Abstract
Objectives
The ultrastructural appearance of retinal capillaries can yield important information about disease mechanisms, but is not well characterised in human post mortem samples. We therefore aimed to create a baseline for the appearance of capillaries and establish how this is influenced by post mortem fixation delays and donor age.
Methods
Electron microscopy was used to characterise retinal capillaries in 20 anonymous donors (with no known eye diseases) of various ages and with various post mortem fixation delays. In addition, samples from six patients with conditions that are known to affect the retinal vasculature (four cases of type 2 diabetes without diabetic retinopathy, one case of diabetic retinopathy and one case of macular telangiectasia type 2) were analysed.
Results
Vacuoles were found in capillary basement membranes at the vessel—glia interface in all samples, from both the normal and disease cases. Vacuole frequency increased with donor age but was not influenced by post mortem fixation delays.
Conclusion
Vacuoles in the basement membrane are a normal feature of adult human retinal capillaries and do not indicate disease. Their incidence increases with age and might be a contributing factor to late-onset pathologies of the retinal vasculature.
INTRODUCTION
Histopathological studies of the retinal vasculature in post mortem human tissue are an essential part of research into retinal disease mechanisms. In diabetic retinopathy, for example, degenerative changes in the microvasculature, such as non-perfused capillaries and the formation of micro-aneurysms, are hallmarks of disease that have been characterised extensively both in living patients and in post mortem material. Ultrastructural analysis of cross-sections of retinal capillaries can provide critical insight into the health of the microvasculature that is not possible with normal light microscopy.
In diabetic retinopathy microvascular pathologies such as reduced pericyte–endothelial cell ratios and thickened capillary basement membranes have been recognised for decades. There are numerous studies in various animal models of diabetes demonstrating degenerating pericytes and capillary basement membranes of irregular and increased thickness, comprising debris-like inclusions and vacuoles, whereas in healthy control animals pericytes appear intact and the basement membrane is thin and of homogenous density and uniform thickness.1–4
Studies attempting to quantify these pathological changes in human tissue specimens are less conclusive. In adult humans the basement membrane of retinal capillaries is irregular, containing numerous vacuoles, and has a ‘Swiss-cheese’ appearance.5,6 Furthermore, evidence of pericyte degeneration is also a common finding in non-diabetic retinas,7 similar in many ways to the phenotypes described in diabetes. Against this background it is more difficult to assess pathological changes in humans in comparison to animal models, which in general maintain pristine capillary ultrastructure throughout adult life and can be processed immediately after death. Fixation procedures in humans are usually less than ideal because in most cases eye tissue can only be retrieved and fixed several hours after death, which can introduce artefacts. The post mortem delays may vary greatly between samples and are difficult to influence and control. Therefore, the majority of eye tissue from anonymous post mortem donations is not ideally suited for ultrastructural analysis.
We have therefore used post mortem retinal samples from anonymous human donors to establish how the ultrastructural appearance of retinal capillaries in post mortem human tissue is influenced by post mortem fixation delays and donor age.
MATERIALS AND METHODS
Donors and tissue processing
Post mortem retinal tissue was obtained from a rhesus macaque (aged 17 years), from 20 human, anonymous donors with no known ocular disease (ages ranging from 1 to 97 years), from four type 2 diabetic donors without and one with diagnosed proliferative diabetic retinopathy and from one donor with diagnosed macular telangiectasia (MacTel) type 2. All retina samples were taken from the mid periphery of nasal-inferior retina. Tissue was fixed immediately in Karnovsky’s fixative or a tannic acid/glutaraldehyde mix. Alternatively, tissue was first fixed in paraformaldehyde (PFA) and later transferred into Karnovsky’s fixative prior to processing (table 1).
Table 1.
