Abstract
Introduction
The size and composition of the European Union healthcare workforce are key drivers of expenditure and performance; it now includes new health professions and enhanced roles for established professions. This project will systematically analyse how this has contributed to health service redesign, integration and performance in 9 European countries (Scotland, England, Netherlands, Germany, Italy, Czech Republic, Poland, Norway, and Turkeyi). This paper describes the protocol for collection of survey data in 3 distinct care pathways, and sets it in the context of the wider programme.
Methods
Questionnaires will be distributed to healthcare professionals (n=14 580), managers (n=3564) and patients (n=19 440) in 3 care pathways (breast cancer; type 2 diabetes; and coronary heart disease) within 12 hospitals and associated primary care settings in each country. Questionnaire topics will include demography, the different professionals working on the care pathway, the tasks they do and the time taken, their decision-making abilities when considering skill mix, specialisation and integration of care. Patient satisfaction, healthcare utilisation and preferences will be explored. In later work, register data and data from patient records will be used to record clinical outcomes. Data will also be collected on workforce and procedure costs. Descriptive analysis will identify the different models of care and multivariate analysis will establish the most clinically and cost-effective models.
Ethics and dissemination
This protocol was approved by ethical committees in each country. Findings will be disseminated through national/international clinical, health services research and health workforce conferences, and publications in national/international peer-reviewed journals.
Introduction
Background and rational
Workforce is the largest single component of healthcare expenditure in European Union (EU) member states.1 The size and composition of the healthcare workforce are key drivers of both expenditure levels and the performance of healthcare systems. Both the size and composition of the healthcare workforce are changing in many European countries in response to measures to contain healthcare expenditures, changing needs for healthcare, and changing working patterns (eg, feminisation of the workforce, with increasing demands of childcare and the move towards part time working, and implementation of working time legislation).
In a number of countries there have also been substantial innovative developments in health workforce skills. New health professions have been introduced (eg, physician associates (PAs)2) and enhanced roles for established professions (such as nurses, and pharmacists) have been developed.3 4 The term ‘new professional roles' is used in the remainder of this paper to describe both these scenarios. New professional roles potentially lead to the delegation of care from doctors to other healthcare professionals (in which case the doctor may still retain a supervisory role and remains responsible for the overall care of the patient5) and the substitution of roles (in which a professional, such as a nurse prescriber,6 assumes full responsibility for a task (prescribing) previously the preserve of a doctor). Both of these have further ramifications as care previously delivered by, for example, a nurse is now delivered by a healthcare assistant.7 New professional roles have the potential to contribute to increased effectiveness and efficiency in service delivery,8–11 and mapping the skills and competencies of the health workforce has been identified as one of the key areas for action by the European Commission (EC).12 As new professional roles become more universal, current approaches to workforce planning will need to be adapted to include these new models of service delivery. Furthermore, at a time when integrated care is regarded as a quality marker, it is important to understand how it is affected, if at all, by the deployment of an increasingly diverse workforce.
This paper describes the protocol for surveys, in nine countries, which are part of a wider programme of work entitled Health Care Reform: The iMpact on practice, oUtcomes and costs of New roles for health pROfeSsionals (MUNROS: http://www.abdn.ac.uk/munros). The ultimate aim of the MUNROS programme is to inform a workforce planning model based on integrated financial and health service planning, and the competencies needed to deliver care, rather than professional qualifications. The programme will systematically study the workforce issues described above in primary and secondary healthcare settings—along the three clinical pathways for breast cancer, type 2 diabetes and coronary heart disease following an ST segment elevation myocardial infarction (STEMI)—in nine European countries (Scotland, England, Netherlands, Germany, Italy, Czech Republic, Poland, Norway, and Turkeyi). The design of the overall MUNROS programme is observational and cross-sectional, combining the questionnaire surveys described in this paper (including a patient-completed discrete choice experiment (DCE)) with patient-level, hospital-level and country-level data on clinical outcomes as available from routinely held databases, and unit costs of care consumption. Economic modelling using multicriteria decision analysis (MCDA) will inform a final synthesis to identify optimal models of care and distinguish the critical elements of these models. The findings will be incorporated into a generic multiprofessional workforce planning tool; this will be developed by mapping the tasks performed to the skills and competences required to undertake these tasks, alongside estimates of projected patient need. In each partner country, a Country Expert Advisory Group (CEAG) has been convened to support and advise the project. The study is also advised by an international Expert Advisory Board (EAB).
There were three pieces of work undertaken in earlier stages of the MUNROS programme which informed the development of the surveys. First, the key features of the health delivery systems in the nine countries of study were detailed through analysis of routinely collected data from international and national statistical offices and national health services, and a systematic review of published research, policy documents and grey literature was conducted.3 Second, again using routinely available data, the skill mix of the health workforce in the primary and secondary care sectors in all European countries was detailed, and then details of new professional roles and the numbers working in them in each sector in the nine partner countries were described.
