optics. Perhaps the most practical approach would be assessment of bleachable rhodopsin using reflectometry.29–32 The cause of photoreceptor loss has been ascribed to changes in the RPE as originally proposed by Hogan7 or in the BM. The major index of aging change in the RPE is considered to be accumulation of lipofuscin, and a relationship between increased autofluorescence and risk for GA has been established.10,11 The nature of the association may be related to free radical generation by lipofuscin or failure to degrade phagosomal material and recycling lipids causing short photoreceptor outer segments.33–36 However, a large area of autofluorescent granules and a multilayered RPE were not invariably associated with photoreceptor loss in our donors (Figure 5). Concerning BM, there is considerable evidence that lipid accumulation in a thickened membrane may impede metabolic exchange between the choroid and RPE.37–39 The inverse relationship between BLD average thickness and the amount of autofluorescence might be explained on the basis of the functional relationship between the 2 entities. Reduction of metabolic exchange between the choroid and RPE would predictably cause local deficiency of vitamin A, as has been suggested in Sorsby fundus dystrophy.40 It has been shown that reduced availability of vitamin A in rodents reduces RPE autofluorescence .41
Photoreceptor loss was also seen in eyes in which neither BM nor the RPE appeared abnormal. It is possible that functional abnormality may occur in the absence of clear structural change but the observation suggests that photoreceptor loss may be unrelated to either BM or RPE disease in some cases. One possible mechanism causing photoreceptor loss in such cases is derived from observations in the cfh knockout mouse in which there was reduced visual function in the absence of BM thickening; the BM was thinner than in aged matched mice.42 There was an excess of C3 protein in the outer retina and the photoreceptor outer segments were severely distorted. It was thought that there might be an imbalance between outer segment formation and outer segment shedding or of phagocytosis of shed outer segment tips. There is no clear concept of the significance of excess complement in the outer retina or the potential importance of complement to photoreceptor health. In this context, it may be relevant that the RPE expresses CFH protein inwards,43 CFH being a major fluid-phase regulator of the complement cascade. In addition, there is some evidence that mitochondrial changes may play a role in AMD,44 and it would not be surprising if such changes may affect photoreceptor cells preferentially because they have major energy requirements.
Conclusions
It is evident from our findings in eyes with GA that AMD does not follow the same course from one case to another. This should not be surprising in a multifactorial disorder in which there are multiple genetic and environmental risk factors. Proposed therapeutic approaches currently are directed toward modulating accumulation of lipofuscin in the RPE or mobilizing proteins and lipids in BM, thus causing it to become thinner.45–47 However, from structural studies, it would appear that changes in BM or the RPE are important in only a proportion of individuals with AMD. Thus, in some cases, photoreceptor loss may be due to BM thickening, and in others, loss may be due to accumulation of lipofuscin in the RPE or loss may be unrelated to BM or RPE changes. If a specific treatment were directed to unselected cases of GA with the objective of slowing or preventing photoreceptor loss, the therapeutic approach would not address the major problem in a large proportion of cases recruited to a study, which would reduce the chance of identifying treatment benefit. As a consequence, an effective treatment of AMD may be discarded. A much greater chance of success would be achieved by robust phenotyping of cases before recruitment to a trial. Measurement of absolute levels of autofluorescence would give some measure of RPE health. It would be good if it were possible to measure the thickness of BM and its lipid content. Choroidal thickness or choroidal blood flow may give some indication as to the biophysical properties of BM. If these conclusions are correct, such measures should be used to select patients for therapeutic trials to ensure that the treatment was appropriate to the nature of the disease in those recruited. These measures would also be used to monitor therapeutic effects. Finally, in some cases, photoreceptor loss may occur in the absence of change in other tissues and may be due to disease processes intrinsic to the neuroretina. Thus, foreknowledge of the distribution and density of photoreceptor cells would be important. Clearly, it would be attractive to have a single treatment for early AMD, whereby transition to late disease were modulated, but the current evidence implies that this is unlikely to occur. It is also the case that accurate phenotyping would allow better phenotype-genotype correlation. In time, it may be shown that genotype is a good predictor of phenotype and likely disease progression. These issues will be addressed in subsequent analyses of this GA cohort.
Acknowledgments
Funding/Support: This research was funded in part by National Institutes of Health grant R24-EY017404 (Dr Hageman), a grant from the American Macular Degeneration Foundation (Dr Hageman), and an unrestricted grant to the University of Utah Department of Ophthalmology and Visual Sciences (Moran Eye Center) from Research to Prevent Blindness Inc.
