Abstract
Background:
Acne vulgaris is the most common skin disease. Local and systemic antimicrobial drugs are used for its treatment. But increasing resistance of Propionibacterium acnes to antibiotics has been reported.
Materials and Methods:
In a double-blind clinical trial, 40 patients with mild to moderate acne vulgaris were recruited. one side of the face was treated with Clindamycin Gel 1% and the other side with Azithromycin Topical Gel 2% BID for 8 weeks and then they were assessed.
Results:
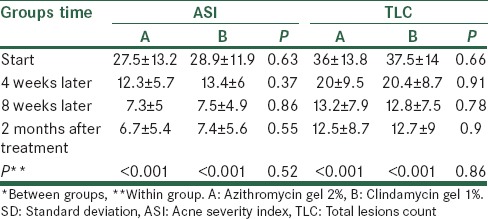
Average age was 21. 8 ± 7 years. 82.5% of them were female. Average number of papules, pustules and comedones was similarly reduced in both groups and, no significant difference was observed between the two groups (P > 0.05, repeated measurs ANOVA). The mean indexes of ASI and TLC also significantly decreased during treatment in both groups, no significant difference was observed between the two groups. (P > 0.05, repeated measurs ANOVA). Also, impact of both drugs on papules and pustules was 2-3 times greater than the effect on comedones. Average satisfaction score was not significant between the two groups (P = 0.6, repeated measurs ANOVA). finally, frequency distribution of complications was not significant between the two groups (P > 0.05, Fisher Exact test).
Conclusion:
Azithromycin gel has medical impact at least similar to Clindamycin Gel in treatment of mild to moderate acne vulgaris, and it may be consider as suitable drug for resistant acne to conventional topical therapy.
Keywords: Acne vulgaris, administration topical drug, azithromycin, clindamycin, drug treatment
INTRODUCTION
Acne vulgaris is a multifactorial disease of the pilosebaceous unit, which significantly affects the economy and psyche of the patient.
According to chronic and pathogenic nature of this disease, different treatments from topical medications to systemic medications are used, such as topical retinoid, benzoyl peroxide, azelaic acid, and oral medications (antibiotics, hormonal drugs, isotretinoin).[1]
Topical antibiotics such as erythromycin, clindamycin, and tetracycline in doses of 1–4% in the various formulations can be used alone or in combination.[2,3] At least 2 months is needed to show maximum effect.[4] Increased resistance of Propionibacterium acnes to antibiotics in the world is alarming. However, the association between colonization and antibiotic-resistant cases is very complex, as there may be drug-resistant strains in vitro, but they may lead to clinical improvement for other reasons, such as its anti-inflammatory effect.[5] Topical tetracycline is used less than other topical antibiotics.[6,7]
In the last 30 years, resistance to erythromycin, clindamycin, and cotrimoxazole has been increased. Furthermore, there are cross-resistance between antibiotic, e.g. erythromycin and clindamycin.[5,7,8]
Propionibacterium acnes sensitivity and drug-resistance patterns are different in different parts of the world. For example, a study in Egypt as in vitro study showed resistance to clindamycin as 65% and 48% resistance to erythromycin and it was almost above 2 times more than azithromycin.[9] And in the North of Mexico, the resistance to azithromycin was reported as 82%, as 68% to cotrimoxazole, as 46% to erythromycin, and clindamycin was in the last place and this is despite the available information.[10] Very high resistance to clindamycin was reported in in Japanese patients with P. acnes carrying germ (x).[11] However, this pattern has not been investigated in our region and background information is not available. Due to the development of drug-resistance, new strategies including the formulation of effective drugs with fewer side effects are needed.
Azithromycin is a more semisynthetic and newer macrolide with superior properties compared to tetracycline, clindamycin, and erythromycin. Azithromycin has no effect on hepatic cytochrome P450.[12] This drug is safe, even in pregnancy and lactation and children can also use it.[13] Azithromycin synthetic construction makes it unique and the level of this drug which reaches to tissues and cells and phagocytes appears to be significantly higher than plasma levels. Clindamycin side effects include gastrointestinal irritation, rash, neutropenia, liver disorders, and pseudomembrane colitis disorder.[12]
Azithromycin gel 2% is currently available in several countries in acne treatment. However, scientific studies about its effects and side effects are not available in the resources. Therefore, in this study, the therapeutic effects and side effects of azithromycin topical gel 2% with clindamycin gel 1% are examined which are common treatments in our country for acne lesions.