Following this high-level analysis, three clinical conditions were selected for more in-depth study in the remainder of the programme. The conditions were selected from a list generated in the earlier scoping work in the nine partner countries that identified the clinical areas in which the new professional roles were employed. This list was supplemented with suggestions from clinical managers and workforce managers who were members of each partner's CEAG and the international EAB. A 2-day face-to-face meeting of a stakeholder group comprising invited expert representatives from the medical and non-medical healthcare professionals, primary and secondary care settings, managers and patients reviewed, scrutinised and made additions to the list of potential conditions and agreed selection criteria (box 1).
Box 1. Clinical criteria for selection of care pathways.
The clinical condition is of high prevalence, significant morbidity and mortality are associated with the condition, and data on these exist (ie, a burden to society)
Data exists on health outcomes that are related to new professional roles and/or the integration of care: outcomes of processes (eg, patient follow-up and integration of care, patient satisfaction), intermediate health outcomes (eg, clinical health outcomes, avoided complications), and final outcomes of care (eg, patient quality of life)
Procedures and clinical management are similar across different national boundaries
Care could be delivered by a range of health professionals—in at least some of the partner countries care is delivered by either new professions or new roles for existing professions. The contribution of different professions varies across partners
Patients have a role in managing the condition
Care is delivered in primary and secondary settings, and desirably in intermediate and tertiary care settings. Overall, at least one care pathway will have a substantial presence in primary care setting while another one will have a substantial presence in a secondary care setting
Applying the criteria resulted in four clinical conditions and associated care pathways being identified: hip replacement/hip fracture, breast cancer, type 2 diabetes and coronary heart disease following a STEMI. These four were then assessed by each of the nine partner countries for use of new healthcare professionals and availability of routine data (required for assessment of clinical outcomes). As a result of this, hip replacement/hip fracture was excluded.
The final three clinical conditions can be considered, respectively, as examples of: a condition requiring a scheduled surgical intervention, postoperative and follow-up care; a long-term condition managed largely in primary care, but with support from secondary care; a condition presenting acutely and requiring unscheduled hospital care, rehabilitation and long-term care. The care pathways for each of these conditions were then identified as the clinical context for all subsequent research.
Following selection of the pathways, case studies were conducted. The case studies sought to understand the new professional roles that were being delivered, the mechanisms and drivers for greater skill mix in the delivery of care, and the delegation of tasks from medical to other members of the healthcare team. Eight of the nine partners conducted case studies in two care pathways that were selected to ensure that across the partners, several case studies were conducted in each of the three pathways.
Objectives of the surveys
The overall aim of the surveys is to describe and quantify the use of new professional roles in primary and secondary care sectors in three care pathways in nine European countries, to understand their effects on the quality of care, and on the delivery of integrated care. Later stages of the project will evaluate their clinical and cost effectiveness; select the most effective and efficient service models as benchmarks; and develop a workforce planning tool based on the competencies required to meet population needs.
Methods
Conceptual framework
The MUNROS project researches the relationship between the inputs to the health service, focusing in particular on the staff input, and the outputs of the health service, focusing on patient outcomes. Where the focus of research is on the quantity and mix of different types of staff, rather than by institution, the appropriate conceptual framework is that of a production function employed in economics. Thus, the relationship which is the focus of the research can most concisely be defined as:
| 1 |
Equation (1) states that clinical outcomes for a sample of patients, i (where i=1…N), in receipt of treatment along care pathway P, in hospital H1, in country C, results from the activities of the workforce, identified by L, in pathway P, at hospital H1, in country C together with all the other non-staff inputs for care, here defined by K.
The project design seeks to distinguish hospitals which employ new professions and those which employ both new and established professions within the same care pathway. Using the above notations, it seeks to distinguish a hospital H1 in which only established professions, L1, are employed and a second hospital H2 in which both established professions, L1, and new professions, L2, are employed. A comparison of the clinical outcomes for patients along this pathway in these two hospitals, as in equation (1) (above) and equation (2) (below) will then distinguish the impact of employing new professions.
| 2 |
The advantages of this specification are that it:
Controls for heterogeneity in the clinical outcome mix, O, by moving from the health service as a whole to defined care pathways identified in the earlier developmental work. Measures of clinical output which are specific to the patients treated along each pathway will be obtained.
Captures differences in service design which result in differences in staff mix.
Controls for heterogeneity in patient characteristics, i, by obtaining details of a wide range of characteristics in the patients' questionnaire and through the use of vignettes in the health professionals' questionnaires. These vignettes present respondents with a standardised clinical episode: a patient presenting at a particular stage in the pathway with a highly specific condition which requires treatment and which is accompanied by a specific set of comorbidities. This eliminates the issue of unmeasured comorbidities in this specific treatment group.