Donor tissue and fixation information
| Age (years) | Cause of death | Post mortem delay |
Initial fixative | Time until Karnovsky’s fixative |
Ophthalmic history |
|---|---|---|---|---|---|
| Rhesus macaque (Macaca mulatta) | |||||
| 17 | Euthanasia | 15 min | Karnovsky’s | – | – |
| Human normal controls | |||||
| 1 | Unknown | 37 h | 2% PFA | 22 months | None reported |
| 10 | Unknown | 25 h | 2% PFA | 18 months | None reported |
| 18 | Cardiac arrest | 13 h | 2% PFA | 2 months | None reported |
| 19 | Motorbike accident | 18 h | 2% PFA | 2 months | None reported |
| 24 | Intracranial haemorrhage | 55 h | Karnovsky’s | – | None reported |
| 34* | Lung cancer | 8 h* | 2% PFA | 3 weeks | None reported |
| 42 | Hanging suicide | 20 h | 2% PFA | 16 months | None reported |
| 43 | Prescription drug overdose | 17 h | 2% PFA | 19 months | None reported |
| 47 | Rectal carcinoma | 8h | 2% PFA | 19 months | None reported |
| 55 | Surgical enucleation | <30 min | Tannic acid–glutaraldehyde | – | Extra ocular tumour |
| 63* | Prostate cancer | 8 h* | 2% PFA | 4 months | None reported |
| 71 | Throat cancer | 11 h | 2% PFA | 4 months | None reported |
| 73 | Cholangiocarcinoma | 7h | 2% PFA | 19 months | None reported |
| 74 | Lung cancer | 14 h | 2% PFA | 3 months | None reported |
| 75 | Unknown | 4h | Karnovsky’s | – | Healthy eye from a unilateral Coats’ disease case |
| 79 | Lung cancer | 13 h | 2% PFA | 19 months | None reported |
| 80 | Chronic obstructive pulmonary disease type II | 13 h | 2% PFA | 19 months | None reported |
| 84 | Lung cancer | 9h | 2% PFA | 19 months | None reported |
| 96 | Unknown | 47 h | 2% PFA | 3 months | None reported |
| 97 | Unknown | 48 h | 2% PFA | 3 months | None reported |
| Type 2 diabetes | |||||
| 74 | Intracranial haemorrhage | 7h | 2% PFA | 13 months | None reported |
| 77 | Pancreatic cancer | 8h | 2% PFA | 3 months | None reported |
| 78 | Stroke | 9h | 2% PFA | 15 months | None reported |
| 79 | Cardiac arrest | 9h | 2% PFA | 14 months | None reported |
| Diabetic retinopathy | |||||
| 71 | Cerebrovascular accident | <12 h | 2% PFA | 6 months | Diabetic retinopathy, laser surgery |
| MacTel type 2 and type 2 diabetes | |||||
| 75 | Unknown | 4h | Karnovsky’s | – | MacTel type 2, no retinopathy |
| Post mortem degradation controls | |||||
| 34* | Lung cancer | 56 h* | 2% PFA | 3 weeks | None reported |
| 63* | Prostate cancer | 56 h* | 2% PFA | 4 months | None reported |
‘Time until Karnovsky’s fixative’ refers to the time between initial fixation of the tissue and its transfer into Karnovsky’s fixative.
Indicates the normal controls from which the other donor eye was used as a post mortem degradation control.
Electron microscopy and image analysis
All tissue was osmium tetroxide-treated, dehydrated and embedded in araldite resin. Microvessels with a diameter of less than 10 µm were imaged using a Jeol 1010 transmission electron microscope (Jeol, Welwyn Garden City, UK) fitted with a Gatan Orius SC1000B camera (Gatan, Abingdon, UK). Epoxy resin-embedded tissue was also serially sectioned and imaged using the Gatan 3VIEW serial block face imaging system (Gatan, Abingdon, UK) fitted to a Zeiss Sigma variable pressure field emission scanning electron microscope (Zeiss, Cambridge, UK). Three-dimensional reconstruction and Boissonnat surface modelling was performed using Digital Micrograph (Gatan UK, Abingdon, UK) and Reconstruct software (Boston University, Boston, MA, USA).8.