MATERIALS AND METHODS
It is a double-blind clinical trial which was conducted during January to December of 2013 in Isfahan.
Inclusion criteria included mild to moderate acne, aged 12–45 years of both sexes, patient satisfaction to participate in the study, lack of reception of topical antibiotics within 2 weeks prior to the study, lack of reception of systemic medication (anti-acne medications including isotretinoin- OCP1-antibiotics, drugs that cause acne) for 1-month prior to the study, the absence of a history of hypersensitivity to any of the drugs (clindamycin-azithromycin). Exclusion criteria included discontinuation of therapy until the end of the study, requiring systemic therapy during the study and lack of patient's referral in the later stages and development of drug allergy.
Following approval of proposal with registration code of 392518 and taking Iranian Registry of Clinical Trials Center (IRCT) code from IRCT (numbered as IRCT2014031216970N1), 40 patients with inclusion criteria were selected and written consent was taken regarding agreement on participation in the study. Acne severity in the patients under study was specified using Food and Drug Administration system as follows:[14]
Mild: Some noninflammatory lesion, no more than a few papules/pustules, but no nodules
Moderate: Up to many noninflammatory lesions, may have some inflammatory lesion, but no more than one small nodule
Severe: Up to many noninflammatory and inflammatory lesion, but no more than a few nodules.
Powder azithromycin and clindamycin was provided from Farabi Pharmacational Institute and two drugs of clindamycin gel 1% and azithromycin gel 2% (each of these drugs were made of powder + gel base of sodium carboxymethyl cellulose 3% and 5 carbomer 0.5%) was produced in Faculty of Pharmacology of Isfahan. Were prepared proportionate to the objective of maximum stability and proper pH with the desired concentration, and were given to patients in similar tubes labeled A and B, and they were asked of the two drug codes, choose one code for the right side of face and the other for the left side. In terms of patient selection, respective side was written on the tubes, so that the wrong conclusion is prevented. Furthermore, the patients were instructed regarding use of the gel and avoiding mixing the two drugs and the prevention of drug use on the opposite side. Dose of the drug was twice daily for 2 months. Severity of disease and improvement was measured by total lesions count (TLC) and acne severity index (ASI) scoring.[15]
TLC: Comedones + papules + pustules;
ASI: (0.25 comedones) + papules + (2 pustules)
Patient's satisfactions: Visual analog score (from 0 to 10).
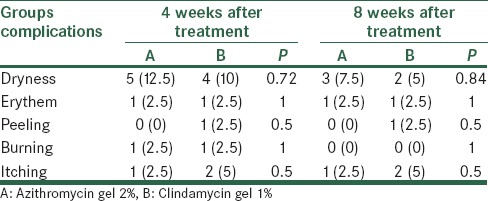
At baseline, 4 weeks and 8 weeks after initiation of treatment and 2 months after treatment were determined. The rate of recurrence and complications (including dryness, erythema, desquamation, burning, and irritation were calculated and recorded) was examined during the period of study and they were written along with other data in data collection forms.
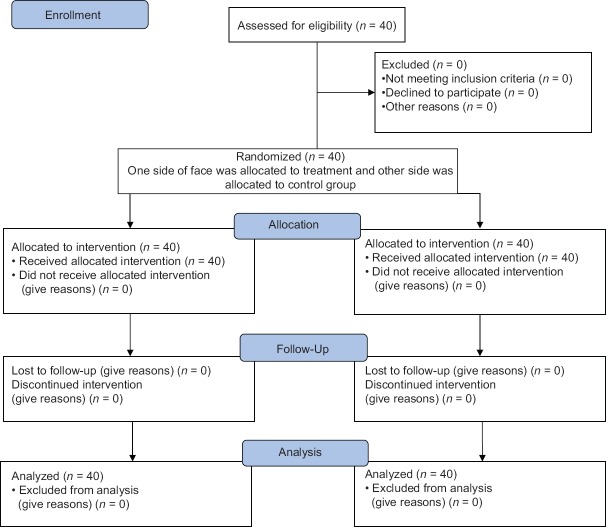
Finally, collected data were analyzed using SPSS software 22 (manufacturwed in BMI company), as well as statistical tests including Chi-square, Fisher's test, and t-test and variance analysis with observation replications. P < 0.05 as significant level [Figure 1].