Clinical protocols reduce heterogeneity in other inputs to health outcomes, as indicated by K, for these determine the management of the disease by prescribing the procedures, drugs and technologies used in treatment.
The core of the surveys requires health professionals, managers and patients to identify who does what at each stage along the three care pathways. The tasks needed to deliver care along each pathway, and the professional(s) undertaking those tasks will be identified together with actual and potential substitutions. When associated ultimately with cost and clinical output data, it will enable the identification of the most efficient combination of skills and competencies to achieve a given level of clinical output, or the combination of skills and competencies that will achieve the highest level of clinical output for a given cost.
Study design
This is a cross-sectional survey using self-completed questionnaires, either distributed by post or handed out at staff meetings or patient clinics for three specific care pathways (breast cancer, type 2 diabetes and coronary heart disease following a STEMI).
Study setting
The study setting is 12 hospitals and 60 associated primary care centres (average 5 per hospital) in each of the nine countries. Careful selection of hospitals enables us to reduce unmeasured heterogeneity. It is reasoned that similar types of hospitals are likely to employ the same technology. Thus, teaching hospitals are likely to employ some of the latest technology available to the health service and are more likely to be engaged in research with associated funding opportunities for new developments. Large hospitals may have similar volumes of throughput along a care pathway (assuming that volume of throughput is one determinant of the quality of clinical outcomes).
Countries were selected to reflect the diversity of systems in Europe and the different stages of reform of healthcare systems. They include those: in the later stages of transition from highly centralised (ex-communist) systems (Czech Republic and Poland), at the forefront of innovation of delivery systems (Netherlands, Scotland and England), with more established and stable systems (Germany, Italy and Norway), and a rapidly developing country (Turkey).
Participants and eligibility
There are two categories of participants who will be identified and recruited from a participating hospital or general practice.
Healthcare professionals and managers: All healthcare professionals providing care to patients within one of the three selected care pathways—from the point of diagnosis to long-term follow-up—will be invited to take part, together with all healthcare managers responsible for decision-making about the workforce providing care for these patients.
Patients: A random sample of patients within one of the three selected care pathways will be eligible to take part as long as they meet the following inclusion criteria:
Male or female patients aged 21 years and over (note there is no upper age limit);
Receiving care in one of the three care pathways: breast cancer; type 2 diabetes; and coronary heart disease;
Having capacity to understand the purpose of the study and complete the questionnaire.
In addition, the following disease-specific inclusion criteria will be applied:
Patients with coronary heart disease—have suffered a STEMI, are stabilised (ie, may still be in initial hospital admission) or up to 2 years in follow-up;
Patients with breast cancer—have been diagnosed and received some treatment for breast cancer, and are between 3 months and 2 years post-surgery;
Patients with type 2 diabetes—have been diagnosed with type 2 diabetes and are at least 3 months post diagnosis to 2 years in follow-up.
Identification and recruitment of sites and participants
Hospitals and primary care centres
Hospitals vary by type, location, size and population served, and the organisation within which they are managed. All of these factors may influence the extent to which new healthcare professionals/new professional roles are employed in the care of patients. Identification and recruitment of the hospitals will be based on the following procedure adapted to local circumstances so as to ensure representation of each of these factors. Thus, all hospitals in each country will be listed, and the list stratified by: type (teaching hospitals and general hospitals), geographical region, rurality (urban, suburban or rural), and sociodemographic characteristics of the catchment area (deprived and less deprived). Eligible hospitals will be invited to consider taking part by mailing an invitation pack (covering letter, participant information sheet, and expression of interest form) to hospital directors or their delegated deputy. From those expressing interest, 12 hospitals will be selected according to the criteria outlined above under ‘Study setting’ section. Hospital consent to participate will obtained by mailing invitation packs (covering letter, participant information sheet, and consent forms) either to hospital directors or clinical leads for each condition (or as appropriate in non-UK countries) according to preference of hospital. Ideally hospitals should be providing care along two of the three selected care pathways.
Primary care centres associated with each hospital will be similarly selected. All primary care providers in the catchment area of the recruited hospitals will be contacted by mail with an invitation pack (covering letter, participant information sheet, and expression of interest forms) and from those expressing interest, a maximum variation sample of average five (and a maximum of 60 per country) will be purposively selected to give representation of different types, locations and socioeconomic factors (eg, deprived and wealthier communities, different ethnicities).