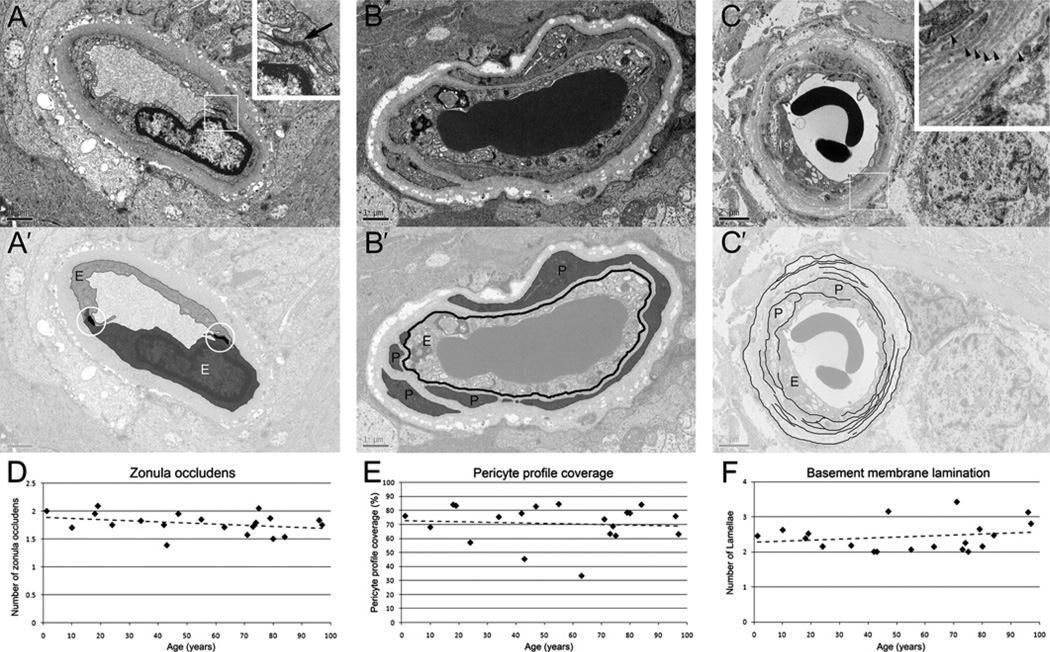
From each donor 29±7 capillaries were analysed. Vessels were chosen randomly in the inner nuclear layer and the retinal ganglion cell layer. The degree of basement membrane vacuolisation was established by assigning a score to each capillary cross-section based on the percentage of the total basement membrane area that is covered by vacuoles (0=0%, 1=1–20%, 2=21–40%, 3=41–60%, 4=61–80% and 5=81–100%, figure 2E,E’). Pericyte profile coverage was assessed by using the percentage of the circumference of the outer surface of the endothelial cell that was covered by a pericyte profiles (figure 3B,B’). Basement membrane lamination was assessed by scoring the highest number radially of separate lamellae per capillary cross-section. (figure 3C,C’). Averages were calculated for each donor. The images were scored independently by two observers and the quantification process was blinded. Spearman’s rank correlation coefficient was used to determine significance levels. The ρ value and p values were calculated using the software Stata10 Intercooled (StataCorp, College Station, TX, USA).
Figure 2.
Basement membrane vacuolisation correlates with donor age but not with post mortem delay. Vacuoles (arrow heads in B–E) are virtually absent in a 14-month-old donor (A) but are more frequent in older donors (B, 19 years old; C, 47 years old; D, 80 years old). Vacuolisation was scored (0 to 5) by assessing the proportion between the area of the vacuoles (arrowheads in E and white areas in E’) and the total area of the basement membrane (black areas in E’). (F) Scores significantly increased with donor age. (G) In contrast, vacuolisation scores (from all normal donors, filled diamonds) did not correlate with post mortem fixation delay. Furthermore, tests with pairs of eyes (squares in G), where one eye was fixed 8 h after death and the other eye was kept in phosphate-buffered saline for an additional 48 h at room temperature, showed no major changes regarding vacuolisation (open squares, 34-year-old donor; filled squares, 63-year-old donor). E, endothelial cell; P, pericyte profile; line in (F) is line of best fit. Scale bars are 1 µm in A–C and E, and 2 µm in D.
Figure 3.