Figure 1.
Consort flow diagram
RESULTS
In this study, 40 patients with average age 21.8 ± 7 including 7 men (17.5%) and 33 women (82.5%) were studied.
Table 1 gives mean and standard deviation (SD) for the number of comedones, papules, and pustules from beginning of the treatment until 2 months after the intervention in both groups. According to t-test, average number of comedones, papules, and pustules showed no significant difference in two groups in all periods (P > 0.05). Variance analysis with replication of observations showed there is no significant difference in two groups in terms of changes in the number of comedones, papules, and pustules during intervention, and it decreased similarly in both groups and no significant difference is observed between two groups (P = 0.83, P = 0.42, and P = 0.49, respectively). However, reduction in the number of lesions was significant in two groups (P < 0.001).
Table 1.
Mean±SD of the number of acne from beginning until 2 months after treatment
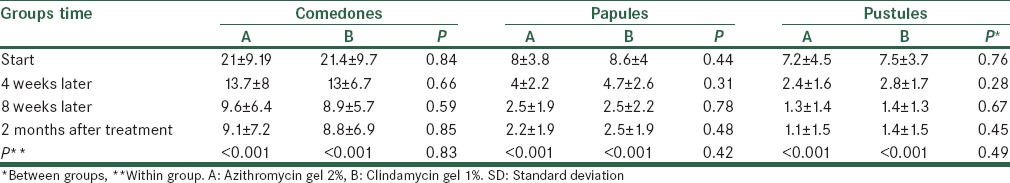
Table 2 gives mean ± SD of ASI and TLC indexes from beginning of treatment until 2 months after intervention in terms of groups receiving clindamycin gel 1% and azithromycin gel 2%. According to t-test, an average of two indexes showed no significant difference in two groups in all periods. Variance analysis with replication of observations showed changes in two indexes were significantly reduced in two groups, but the reduction was similar in two groups. Diagrams 1 and 2 show changes trends for ASI and TLC indexes in two groups.
Table 2.
Mean and SD of ASI and TLC indexes from beginning until 2 months after treatment
Average satisfaction score in patients 4 weeks after treatment in clindamycin gel 1% group and azithromycin gel 2% was 6.98 ± 1.4 and 6.88 ± 0.99, and no significant difference was observed between the two groups according to t-test results (P = 0.71). Eight weeks after treatment, average satisfaction score was 7.5 ± 1.4 and 7.3 ± 1.24 in two groups, respectively, and the difference between two groups was not significant (P = 0.51). Two months after treatment, average of this score was 7.7 ± 1.1 and 7.8 ± 1.1 in two groups, respectively and no significant difference was observed between them (P = 0.8). Variance analysis with replication of observations showed also no significant difference in two groups (P = 0.6). The recurrence of disease was not observed in the 2 months after stopping treatment [Figures 2 and 3].
Figure 2.