Role of the Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Bird and Hageman had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bird, Hageman.
Acquisition of data: All authors.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: Bird, Hageman.
Statistical analysis: Bird, Phillips.
Obtained funding: Hageman.
Administrative, technical, and material support: Phillips, Hageman.
Study supervision: Bird, Hageman.
Conflict of Interest Disclosures: Dr Hageman is a CAB member for Sequenom lnc; scientific founder and shareholder for Optherion Inc; SAB member for AGTC Inc; and scientific founder and shareholder for Voyant Biotherapeutics LLC. No other disclosures were reported.
Additional Contributions: We are extremely grateful to Lisa Hancox, BS, Ryan Lee, DDS, Heather Stockman, BA, COT, Jill Hageman, RN, Sheri McCormick, BA, and Julie Donahue, BA, for their assistance in the processing of tissues and acquisition of donor records. Their salaries were paid, in part, by National Institutes of Health grant R24-EY017404 (Dr Hageman). We also thank the Iowa Lions and Utah Lions Eye Banks for their dedication to eye and tissue donation. This study could not have been conducted without the precious gift of eyes from donors and their families.
Previous Presentation: The findings in this study formed the basis of the Charles Schepens Lecture presented at the Annual Meeting of the American Academy of Ophthalmology; October 9, 2012; Chicago, Illinois.
REFERENCES
- 1.Gass JDM. Drusen and disciform macular detachment and degeneration. Arch Ophthalmol. 1973;90(3):206–217. doi: 10.1001/archopht.1973.01000050208006. [DOI] [PubMed] [Google Scholar]
- 2.Sarks SH. Drusen patterns predisposing to geographic atrophy of the retinal pigment epithelium. Aust J Ophthalmol. 1982;10(2):91–97. doi: 10.1111/j.1442-9071.1982.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 3.Maguire P, Vine AK. Geographic atrophy of the retinal pigment epithelium. Am J Ophthalmol. 1986;102(5):621–625. doi: 10.1016/0002-9394(86)90535-0. [DOI] [PubMed] [Google Scholar]
- 4.Solomon SD, Jeffreys JL, Hawkins BS, et al. Submacular Surgery Trials Research Group. Risk factors for second eye progression to advanced age-related macular degeneration: SST report No. 21. Retina. 2009;29(8):1080–1090. doi: 10.1097/IAE.0b013e3181b1baeb. [DOI] [PubMed] [Google Scholar]
- 5.Maguire MG, Ying GS, McCannel CA, Liu C, Dai Y. Complications of Age-related Macular Degeneration Prevention Trial (CAPT) Research Group. Statin use and the incidence of advanced age-related macular degeneration in the Complications of Age-related Macular Degeneration Prevention Trial. Ophthalmology. 2009;116(12):2381–2385. doi: 10.1016/j.ophtha.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonasson F, Arnarsson A, Sasaki H, Peto T, Sasaki K, Bird AC. The prevalence of age-related maculopathy in Iceland: Reykjavik Eye Study. Arch Ophthalmol. 2003;121(3):379–385. doi: 10.1001/archopht.121.3.379. [DOI] [PubMed] [Google Scholar]
- 7.Hogan MJ. Role of the retinal pigment epithelium in macular disease. Trans Am Acad Ophthalmol Otolaryngol. 1972;76(1):64–80. [PubMed] [Google Scholar]
- 8.Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976;60(5):324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye (Lond) 1988;2(pt 5):552–577. doi: 10.1038/eye.1988.106. [DOI] [PubMed] [Google Scholar]
- 10.von Ruckmann A, Fitzke FF, Bird AC. Fundus autofluorescence in age-related disease imaged with a scanning laser ophthalmoscope. Invest Ophthalmol Vis Sci. 1997;38(2):478–486. [PubMed] [Google Scholar]
- 11.Holz FG, Bellmann C, Margaritidis M, Schütt F, Otto TP, Völcker HE. Patterns of increased in vivo fundus autofluorescence in the junctional zone of geographic atrophy of the retinal pigment epithelium associated with age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1999;237(2):145–152. doi: 10.1007/s004170050209. [DOI] [PubMed] [Google Scholar]
- 12.Wolf-Schnurrbusch UE, Enzmann V, Brinkmann CK, Wolf S. Morphologic changes in patients with geographic atrophy assessed with a novel spectral OCT-SLO combination. Invest Ophthalmol Vis Sci. 2008;49(7):3095–3099. doi: 10.1167/iovs.07-1460. [DOI] [PubMed] [Google Scholar]