Other features. (A) Endothelial cell numbers were quantified by counting tight junctions (black arrow in inset in A and circled in A’) per capillary profile, but plotting against donor age showed no age-related changes (D). (B) The percentage of endothelial cell basement membrane (black line in B’) covered by pericyte profiles (in grey) was used as an indication of pericyte coverage; plotting against donor age showed no correlation (E). (C) Basement membrane lamination (arrowheads in inset in C) was quantified by counting the maximum number of lamellae (black lines in C’) across a given capillary radius, but showed no donor age-related changes (F). E, endothelial cell; P, pericyte profile; stippled lines in D, E and F are lines of best fit. Scale bar is 1 µm in A and B, and 2 µm in C.
RESULTS
Fixation and post mortem delays
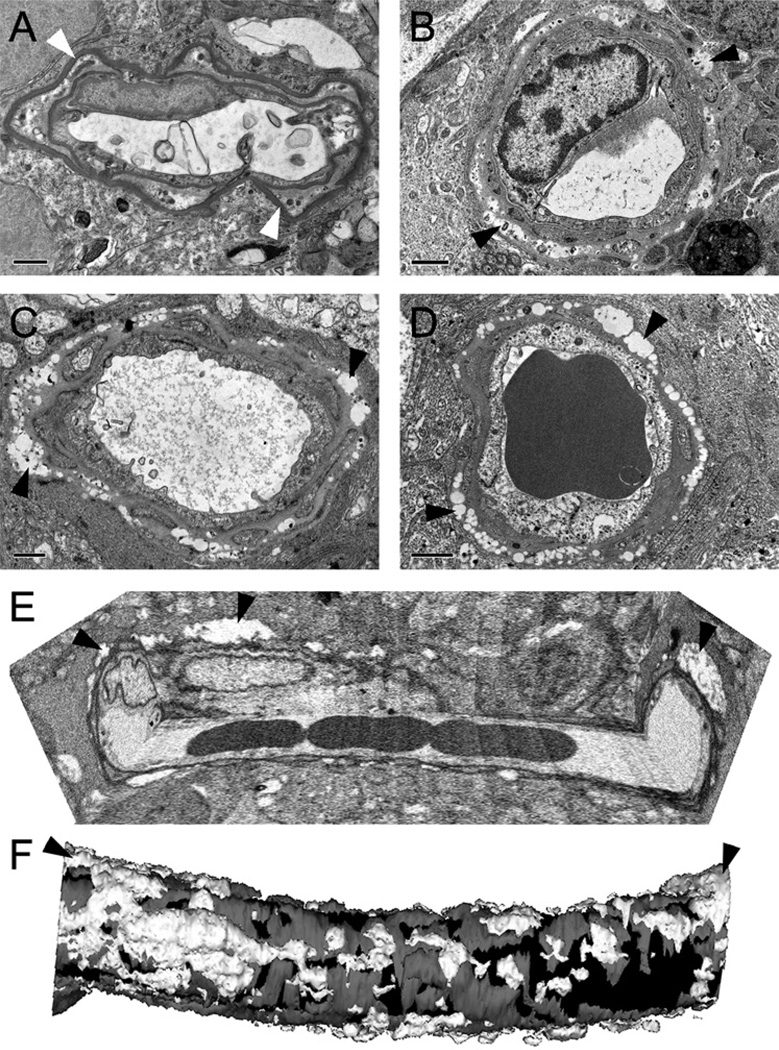
Since the cellular and ultrastructural changes that occur after death are not well characterised, it could be argued that apparent abnormalities in post mortem human retinal capillaries may be artefacts. In order to establish how post mortem delays and fixation methods may influence the ultrastructural appearance of retinal capillaries we used a well-preserved monkey retina as an example to demonstrate the normal appearance of capillaries and compared this with three differently fixed human samples. The monkey eye was fixed within 15 min of death in Karnovsky’s fixative (a fixative containing glutaraldehyde and para-formaldehyde). Retinal capillaries displayed normal basement membranes, and endothelial and pericyte profiles (figure 1A). In contrast, a human sample (donor aged 80 years) contained large vacuoles in the basement membrane near the glia interface (arrowheads in figure 1B). However, this sample was fixed 13 h after death with paraformaldehyde, stored for 19 months and then post-fixed in Karnovsky’s. It is therefore possible that the vacuoles may have been caused by the long post mortem delay or by suboptimal fixation. We therefore analysed a sample (donor aged 75 years) that had been fixed within 4 h of death directly in Karnovsky’s fixative. Remarkably, the same vacuoles could be found in the capillary basement membrane (arrowheads figure 1C).