Healthcare professionals and managers
Within each clinical team (ie, the team providing care to people with one of the three conditions) at each hospital a key contact will be identified. This is likely to be the clinical lead. They will advise on the best method of questionnaire distribution. Invitation packs (covering letter, participant information leaflet (PIL), and questionnaire) will be sent to identified participants using one or a combination of the following methods tailored to national and local arrangements: (1) where names are in the public domain, participants may be contacted directly by the researchers; (2) where this is not possible, key contacts or their depute will inform their team about the study and ask those interested in participating to send their contact details to the researchers so that the questionnaire packs can be mailed directly; (3) alternatively, key contacts will distribute questionnaires on behalf of the researchers, with a request to mail the completed questionnaire back to the researchers in a reply-paid envelope and (4) finally, face-to-face launch meetings will be arranged at each site, and a member of the research team will give a short summary of the purpose and structure of the project, encourage participation, and distribute questionnaires to those attending the meeting. All questionnaires will be identified with a secure identification number that is linked to the identity of the recipient, and recorded on a paper log that will be subsequently transcribed to an electronic log. This will allow up to two targeted reminders to be sent to non-responding healthcare professionals and managers by clinical managers/link people.
The first three of the above four approaches will be adopted in primary care centres. Where there is no primary care doctor with a special interest in one of the three conditions, specific questionnaires will be randomly allocated.
Patients
For each care pathway, patients meeting the inclusion criteria will be identified either prospectively as they present in clinic or from clinic lists, according to local preference. Those identified in the clinic will be handed an invitation pack (covering letter, PIL, and questionnaire) by the responsible clinician. They will be encouraged to complete the questionnaire while waiting for their appointment. Patients will be asked to complete and return the questionnaires directly to the researchers via a box in the clinic or mail it directly in a reply-paid envelope. Those identified from clinic lists will be mailed the invitation pack by the clinical staff or their designated representative. A log of patients given the questionnaire, and their contact details will be maintained by clinic staff to allow response rates to be assessed and one reminder to be sent to non-responders.
Sample size
In each country, 12 hospitals will be selected and thereafter, three care pathways within each of these hospitals; this gives 36 care pathways and a total of 324 (36×9) care pathways across all partners. We estimate that the average number of healthcare professionals on a pathway will be 30 giving a total of 9720 questionnaires distributed (324×30) to healthcare professionals across all partners. We further estimate that there will be an average of 6 healthcare managers per pathway giving a total of 1944 (6×324). There will be 540 (60×9) primary care centres taking part with an estimated 4860 (9×540) questionnaires distributed to healthcare professionals across all partners, and 1620 (3×540) questionnaires distributed to healthcare managers. The above distribution is designed to generate a sample large enough to capture representation of a range of site characteristics likely to affect workforce diversification while recognising the differences between the three clinical conditions.
Using the procedures described above and by extrapolating from researchers' recent experience,13 we estimate a 40% response rate to the health professional and manager questionnaires, thus giving a total of 5832 and 1425 returned health professional and manager questionnaires, respectively.
Patient recruitment will continue at each of the 324 hospitals until 30 patients have been approached in total per condition, and at each primary care centre until an average of six patients per centre have been approached per condition (or 30 per hospital area). With an estimate of a 50% response rate (based on recent work of the applicants14), this will produce 9720 completed patient questionnaires. These numbers are judged sufficient to allow estimation of the main outcomes, and facilitate comparison of the main outcomes by country and condition.
Data collection
Data collection will close at the end of 2015.
Questionnaires
Three questionnaires, each with three versions tailored to the three care pathways, were designed to be completed by: (1) healthcare professionals; (2) healthcare managers of these professionals; (3) patients receiving care from these professionals. A fourth questionnaire, a DCE survey was sent to patients who had agreed in (3) to participate further. Draft questionnaires were developed, in English, by an expert group drawn from those partners with the most extensive research experience in the area. Questionnaires were translated and validated through back translation into each of the partner country languages.
Final versions of the questionnaires (1) to (3) above were agreed after feedback from partners and the CEAG, pre-pilots with local colleagues and a formal pilot in which each country piloted the three questionnaires in one hospital for two of the three target conditions (∼20 healthcare professionals, 3 healthcare managers and 5 patients). Where available, standard instruments and scales have been incorporated. The resource use questions are based on those developed in and widely applied in other research undertaken by partners. Overall design drew on the Cochrane review,15 and uses methods known to encourage high response rates.
The questionnaires are as follows:
Healthcare professional questionnaire: This questionnaire includes sections on respondent demography, roles, and education (closed questions), who they work with (fixed choice options based on a list generated in consultation with local clinical colleagues to ensure all those providing healthcare along the care pathway are included, and including an ‘Other’ option), the tasks undertaken at different stages of the care pathway (based on detailed discussions with local clinical colleagues), the frequency with which these are undertaken and the time taken for both a standardised patient based on a vignette and for a patient they would typically treat (combination of yes/no questions and open responses), their opportunity to undertake new roles, the barriers and facilitators to undertaking new roles (combination of yes/no questions, Likert scales and open responses), the drivers for new roles (combination of yes/no questions, Likert scales and open responses), the integration16 and specialisation of care on the relevant care pathway, and whether care was seen as being team based or doctor led.