- 13.Fleckenstein M, Charbel Issa P, Helb HM, et al. High-resolution spectral domain-OCT imaging in geographic atrophy associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49(9):4137–4144. doi: 10.1167/iovs.08-1967. [DOI] [PubMed] [Google Scholar]
- 14.Bearelly S, Chau FY, Koreishi A, Stinnett SS, Izatt JA, Toth CA. Spectral domain optical coherence tomography imaging of geographic atrophy margins. Ophthalmology. 2009;116(9):1762–1769. doi: 10.1016/j.ophtha.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen JC, Fitzke FW, Pauleikhoff D, Bird AC. Functional loss in age-related Bruch's membrane change with choroidal perfusion defect. Invest Ophthalmol Vis Sci. 1992;33(2):334–340. [PubMed] [Google Scholar]
- 16.Owsley C, McGwin G, Jr., Jackson GR, Kallies K, Clark M. Cone- and rod-mediated dark adaptation impairment in age-related maculopathy. Ophthalmology. 2007;114(9):1728–1735. doi: 10.1016/j.ophtha.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 17.Jackson GR, Owsley C, Curcio CA. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res Rev. 2002;1(3):381–396. doi: 10.1016/s1568-1637(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 18.Scholl HPN, Bellmann C, Dandekar SS, Bird AC, Fitzke FW. Photopic and scotopic fine matrix mapping of retinal areas of increased fundus autofluorescence in patients with age-related maculopathy. Invest Ophthalmol Vis Sci. 2004;45(2):574–583. doi: 10.1167/iovs.03-0495. [DOI] [PubMed] [Google Scholar]
- 19.Dimitrov PN, Robman LD, Varsamidis M, et al. Visual function tests as potential biomarkers in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(13):9457–9469. doi: 10.1167/iovs.10-7043. [DOI] [PubMed] [Google Scholar]
- 20.Bird AC, Bressler NM, Bressler SB, et al. The International ARM Epidemiological Study Group. An International Classification and Grading System for Age-Related Maculopathy and Age-Related Macular Degeneration. Surv Ophthalmol. 1995;39(5):367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- 21.Hageman GS, Mullins RF, Russell SR, Johnson LV, Anderson DH. Vitronectin is a constituent of ocular drusen and the vitronectin gene is expressed in human retinal pigmented epithelial cells. FASEB J. 1999;13(3):477–484. doi: 10.1096/fasebj.13.3.477. [DOI] [PubMed] [Google Scholar]
- 22.Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14(7):835–846. [PubMed] [Google Scholar]
- 23.Hageman GS, Mullins RF. Molecular composition of drusen as related to substructural phenotype. Mol Vis. 1999;5:28. [PubMed] [Google Scholar]
- 24.Russell SR, Mullins RF, Schneider BL, Hageman GS. Location, substructure, and composition of basal laminar drusen compared with drusen associated with aging and age-related macular degeneration. Am J Ophthalmol. 2002;129(5):205–214. doi: 10.1016/s0002-9394(99)00345-1. [DOI] [PubMed] [Google Scholar]
- 25.Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102(20):7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang K, Hopkins JJ, Heier JS, et al. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc Natl Acad Sci U S A. 2011;108(15):6241–6245. doi: 10.1073/pnas.1018987108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Göbel AP, Fleckenstein M, Schmitz-Valckenberg S, Brinkmann CK, Holz FG. Imaging geographic atrophy in age-related macular degeneration. Ophthalmologica. 2011;226(4):182–190. doi: 10.1159/000330420. [DOI] [PubMed] [Google Scholar]
- 28.Brader HS, Ying GS, Martin ER, Maguire MG. Complications of Age-Related Macular Degeneration Prevention Trial-CAPT Research Group. New grading criteria allow for earlier detection of geographic atrophy in clinical trials. Invest Ophthalmol Vis Sci. 2011;52(12):9218–9225. doi: 10.1167/iovs.11-7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kemp CM, Jacobson SG, Faulkner DJ. Two types of visual dysfunction in autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1988;29(8):1235–1241. [PubMed] [Google Scholar]
- 30.Kemp CM, Jacobson SG, Faulkner DJ, Walt RW. Visual function and rhodopsin levels in humans with vitamin A deficiency. Exp Eye Res. 1988;46(2):185–197. doi: 10.1016/s0014-4835(88)80076-9. [DOI] [PubMed] [Google Scholar]