Figure 1.
Vacuolisation of the basement membrane in human retinal microvessels. A capillary from a rhesus macaque retina (A) showed intact basement membranes and good pericyte coverage. In contrast, in humans (B–F) numerous vacuoles were present within the vascular basement membrane (arrowheads in B–F) irrespective of fixation methods and post mortem delays. The tissue in B was fixed 13 h after death and stored in 2% paraformaldehyde for 19 months before fixation in Karnovsky’s fixative for electron microscopy processing, whereas tissue in C was fixed directly in Karnovsky’s 4 h after death, and tissue in D–F was fixed within 30 min after surgical enucleation in tannic acid–glutaraldehyde solution. (E) Reconstruction of a vessel (from the enucleated eye) using 300 consecutive sections. (F) Three-dimensional reconstruction of the vessel in (E); endothelial cells and lumen (black), pericytes (grey) and basement membrane vacuoles (white). Scale bars are 1 µm in A–D.
To further exclude post mortem delay as factor that causes the basement membrane vacuoles we studied a sample from an enucleated eye (donor aged 55 years) that was fixed within less than 30 min of extraction in tannic acid and glutaraldehyde. Extensive basement membrane vacuolisation was also found in all capillaries (figure 1D), demonstrating that basement membrane vacuolisation is not a post mortem artefact but a normal feature present in adult human retinal capillaries.
To obtain more insight into the morphology of the basement membrane vacuoles we serially sectioned a 30 µm length of a retinal capillary from our best preserved sample (enucleated eye) using the Gatan 3VIEW system (figure 1E). The outer surface of endothelial cells, pericyte profiles and basement vacuoles were traced in each section, reconstructed and displayed as smoothened Boissonnat surfaces8 (figure 1F). This showed that the vacuoles were isolated or connected patches of varying size and shape, distributed seemingly randomly along the vessel.
Basement membrane vacuolisation
Since it has been reported that basement membrane vacuoles are absent in children,9,10 we studied donors of different ages. In the retina from a 14-month-old donor (with a 37 h post mortem delay) we found that the basement membranes from most capillaries did not contain any vacuoles (figure 2A). Due to the very long post mortem delay in this case, the tissue surrounding the capillaries was not well preserved. But importantly, this had no effects on the integrity of the capillary basement membrane, which further demonstrates that basement membrane vacuoles are not a post mortem or fixation artefact. In samples from older donors (19, 47 and 80 years old) basement membrane vacuoles could be readily detected (figure 2C,D). In some instances, in particular in older donors, capillaries were entirely surrounded by vacuoles (figure 2D).
In order to test whether basement vacuolisation correlates with donor age we analysed 20 anonymous donors (with no known eye pathologies) aged between 1 and 97 years. Vacuolisation was quantified on a scale from 0 to 5 (figure 2E,E’) and correlated clearly with the donor age (figure 2F, Spearman’s rank correlation coefficient, r=0.7203, p=0.0003).
Most of our samples were fixed within 24 h of death but we also included three samples that were fixed 50 h after death (table 1). Plotting the amount of basement membrane vacuolisation against post mortem fixation delays revealed no obvious correlation (figure 2G, Spearman’s rank correlation coefficient, r=−0.0674, p=0.7657). To further exclude the possibility of post mortem artefacts we included a controlled fixation delay by using a pair of eyes from a young donor (34 years old) with relatively low vacuolisation. One eye was fixed immediately (post mortem delay of 8 h) and the other eye was kept in phosphate-buffered saline for an additional 48 h at room temperature. This artificial delay did not introduce any major changes in the vacuolisation score. Similarly, the eyes from an older donor (63 years old) scored in the same range irrespective of fixation delays (figure 2G).