Healthcare manager questionnaire: The healthcare manager questionnaire was constructed in a similar manner to the healthcare professional questionnaire, and includes sections on respondent demography, roles, and education (closed questions), the staff they manage (fixed choice options, as above), the tasks undertaken at different stages of the care pathway by different professionals (fixed choice options), the influences on their decision-making about staffing changes in the mix of staff working on the relevant care pathway (Likert scales), the drivers for these (combination of yes/no questions, Likert scales and open responses), and the integration16 and specialisation of care on the relevant care pathway.
Patient questionnaire: The patient questionnaire includes sections on: the patient's health including confirmation of eligibility, the Charlson Index for comorbidities17 and the EQ5D-5L as a quality-of-life instrument,18 the care they have received and the professionals who provided the care (tick box yes/no options), their experience of care (Likert scale responses to a series of statements), their satisfaction with care (Likert scale responses to various parameters of care), and their perceptions of the importance of specific characteristics of care, continuity of care and how care was organised (team based or doctor led), their use of healthcare services and who they saw (tick box and open questions), the value they place on their care (a willingness to pay question), demographic questions (age, weight, education, employment, income, lifestyle), and effect of condition on daily life. A final question asks them to provide contact details if they would be willing to be contacted again for subsequent stages of the research.
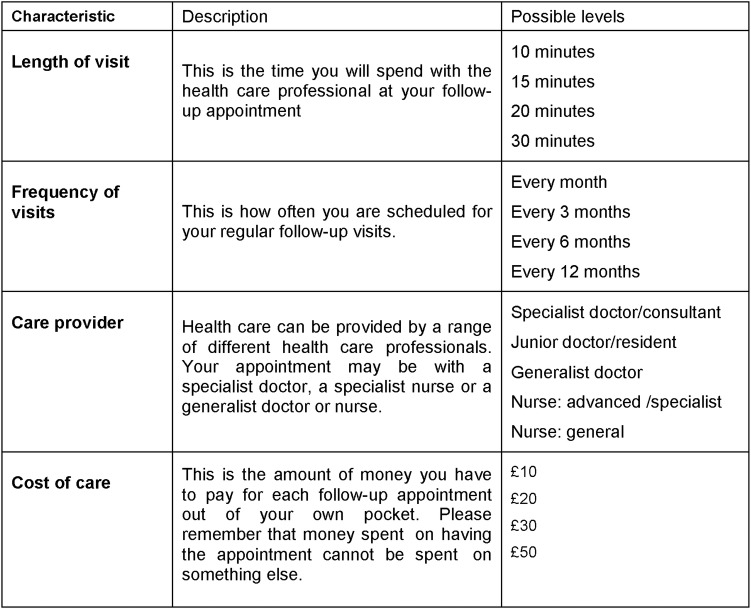
DCE: A fourth questionnaire, a DCE survey, will explore patients' preferences and trade-offs for different aspects of care. The questionnaire will elicit preferences for treatment by new healthcare professionals compared to traditional approaches. The DCE will be sent to those patients who in the initial questionnaire give their consent to be contacted about further research and provide contact details. The attributes and levels will be based on the literature and the responses to relevant items in the patient questionnaire. Based on pilot data, these are likely to be as shown in figure 1. The respondents will be asked to imagine a scenario in which their acute condition has been stabilised and they are in follow-up care. The questionnaire will also include questions to confirm eligibility, basic demographic questions (sex, date of birth, household members, educational level, household income), and questions about the way they have completed the choice sets, their attitudes to health, their health status (excellent, very good, good, fair poor), their health expectations in the next 2 years if they have and do not have follow-up care, the importance of each of the attributes to them (rated from 1, not important to 5 very important), and their willingness to pay for an ideal follow-up visit. The DCE will be distributed by mail or email according to national preferences and one reminder will be sent.
Figure 1.
Potential DCE attributes/characteristics, descriptors and levels.
Outcomes
The survey outcomes are a description of the:
Healthcare professionals involved in the delivery of care;
Tasks on the care pathway, the frequency with which these are delivered and by whom;
Patients' expectations, experiences, and preferences for care;
Integration of care;
Drivers for skill-mix changes in the team delivering care.