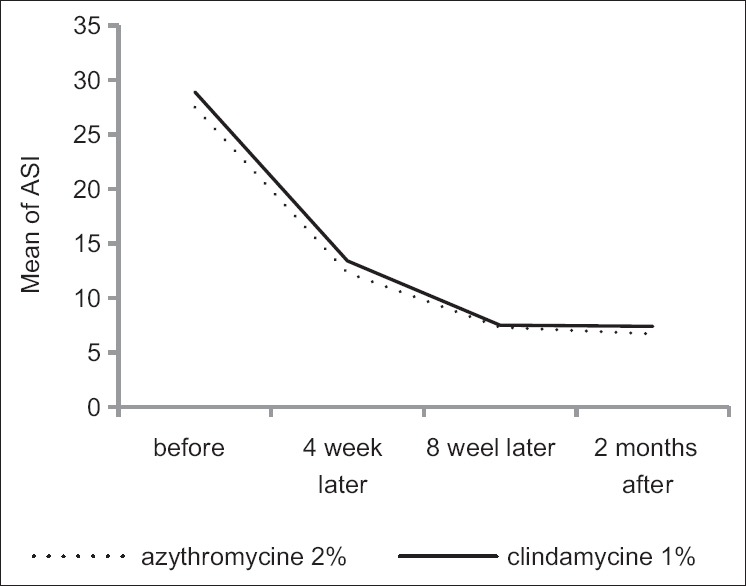
Acne severity index changes from before treatment to after 2 months of treatment in both groups
Figure 3.
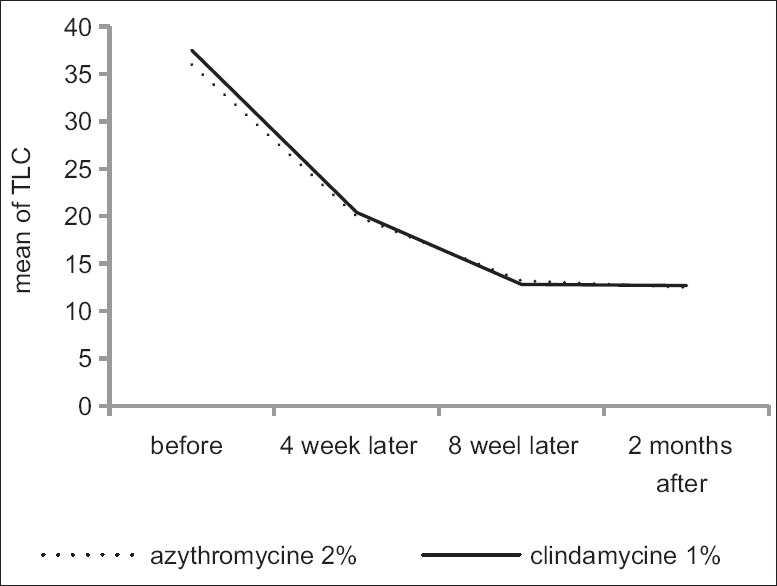
Total lesions count index changes from before treatment to after 2 months of treatment in both groups
Table 3 gives frequency distribution of complications during treatment, at 4 weeks and 8 weeks after treatment in both groups. According to Fisher's exact test, frequency distribution of complications in the two groups was not significantly different at 2 times (P > 0.05).
Table 3.
Frequency distribution of complications in the two groups
Effects of both drugs on papules and pustules were 2–3 times more than the effects on comedone.
DISCUSSION
Current work aimed at comparing the efficacy of azithromycin gel 2% and clindamycin gel 1% in the treatment of mild to moderate acne vulgaris. Findings showed both combinations, that is, azithromycin gel 2% and clindamycin gel 1%, have acceptable medical impacts in the treatment of mild to moderate acne vulgaris. Furthermore, no significant difference was observed between the 2 groups in terms of occurrence of side effects and complications. Azithromycin is an effective antibiotic in acne treatment,[16] which is more effective especially in pulse therapy form and shows fewer complications compared to other common antibiotics.[17,18,19] However, a few studies have been conducted on its topical formulation for acne treatment. In the studies by Tan and Hugh, azithromycin alcoholic solution was used to treat acne vulgaris with reasonably effectiveness.[6,20] In the study by Sandoval et al., pulse azithromycin as monotherapy was effective for acne during puberty.[16]
In the study by Shirazpour et al.: A double-blinding randomized clinical trial in Iran university, 32 patient with mild to moderate acne were treated with alcoholic solution 2% azithromycin BID and 30 patients were matched (age - sex and grading acne) treated with alcoholic solution 1% of clindamycin BID for 12 weeks and topical adapalene gel 1% for both groups at night.