Other ultrastructural features
To assess whether retinal capillaries lose cellular components with increasing donor age we also quantified endothelial cell numbers and pericyte coverage. However, the number of tight junctions, used as an indication of endothelial cells numbers (figure 3A,A’), was not affected by donor age (Spearman’s rank correlation, r=−0.2742, p=0.2420, figure 4D). Similarly, a measure of pericyte coverage (figure 3B,B’) did not correlate with donor age (Spearman’s rank correlation, r=−0.0602, p=0.8008, figure 3E), suggesting the basement membrane vacuoles are not remnants of degenerated pericytes. Furthermore, we also noticed sporadic basement membrane lamination (figure 3C,C’) in most samples studied. This feature could conceivably be the result of successive rounds of pericyte degeneration and regeneration, but did not correlate with donor age either (Spearman’s rank correlation coefficient, r=0.1469, p=0.5364). Furthermore, there was no significant correlation between the level of pericyte coverage and basement lamination in our samples (Spearman’s rank correlation coefficient, r=0.3004, p=0.1982), nor was there any significant correlation between these two features and post mortem delays (not shown).
Figure 4.
Retinal capillary phenotypes in cases with known retinal diseases. (A–B) The retina from a 71-year-old donor with diabetic retinopathy showed acellular capillaries (A) and capillaries with no discernable pericyte profiles (B). Basement membrane vacuolisation (arrow heads in B was also observed. C shows a capillary from a 74-year old donor with type 2 diabetes but no diabetic retinopathy. (D) Capillaries from a macular telangiectasia (MacTel) type 2 donor also showed basement membrane lamination (arrow in D) and vacuolisation (arrowheads in D) and lack of pericyte profiles. E–H shows quantification of individual disease cases compared with the line of best fit previously determined for normal healthy donors. Open triangles, type 2 diabetic patients; filled triangles, diabetic retinopathy; open circles, MacTel type 2. Stippled line is line of best fit through normal donors only. Scale bars are 1 µm in A–D.
Capillary phenotypes in individual cases with known disease
After having established a baseline for the ultrastructural appearance of capillaries in a presumed normal donor population we wanted to test how samples from individual disease cases compare. A case with known diabetic retinopathy (71 years old) displayed well known characteristic of diabetic retinopathy such as acellular capillaries (figure 4A) and lack of pericytes (figure 4B). Not surprisingly, this case scored very low in our pericyte coverage measurements (black triangle in figure 4G). However the other traits (basement membrane vacuolisation and lamination and endothelial cell numbers) were found at equal frequency as in our control population (black triangles in figure 4E–F,H). Four cases with reported diabetes but no diabetic retinopathy (figure 4C) scored very similarly to our control population in all four measurements (open triangles in figure 4E – H).
We also studied a peripheral retina sample from a case that had been diagnosed with MacTel type 2. Two previous case reports from patients with MacTel type 2 have demonstrated degenerative changes of capillaries in the retinal periphery, including pericyte depletion and basement membrane lamination.11 12 In our MacTel type 2 sample we also found many capillaries that lacked pericytes and displayed basement membrane vacuolisation and lamination (figure 4D). However, these were extreme examples and quantification of multiple capillaries (circle in figure 4E – H) showed that peripheral capillaries in our MacTel type 2 case were normal overall.
DISCUSSION
Our study has revealed that with increasing age retinal capillaries in adult humans accumulate vacuoles in the basement membrane at the glia interface. Several lines of evidence demonstrate that these vacuoles are not post mortem or fixation artefacts. First, they were very rare in the 14-month-old donor whose tissues were fixed 37 h after death (one of the longest post mortem delays in our study). Second, the vacuoles were widespread in our two samples that were fixed within 30 min and 4 h after enucleation. Third, even the artificial introduction of post mortem delays had no noticeable influence on capillary morphology. Last, there was a statistically highly significant correlation between the amount of vacuolisation and donor age.
It should be kept in mind that fixation procedures for human post mortem tissue are rarely ideal and, particularly with samples from specific diseases, investigators are usually forced to work with whatever tissue is obtainable. In that respect, the samples used in our study are representative of the type of material that is typically available to investigators and can provide a baseline against which disease-specific features can be identified in suboptimally fixed tissue.