Data management and analysis
Data from returned questionnaires will be entered into an Excel spreadsheet by each partner following agreed data coding rules and data cleaning protocols (eg, for missing data). Double data entry on 10% of returned questionnaires will be used to check for accuracy. The final data set will be exported into a STATA database for analysis, using a standard syntax and according to an a priori data analysis plan agreed to by all partners. Any identifying data (eg, hospital name, care pathway) will be anonymised by coding to allow for clustering in the analyses while maintaining confidentiality. Where terms for different healthcare professionals vary in the different partner countries, these will be coded to internationally recognised high-level categories (eg, consultant doctor, junior doctor, nurse, advanced practice nurse). Partners will hold country-level databases and a cross-country data set will be created for Europe wide analyses to be led by named researchers (ie, the database will not be made generally available to the whole team). Data will be stored securely on password-protected computers and a single study Master File stored on a shared drive (Sharepoint).
Initial analyses will include simple descriptive frequencies, and associations between dependent and independent variables using appropriate multivariate techniques. The pooled country database will be analysed using multivariate and multilevel modelling methods, and made available to partners to undertake an agreed plan of analysis. Country-specific and intercountry analyses will model the relationships between the central dependent and independent variables as specified in equations (1) and (2) of the conceptual framework, within and across countries. Analysis of the results of the DCEs will distinguish how the preferences of respondents for different care pathways are to be measured and weighted, and what intercountry differences exist.
Planned work to follow the questionnaires
Additional outcome measures not collected by the patient questionnaires will be extracted from register data at the hospital and/or national level; the data source will vary by country because of different clinical recording systems and health service systems. These data will include standard relevant health and healthcare indicators (eg, morbidity and mortality), and measures of patient safety, patient turnover, length of inpatient stay, and number of readmissions. Process productivity will then be calculated, measured as consultation times per type of professional and consultation rates per hour. The data will also be used to assess the representativeness of the survey respondents compared with the wider hospital population of patients receiving care along the same pathway and, in countries where there are aggregated national data, the representativeness of the hospital sample compared with all hospitals.
The economic evaluation will take a healthcare perspective of the costs and effects associated with the new professional roles using a state-of-the-art economic evaluation (including a Markov modelling exercise) and MCDA. Only (changes in) costs within the healthcare system and clinical effects will be considered. The analysis plan will exploit the size and variation in data across all participating countries, and the comparability in level of detail, completeness and quality of data across these countries. The analyses will explore whether service redesign leads to cost containment, investigate the balance of cost and benefits, and identify incentives for policymakers when increased roles for the new professional roles are introduced.
Optimal models of care will be identified and the critical elements of these distinguished. The analysis is aimed to identify optimal models for ‘best’ care delivered cost effectively. It will present examples of care integration and of the costs associated with financing these pathways. It will suggest solutions to barriers identified at organisational and team level that are informed by examples of good practice using standard theoretical models.
Finally, a workforce planning model for each care pathway will be developed reflecting the dynamic interaction between the number and type of health professionals (allowing for different approaches to labour substitution), and the quality and cost of care for patients and projected patient need. Algorithms and computer modelling will be used to develop the final tool. The information requirements of the planning models will be detailed, and the methodological and data improvements required for improved workforce planning models will be distinguished.
The models so developed will enable workforce planners to optimise care delivery along care pathways, taking into account the needs of the population, the tasks required to deliver care to meet these needs, and the availability (actual and potential) of various health professions with the competences to deliver these tasks. Service providers will be able to benchmark against these to evaluate the efficiency of existing provision and identify the modifiable areas offering the largest efficiency gains.
Discussion
In most healthcare teams, roles of healthcare professionals are evolving in different ways. Some traditional roles are being extended, new healthcare professions are being introduced, tasks are being delegated from or substituted by one professional to another, and new roles evolve as new technologies are introduced. The nature and detail of this delegation has not been previously documented, and the clinical and cost effectiveness of the new healthcare workforce configurations has not been systematically explored. Our hypotheses are that increasing skill mix in this way is likely to be cost effective and that there is potential for wider implementation of these workforce configurations. Our main objective is to inform evidence-based workforce planning.
The current research evidence suggests that new professional roles can help improve access to care and the quality of care.3 19 20 The greater deployment of new professional roles could facilitate increased flexibility, and offer new solutions to the challenges of delivering healthcare to populations with changing and escalating needs. Existing research has failed to show how changing skill mix enhances or inhibits the integration of care within and between organisations, and has largely focused on process rather than clinical outcome measures. It has failed to benchmark best practices regarding the composition of healthcare teams and has also failed to show the effects on care as the new professional roles change care processes and care pathways. There appears to be little robust evidence of how new professional roles might reduce the costs of healthcare services, and no evidence of the impact on efficiency of care. We will fill these lacunae.