Response to treatment and decreasing the grade (Leeds system) of the disease and number of nodules, papules were not significantly different in the 1st month while just the number of nodules in the group on azithromycin showed more decrease in the last months side effect in both groups are little and the rate of satisfaction with both drug, were high and no difference.[21]
In our study treatment response and satisfaction are simulation to mention study but we didn’t evaluate therapeutic effect on nodules, but Shirazpour et al. study reports more side effects which could be because of longer duration of their study, alcoholic base solution, and adding adapalene gel to therapeutic protocol.
In the study by Hajheydari et al., a randomized double-blind clinical trial an 96 patient 9 with mild to moderate acne for 20 weeks in Sari (Iran). They were randomly divided into three groups and treated with 2% alcoholic solution of azithromycin and clindamycin twice daily for 16 weeks.
For each three groups, decreased TLC and ASI were significant (P < 0.05). Azithromycin was more effective on comedone than the other drugs (P < 0.05), hat it has more local side effects.[22]
However in our study the effects of both drugs were similar and there were no side effects. This lesser side effect could be related to shorter study period, gel-based drug and unlike this study the effects of both drugs on inflammatory lesions of 2–3 times more than comedones. The anti-inflammatory action of macrolides has been shown in various studies.[23]
In our study, the effects of both drugs on papules and pustules were 2–3 times more than the effect on comedones.
CONCLUSION
Considering findings in the current research and comparing it with other studies it can be concluded azithromycin gel 2% has optimal medical impact (at least as effective as clindamycin) in treatment of mild to moderate acne vulgaris, and this is particularly the case of P. acnes resistance to current antibiotics, which can be promising. In addition, P. acnes resistance to clindamycin is not evident in our region, but due to limitations of this study including use of two medications in one patient and treatment and follow-up period, it is suggested this research is conducted in higher sample size at broader scope.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Oral contraceptive
REFERENCES
- 1.Zaenglein A, Thiboutot D. Acne Vulgaris. In: Bolognia J, Jorizzo J, editors. Dermatology. 3rd ed. International. [Philadelphia]: Elsevier; 2012. pp. 545–58. [Google Scholar]
- 2.James W, Berger TG. 11th ed. International[London]: Elsevier Saunders; 2011. Andrews Diseases of the Skin Clinical Dermatology; p. 231. [Google Scholar]
- 3.Grove G, Zerweck C, Gwazdauskas J. Tolerability and irritation potential of four topical acne regimens in healthy subjects. J Drugs Dermatol. 2013;12:644–9. [PubMed] [Google Scholar]
- 4.Habif TP, Campbell JR, compbell Jl, chapman MS, Dinulos J. 3th ed. UK/USA: Eisevier; 2011. Skin Disease; pp. 104–5. [Google Scholar]
- 5.Burns T, Breathnach S. 8th ed. UK/USA: Wiley-Blackwell; 2010. Rook's Text book of Dermatology; pp. 41–2. [Google Scholar]
- 6.Tan HH. Topical antibacterial treatments for acne vulgaris: Comparative review and guide to selection. Am J Clin Dermatol. 2004;5:79–84. doi: 10.2165/00128071-200405020-00002. [DOI] [PubMed] [Google Scholar]
- 7.Schafer F, Fich F, Lam M, Gárate C, Wozniak A, Garcia P. Antimicrobial susceptibility and genetic characteristics of Propioni bacterium acnes isolated from patients with acne. Int J Dermatol. 2013;52:418–25. doi: 10.1111/j.1365-4632.2011.05371.x. [DOI] [PubMed] [Google Scholar]