Interestingly, even capillaries with very high levels of vacuolisation did not seem to be disease-specific and were common in the elderly. Obviously, we cannot exclude underlying disease in anonymous donors. However, the fact that the vacuolisation appeared in all of the samples we tested and that it increased with age strongly suggests that this is a normal ageing process. Although it was demonstrated in the early 1960s that basement membranes in adult human retinal capillaries contain vacuoles,9 this is not widely reported in the literature. Therefore it is not well known that retinal capillaries in normal adult humans appear to have a phenotype that normally would be considered as pathological. In fact, vacuole-free capillaries in humans are only present in children,9 whereas capillaries in mature animals of several species (monkeys, dogs, cats and rats) do not display the basement membrane vacuoles seen in adult humans.13–15 In keeping with this, the macaque eye used in this study came from an elderly female (past her reproductive age, 17 years old) but showed no vacuoles. It is therefore possible that basement membrane vacuolisation relates to absolute lifespan or alternatively, it might be a phenomenon specific to the human species.
So far the chemical nature of the basement membrane vacuoles is not clear. Their morphology is reminiscent of age-related lipid inclusions previously describe in Bruch’s membrane.16 Furthermore, it has been shown that lipids can accumulate in larger retinal vessels in diabetic retinopathy.17 However, the molecular composition of vacuoles in capillary basement membrane has yet to be established.
It is not clear by what mechanism basement membrane vacuoles are created. Long-term stress, such as hypoxia, might be a contributing factor; in particular in the outer retina, which is more prone to experience hypoxic stress.18 However, we have not noticed any obvious differences between the inner and outer vascular plexus. Furthermore, our analysis was limited to the retinal periphery where hypoxic stress is likely to be less pronounced than in the macula.
It is also possible that the vacuoles are remnants of degenerated pericytes. This would imply a turnover of degenerating and regenerating pericytes throughout the human lifespan, which could also explain the multiple basement membrane sheaths. However, so far no studies have tested such a hypothesis specifically and we did not find a correlation between basement membrane lamination, pericyte coverage and donor age. Traditionally, pericyte coverage has been assessed by counting cell nuclei in trypsin digests of entire retinas.19 Nevertheless, our electron microscopy-based method of quantifying pericyte coverage seems to be suitable to identify the case of diabetic retinopathy, confirming the validity of this approach.
Our study suggests that caution should be exercised in the interpretation of ultrastructural capillary abnormalities in case studies of retinal vasculature diseases. For example, in our case of MacTel type 2 we found in the peripheral retina capillaries with strong basement membrane vacuolisation and lamination confirming the findings of two previous MacTel type 2 case reports.11 12 However, our quantification showed that these two ultrastructural features were within a normal range of capillary phenotypes typical for a person of that age, suggesting that MacTel type 2 does not induce any disease-specific changes in peripheral retinal capillaries, although we cannot exclude that some focal changes may be masked by global quantification. On the other hand, we also readily found similarly affected capillaries in all the control samples of that age group, further arguing against disease-specific changes in the periphery of the MacTel type 2 sample.
The question remains how capillaries with such grossly distended basement membranes, as we observed in some elderly, normal donors, can remain functional at all. So far it is not even known whether these changes are beneficial or detrimental to vascular function. If the latter were the case, such a progressively worsening function of the glia–vascular unit might contribute to age-related pathologies in the retina.
Acknowledgments
Funding Lowy Medical Research Institute, NIH R24 EY017404 (GSH), Fight for Sight (UK) and an unrestricted grant to the University of Iowa Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness.
Footnotes
Competing interests None to declare.