Dissemination and ethics
Dissemination
Each partner will produce a Country Report on Service Design and Professional Roles which will include an analysis of basic descriptive statistics by country and care pathway. The Country Reports will serve as the basis for producing a Country Briefing Paper for each country studied. This will inform key stakeholders and policymakers in each country of the initial, country-specific findings from the project. A Cross-Country Report will also be produced drawing wider conclusions by comparing and contrasting across the different health systems. A Europe-wide stakeholder meeting for invited policymakers, workforce planners and academics will be held near the end of the project. A final report will be submitted to the EC and will be available on the MUNROS project website. In addition, findings will be presented at appropriate national and international clinical, health services research and health workforce conferences, and publications submitted to peer-reviewed journals in these same fields.
Acknowledgments
The authors wish to thank the European Commission for funding this research programme Health Care Reform: The iMpact on practice, oUtcomes and cost of New ROles for health profeSsionals (MUNROS), under the European Union's Seventh Framework Programme (FP7 HEALTH-2012-INNOVATION-1) grant agreement number HEALTH-F3-2012-305467EC. The authors also wish to thank all those who supported and guided this work, both within the MUNROS research project team and as external associates. The authors also wish to thank all the MUNROS research and project partners for their continuing collaboration in this research.
Footnotes
Collaborators: The MUNROS team which includes the named authors and in addition: Czech Republic: Charles University Prague (Frantisek Vlcek, Marie Zvoníčková, Daniel Hodyc, Hana Svobodová). England: University of Manchester (Jonathan Gibson, James McDonald, and Steve Birch). Germany: Berlin University of Technology (Britta Zander, Julia Köppen, Juliane Stahl). Italy: Catholic University of Sacred Heart, Rome (Silvia Coretti, Paola Codella, Matteo Ruggeri). Netherlands: Erasmus University Rotterdam (Marianne Luyendjk, Iris Wallenburg, Apostolos Tsiachristas, Maarten Janssen, Mathijs Kelder, Maureen Rutten-van Molken). Norway: University of Bergen (Jon Opsahl, Linda Ostergren, Muhammad Kamrul Islam, Nina Berven, Kjell Haug, Bjarte Folkestad, Kari Ludvigsen, Bodil Ravneberg, Jan Erik Askildsen). Poland: University of Warsaw (Alicja Sobczak, Grażyna Dykowska, Małgorzata Winter, Sabina Ostrowska, Michal Mijal). Scotland: University of Aberdeen (Daryll Archibald, Debbie MaClaggan, Mandy Ryan, Diane Skatun, Sebastian Heidenreich ). Turkey: Economic Policy Research Foundation of Turkey (Sinem Erincç, Seda Basihos, Meryem Dogan, Z Güldem Ökem).
Contributors: RE and CB are co-principle investigators with joint responsibility for overall coordination of project (via Project Management Team (PMT)), led the writing of the funding proposal and of this manuscript. HB acted as the lead researcher and contributed to developing the protocol and writing of the manuscript. AdB, JvE, RB and MS contributed to the writing of the funding proposal and commented on this manuscript.
Funding: This research has received funding of €2 999 660 under the European Union's 7th Framework Programme for research, technological development and demonstration under, Grant Agreement no: 305467.
Competing interests: None declared.
Ethics approval: This study protocol was approved by the respective ethical committees in each country. Protocol amendments will be submitted as needed, and communicated to research sites by the Research Fellows in each country. UK (Scotland and England): Leeds East NRES Committee UK and area NHS Research and Development Departments; Netherlands: METC Isala Hospital, Zwolle, Academic Medical Centre Amsterdam, Radboud Academic Medical Centre, Erasmus Medical Centre, UMC Utrecht, Maasstad Hospital, Reinier de Graaf Ziekenhuis Delft, Flevoziekenhuis (samenwerking met AMC voor borstkanker), Maastricht UMCU, St Elizabeth Ziekenhuis, Martini Ziekenhuis Groningen, Ikazia Hospital; Germany: Ethikkommission des Instituts für Psychologie und Arbeitswissenschaft (IPA); Italy: Gemelli teaching hospital, Milan area A, area B, area C and IRCCS ethical committees; Czech Republic: Individual hospital ethical committees; Norway: Regional Ethics Committee, REK vest; Turkey: Individual hospital ethical committees.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: English language versions of consent forms and other related documentation given to participants (eg, questionnaires) are available on request from the authors.
Turkey straddles both Europe and Asia; it is an associate country of the EU, and accession negotiations for full membership are ongoing. For the purposes of this research, Turkey is referred to as a European country while recognising that geographically, some data will be collected from locations in Asia.