- 8.Brunton LL. 12th ed. International New York: Mc Graw Hill Medical; 2011. Goodman and Gilman's. The Pharmacological Basis of Therapeutics. [Google Scholar]
- 9.Abdel Fattah NS, Darwish YW. In vitro antibiotic susceptibility patterns of Propionibacterium acnes isolated from acne patients: An Egyptian university hospital-based study. J Eur Acad Dermatol Venereol. 2013;27:1546–51. doi: 10.1111/jdv.12057. [DOI] [PubMed] [Google Scholar]
- 10.González R, Welsh O, Ocampo J, Hinojosa-Robles RM, Vera-Cabrera L, Delaney ML, et al. In vitro antimicrobial susceptibility of Propionibacterium acnes isolated from acne patients in northern Mexico. Int J Dermatol. 2010;49:1003–7. doi: 10.1111/j.1365-4632.2010.04506.x. [DOI] [PubMed] [Google Scholar]
- 11.Nakase K, Nakaminami H, Noguchi N, Nishijima S, Sasatsu M. First report of high levels of clindamycin-resistant Propionibacterium acnes carrying erm (X) in Japanese patients with acne vulgaris. J Dermatol. 2012;39:794–6. doi: 10.1111/j.1346-8138.2011.01423.x. [DOI] [PubMed] [Google Scholar]
- 12.Trevor A, Katzung BG, Hall MK, Masters SB. 10th ed. International Edition New York: Mc Graw Hill Medical; 2013. Pharmacology Katzung and Trevor's; pp. 391–395. [Google Scholar]
- 13.Kong YL, Tey HL. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779–87. doi: 10.1007/s40265-013-0060-0. [DOI] [PubMed] [Google Scholar]
- 14.Pochi PE, Shalita AR, Strauss JS, Webster SE. report of the consensus conference on acne classification. J Am acad dermatol. 1991;24:495–500. doi: 10.1016/s0190-9622(08)80076-x. [DOI] [PubMed] [Google Scholar]
- 15.Adityan B, Kumari R, Thappa DM. Scoring systems in acne vulgaris. Indian J Dermatol Venereol Leprol. 2009;75:323–6. doi: 10.4103/0378-6323.51258. [DOI] [PubMed] [Google Scholar]
- 16.Sandoval LF, Hartel JK, Feldman SR. Current and future evidence-based acne treatment: A review. Expert Opin Pharmacother. 2014;15:173–92. doi: 10.1517/14656566.2014.860965. [DOI] [PubMed] [Google Scholar]
- 17.Antonio JR, Pegas JR, Cestari TF, Do Nascimento LV. Azithromycin pulses in the treatment of inflammatory and pustular acne: Efficacy, tolerability and safety. J Dermatolog Treat. 2008;19:210–5. doi: 10.1080/09546630701881506. [DOI] [PubMed] [Google Scholar]
- 18.Maleszka R, Turek-Urasinska K, Oremus M, Vukovic J, Barsic B. Pulsed azithromycin treatment is as effective and safe as 2-week-longer daily doxycycline treatment of acne vulgaris: A randomized, double-blind, noninferiority study. Skinmed. 2011;9:86–94. [PubMed] [Google Scholar]
- 19.Hasibur MR, Meraj Z. Combination of low-dose isotretinoin and pulsed oral azithromycin for maximizing efficacy of acne treatment. Mymensingh Med J. 2013;22:42–8. [PubMed] [Google Scholar]
- 20.McHugh RC, Rice A, Sangha ND, McCarty MA, Utterback R, Rohrback JM, et al. A topical azithromycin preparation for the treatment of acne vulgaris and rosacea. J Dermatolog Treat. 2004;15:295–302. doi: 10.1080/09546630410033808. [DOI] [PubMed] [Google Scholar]
- 21.Shirazpour M, Firooz A, Pazooki H, Resaei S. Comparison of topical azithromycin and clindamycin in the treatment of mild to moderate acne vulgaris. Iran J Dermatol. 2008;11:67–72. [Google Scholar]
- 22.Hajiheydari Z, Mahmoudi M, Vahidshahi K, Nozari A. Comparison of efficacy of azithromycin vs. clindamaycin and erythromycin in the treatment of mild to moderate acne vulgaris. Pak J med sci. 2011;27:68–72. [Google Scholar]
- 23.Labro MT. Anti-inflammatory activity of macrolides: A new therapeutic potential? J Antimicrob Chemother. 1998;41(Suppl B):37–46. doi: 10.1093/jac/41.suppl_2.37. [DOI] [PubMed] [Google Scholar]