Ethics approval Institutional Review Board/Ethics Committee approval for post mortem eye tissue collection and storage was in place at the UCL Institute of Ophthalmology, University of Sydney, University of Iowa Department of Ophthalmology and the Centre for Macaques, Salisbury, UK.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Hainsworth DP, Katz ML, Sanders DA, et al. Retinal capillary basement membrane thickening in a porcine model of diabetes mellitus. Comp Med. 2002;52:523–529. [PubMed] [Google Scholar]
- 2.Stitt AW, Anderson HR, Gardiner TA, et al. Diabetic retinopathy: quantitative variation in capillary basement membrane thickening in arterial or venous environments. Br J Ophthalmol. 1994;78:133–137. doi: 10.1136/bjo.78.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson HR, Stitt AW, Gardiner TA, et al. Diabetic retinopathy: morphometric analysis of basement membrane thickening of capillaries in different retinal layers within arterial and venous environments. Br J Ophthalmol. 1995;79:1120–1123. doi: 10.1136/bjo.79.12.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson EC. Scanning and transmission electron microscopic studies of normal and diabetic acellular glomerular and retinal microvessel basement membranes. Microsc Res Tech. 1994;28:165–177. doi: 10.1002/jemt.1070280302. [DOI] [PubMed] [Google Scholar]
- 5.Bloodworth JM, Jr, Molitor DL. Ultrastructural aspects of human and canine diabetic retinopathy. Invest Ophthalmol. 1965;4:1037–1048. [PubMed] [Google Scholar]
- 6.Hogan MJ, Feeney L. The ultrastructure of the retinal blood vessels. II. The small vessels. J Ultrastruct Res. 1963;49:29–46. doi: 10.1016/s0022-5320(63)80034-9. [DOI] [PubMed] [Google Scholar]
- 7.Addison DJ, Garner A, Ashton N. Degeneration of intramural pericytes in diabetic retinopathy. Br Med J. 1970;1:264–266. doi: 10.1136/bmj.1.5691.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiala JC. Reconstruct: a free editor for serial section microscopy. J Microsc. 2005;218:52–61. doi: 10.1111/j.1365-2818.2005.01466.x. [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa T. Fine structure of retinal vessels in man and the macaque monkey. Invest Ophthalmol. 1963;2:1–15. [PubMed] [Google Scholar]
- 10.Hogan MJ, Alvarado JA, Weddell JE. Histology of the Human Eye: An Atlas and Textbook. Philadelphia: WB Saunders; 1971. pp. 508–519. [Google Scholar]
- 11.Eliassi-Rad B, Green WR. Histopathologic study of presumed parafoveal telangiectasis. Retina. 1999;19:332–335. doi: 10.1097/00006982-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Green WR, Quigley HA, De la CZ, et al. Parafoveal retinal telangiectasis. Light and electron microscopy studies. Trans Ophthalmol Soc U K. 1980;100:162–170. [PubMed] [Google Scholar]
- 13.Gardiner TA, Anderson HR, Degenhardt T, et al. Prevention of retinal capillary basement membrane thickening in diabetic dogs by a non-steroidal anti-inflammatory drug. Diabetologia. 2003;46:1269–1275. doi: 10.1007/s00125-003-1147-z. [DOI] [PubMed] [Google Scholar]
- 14.Mansour SZ, Hatchell DL, Chandler D, et al. Reduction of basement membrane thickening in diabetic cat retina by sulindac. Invest Ophthalmol Vis Sci. 1990;31:457–463. [PubMed] [Google Scholar]
- 15.Stitt AW, Bhaduri T, McMullen CB, et al. Advanced glycation end products induce blood-retinal barrier dysfunction in normoglycemic rats. Mol Cell Biol Res Commun. 2000;3:380–388. doi: 10.1006/mcbr.2000.0243. [DOI] [PubMed] [Google Scholar]
- 16.Ruberti JW, Curcio CA, Millican CL, et al. Quick-freeze/deep-etch visualization of age-related lipid accumulation in Bruch’s membrane. Invest Ophthalmol Vis Sci. 2003;44:1753–1759. doi: 10.1167/iovs.02-0496. [DOI] [PubMed] [Google Scholar]
- 17.Cusick M, Chew EY, Chan CC, et al. Histopathology and regression of retinal hard exudates in diabetic retinopathy after reduction of elevated serum lipid levels. Ophthalmology. 2003;110:2126–2133. doi: 10.1016/j.ophtha.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003;121:547–557. doi: 10.1001/archopht.121.4.547. [DOI] [PubMed] [Google Scholar]
- 19.Kuwabara T, Cogan DG. Studies of retinal vascular patterns. I. Normal architecture. Arch Ophthalmol. 1960;64:904–911. doi: 10.1001/archopht.1960.01840010906012. [DOI] [PubMed] [Google Scholar]