Contributor Information
Collaborators: Frantisek Vlcek, Marie Zvoníčková, Daniel Hodyc, Hana Svobodová, Jonathan Gibson, James McDonald, Steve Birch, Britta Zander, Julia Köppen, Juliane Stahl, Silvia Coretti, Paola Codella, Matteo Ruggeri, Marianne Luyendjk, Iris Wallenburg, Apostolos Tsiachristas, Maarten Janssen, Mathijs Kelder, Maureen Rutten-van Molken, Jon Opsahl, Linda Ostergren, Muhammad Kamrul Islam, Nina Berven, Kjell Haug, Bjarte Folkestad, Kari Ludvigsen, Bodil Ravneberg, Jan Erik Askildsen, Alicja Sobczak, Grażyna Dykowska, Małgorzata Winter, Sabina Ostrowska, Michal Mijal, Daryll Archibald, Debbie MaClaggan, Mandy Ryan, Diane Skatun, Sebastian Heidenreich, Sinem Erincç, Seda Basihos, Meryem Dogan, and Z Güldem Ökem
References
- 1.Hernandez P, Dräger S, Evans DB et al. . Measuring expenditure for the health workforce: evidence and challenges . In: Evidence and information for policy. Geneva: World Health Organization, 2006. http://www.who.int/hrh/documents/measuring_expenditure.pdf (accessed 25 Oct 2015). [Google Scholar]
- 2.Ross N, Parle J, Begg P et al. . The case for the physician assistant. Clin Med (Lond) 2012;12:200–6. 10.7861/clinmedicine.12-3-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsiachristas A, Wallenburg I, Bond CM et al. , MUNROS Team. Costs and effects of new professional roles: evidence from a literature review. Health Policy 2015;119:1176–87. 10.1016/j.healthpol.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 4.Weiss MC, Sutton J. The changing nature of prescribing: pharmacists as prescribers and challenges to medical dominance. Sociol Health Illn 2009;31:406–21. 10.1111/j.1467-9566.2008.01142.x [DOI] [PubMed] [Google Scholar]
- 5.http://www.gmc-uk.org/guidance/ethical_guidance/21187.asp (accessed 13 Jan 2016).
- 6.Aronson JK. Nurse prescribers and reporters. Br J Clin Pharmacol 2003;56:585–7. 10.1046/j.1365-2125.2003.02023.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosley S, Dale J. Healthcare assistants in general practice: practical and conceptual issues of skill-mix change. Br J Gen Pract 2008;58:118–24. 10.3399/bjgp08X277032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latter S, Blenkinsopp A, Smith A et al. . An evaluation of nurse and pharmacist independent prescribing. University of Southampton and Keele University: Final Report for the Policy Research Programme at the Department of Health UK, 2010. [Google Scholar]
- 9.Delamaire M, Lafortune G. Nurses in advanced roles: a description and evaluation of experiences in 12 developed countries. OECD Health Working Papers, No. 54 OECD Publishing, 2010. 10.1787/5kmbrcfms5g7-en [DOI] [Google Scholar]
- 10.Farmer J, Currie M, West C et al. . ‘Evaluation of physician assistants to NHS Scotland’. Report to NHS Scotland, 2008. [DOI] [PubMed]
- 11.Laurant M, Harmsen M, Wollersheim H et al. . The Impact of non physician clinicians: do they improve the quality and cost-effectiveness of health care services? Med Care Res Rev 2009;66:36S–89S. 10.1177/1077558709346277 [DOI] [PubMed] [Google Scholar]
- 12.Sermeus W, Bruyneel L. Investing in Europe's health workforce of tomorrow: scope for innovation and collaboration. Summary report of the three Policy Dialogues. Leuven, Belgium: 26–30 April 2010, European Observatory on Health Systems and Policies, 2010. [Google Scholar]
- 13.Cristin R, Sarah R, Davey P et al. . Prevalence and causes of prescribing errors: the PRescribing Outcomes for Trainee doctors Engaged in Clinical Training (PROTECT) study. PLoS ONE 2014;9:e79802 10.1371/journal.pone.0079802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruhn H, Bond CM, Elliott AM et al. . Pharmacist led management of chronic pain in primary care: results from a randomised controlled exploratory trial. BMJ Open 2013;3:e002361 10.1136/bmjopen-2012-002361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards PJ, Roberts I, Clarke MJ et al. . Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev 2009;(3):MR000008 10.1002/14651858.MR000008.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukas CVD, Meterko M, Lowcock S et al. . Monitoring the progress of system integration. Qual Manag Health Care 2002;10:1–11. 10.1097/00019514-200210020-00004 [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 18.http://www.euroqol.org/eq-5d-products/eq-5d-5l.html (accessed 25 Oct 2015).
- 19.Drennan VM, Chattopadhyay K, Halter M et al. . Physician assistants in English primary care teams: a survey. J Interprof Care 2012,26:416–18. 10.3109/13561820.2012.686538 [DOI] [PubMed] [Google Scholar]
- 20.Nkansah N, Mostovetsky O, Yu C et al. . Effect of outpatient pharmacists’ non-dispensing roles on patient outcomes and prescribing patterns. Cochrane Database Syst Rev 2010;(7):CD000336 10.1002/14651858.CD000336.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]