- 31.Van Norren D, Tiemeijer LF. Spectral reflectance of the human eye. Vision Res. 1986;26(2):313–320. doi: 10.1016/0042-6989(86)90028-3. [DOI] [PubMed] [Google Scholar]
- 32.Morgan JI, Pugh EN., Jr. Scanning laser ophthalmoscope measurement of local fundus reflectance and autofluorescence changes arising from rhodopsin bleaching and regeneration. Invest Ophthalmol Vis Sci. 2013;54(3):2048–2059. doi: 10.1167/iovs.12-11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boulton M, Rozanowska M, Rozanowski B, Wess T. The photoreactivity of ocular lipofuscin. Photochem Photobiol Sci. 2004;3(8):759–764. doi: 10.1039/b400108g. [DOI] [PubMed] [Google Scholar]
- 34.Schütt F, Bergmann M, Holz FG, Kopitz J. Isolation of intact lysosomes from human RPE cells and effects of A2-E on the integrity of the lysosomal and other cellular membranes. Graefes Arch Clin Exp Ophthalmol. 2002;240(12):983–988. doi: 10.1007/s00417-002-0558-8. [DOI] [PubMed] [Google Scholar]
- 35.Finnemann SC, Leung LW, Rodriguez-Boulan E. The lipofuscin component A2E selectively inhibits phagolysosomal degradation of photoreceptor phospholipid by the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2002;99(6):3842–3847. doi: 10.1073/pnas.052025899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okubo A, Sameshima M, Unoki K, Uehara F, Bird AC. Ultrastructural changes associated with accumulation of inclusion bodies in rat retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2000;41(13):4305–4312. [PubMed] [Google Scholar]
- 37.Pauleikhoff D, Harper CA, Marshall J, Bird AC. Aging changes in Bruch's membrane: a histochemical and morphologic study. Ophthalmology. 1990;97(2):171–178. [PubMed] [Google Scholar]
- 38.Moore DJ, Hussain AA, Marshall J. Age-related variation in the hydraulic conductivity of Bruch's membrane. Invest Ophthalmol Vis Sci. 1995;36(7):1290–1297. [PubMed] [Google Scholar]
- 39.Starita C, Hussain AA, Patmore A, Marshall J. Localization of the site of major resistance to fluid transport in Bruch's membrane. Invest Ophthalmol Vis Sci. 1997;38(3):762–767. [PubMed] [Google Scholar]
- 40.Jacobson SG, Cideciyan AV, Regunath G, et al. Night blindness in Sorsby's fundus dystrophy reversed by vitamin A. Not Genet. 1995;11(1):27–32. doi: 10.1038/ng0995-27. [DOI] [PubMed] [Google Scholar]
- 41.Katz ML, Norberg M. Influence of dietary vitamin A on autofluorescence of leupeptin-induced inclusions in the retinal pigment epithelium. Exp Eye Res. 1992;54(2):239–246. doi: 10.1016/s0014-4835(05)80213-1. [DOI] [PubMed] [Google Scholar]
- 42.Coffey PJ, Gias C, McDermott CJ, et al. Complement factor H deficiency in aged mice causes retinal abnormalities and visual dysfunction. Proc Natl Acad Sci U S A. 2007;104(42):16651–16656. doi: 10.1073/pnas.0705079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim YH, He S, Kase S, Kitamura M, Ryan SJ, Hinton DR. Regulated secretion of complement factor H by RPE and its role in RPE migration. Graefes Arch Clin Exp Ophthalmol. 2009;247(5):651–659. doi: 10.1007/s00417-009-1049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jarrett SG, Lin H, Godley BF, Boulton ME. Mitochondrial DNA damage and its potential role in retinal degeneration. Prog Retin Eye Res. 2008;27(6):596–607. doi: 10.1016/j.preteyeres.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Radu RA, Han Y, Bui TV, et al. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: a potential therapy for treatment of lipofuscin-based retinal diseases. Invest Ophthalmol Vis Sci. 2005;46(12):4393–4401. doi: 10.1167/iovs.05-0820. [DOI] [PubMed] [Google Scholar]
- 46.Mandal MN, Moiseyev GR, Elliott MH, et al. Alpha-phenyl-N-tert-butylnitrone (PBN) prevents light-induced degeneration of the retina by inhibiting RPE65 protein isomerohydrolase activity. J Biol Chem. 2011;286(37):32491–32501. doi: 10.1074/jbc.M111.255877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding JD, Johnson LV, Herrmann R, et al. Anti-amyloid therapy protects against retinal pigmented epithelium damage and vision loss in a model of age-related macular degeneration. Proc Natl Acad Sci U S A. 2011;108(28):279–287. doi: 10.1073/pnas.1100901108. [DOI] [PMC free article] [PubMed] [Google Scholar]