Abstract
BACKGROUND:
In April 2014, a surge in cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection was seen in Jeddah, Saudi Arabia. The aim of this study is to describe the demographic and clinical features, laboratory and radiological findings of MERS-CoV patients identified during this outbreak in a single tertiary hospital.
METHODS:
All laboratory-confirmed MERS-CoV cases who presented to King Faisal Specialist Hospital from March 1, 2014, to May 30, 2014, were identified. Patients' charts were reviewed for demographic information, comorbidities, clinical presentations, and outcomes.
RESULTS:
A total of 39 patients with confirmed MERS-CoV infection were identified. Twenty-one were male (54%), aged 40 ± 19 years and included 3 (8%) pediatric patients (<18-year-old). 16 (41%) patients were health care workers. Twenty-one (53%) patients were previously healthy whereas eighteen (47%) had at least one comorbidity. The predominant comorbidities included hypertension (31%), diabetes (26%), respiratory (23%), and renal disease (18%). Thirty patients (81%) were symptomatic at presentation, fever (69%) being the most common complaint. The overall mortality rate was 28%. In univariate analysis, older age, hypertension, and chronic kidney disease were associated with mortality.
CONCLUSIONS:
MERS-CoV presentation varies from asymptomatic infection to severe respiratory disease causing death. Future studies to identify the risk factors for worse outcome are needed.
Keywords: Characteristics, Middle East respiratory syndrome coronavirus, patient
In September 2012, a new corona virus was reported in a Saudi patient with pneumonia.[1] This virus was subsequently named Middle East respiratory syndrome coronavirus (MERS-CoV).[2] As of May 18, 2015, 1118 laboratory-confirmed cases of infection with MERS-CoV, including at least 423 deaths, have been confirmed worldwide.[3] MERS-CoV causes acute respiratory disease and is associated with high mortality.[4] Several investigations of healthcare-associated clusters have been reported to date.[5,6] Ongoing sporadic infections in the community are still reported, with potential amplification in healthcare setting.[5,6,7]
Some reports from previous clusters indicated that MERS-CoV infection presents primarily with respiratory symptoms.[8] Clinical presentation ranges from asymptomatic or mild respiratory symptoms to severe respiratory syndrome or death.[6,8,9] Patients with comorbidities tend to have more severe disease and higher case fatality rate.[8] The risk factors associated with more severe disease and death still not clearly defined. One study found that low serum albumin and concomitant infection were associated with Intensive Care Unit (ICU) admission where age >65 years was associated with mortality.[9]
In April 2014, a sharp increase in number of cases reported from Jeddah, Saudi Arabia, heightened international concerns.[10,11] The majority of MERS-CoV transmission occurred in health care facilities.[12] The King Faisal Specialist Hospital (Jeddah) is a tertiary care hospital in Western area of Saudi Arabia. The hospital experienced the second largest health care outbreak in Jeddah. The aim of this study is to describe demographic and clinical features, laboratory and radiological findings of MERS-CoV patients diagnosed during the hospital outbreak.
Methods
Study site and patients population
Patients with suspected MERS-CoV infection were tested in the Ministry of Health regional laboratory in Jeddah and/or King Faisal Specialist Hospital laboratory. Contact tracing was done for close contacts with confirmed MERS-CoV cases even if they were asymptomatic.
All laboratory-confirmed MERS-CoV cases presented to King Faisal Specialist Hospital from March 1, 2014, to May 30, 2014, were identified. Patient's charts and electronic medical records were reviewed for demographic information, comorbidities, clinical features, length of stay, and outcomes. All patients with confirmed MERS-CoV infection were admitted to private rooms equipped with negative pressure ventilation and were followed until death or discharge from hospital.
Definitions
A confirmed case was defined as any person with laboratory confirmation of MERS-CoV infection based on a positive real-time polymerase chain reaction targeting two genes - the E gene and open reading frame gene 1a.[13] Samples were collected from respiratory tract included nasopharyngeal swab, sputum, tracheal aspirate, or bronchoalveolar lavage.
Statistical analysis
Data were described using mean, median, and interquartile range for continuous variables; frequencies and percentages for categorical variables and Chi-square test or Fisher exact test were used to compare categorical variables. Univariate analysis was performed to identify risk factors associated with mortality. P < 0.05 was considered statistically significant.
Results
The first confirmed MERS-CoV case at KFSHRC-J was a patient with end-stage renal disease who underwent hemodialysis few days before presentation at another facility where active transmission of MERS-CoV was ongoing. Few days later, widespread transmission to health care workers (HCWs) and other hospitalized patients in our institution occurred [Figure 1]. The department of infection control conducted aggressive contact tracing that resulted in identification of several asymptomatic cases. Several HCWs were infected early in the outbreak. Seven of the infected HCW reported contact to the index case. With implementation of aggressive infection control practices and isolation of confirmed and suspected cases, number of cases started to decrease. The last confirmed case was on May 7, 2014.
Figure 1.
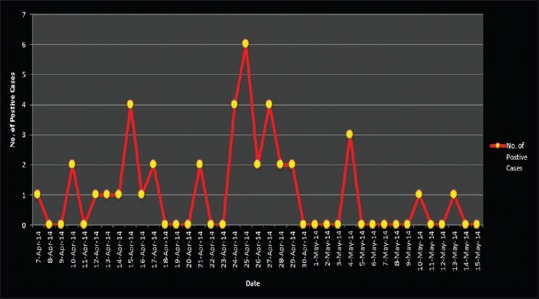
Middle East respiratory syndrome coronavirus epidemic curve at King Faisal Specialist Hospital-Jeddah
Patient characteristics
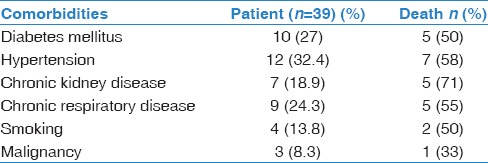
Complete demographic and baseline characteristics are shown in Table 1. A total of 39 patients were confirmed to have MERS-CoV infection. Twenty-one patients were male (54%). The mean age was 40 ± 19. Three of the patients (8%) were younger than 18-year-old. Sixteen patients (41%) were HCW. Of 39 patients, 18 (46%) patients had one or more comorbidities at baseline [Table 2].
Table 1.
Baseline characteristics of confirmed Middle East respiratory syndrome coronavirus patients
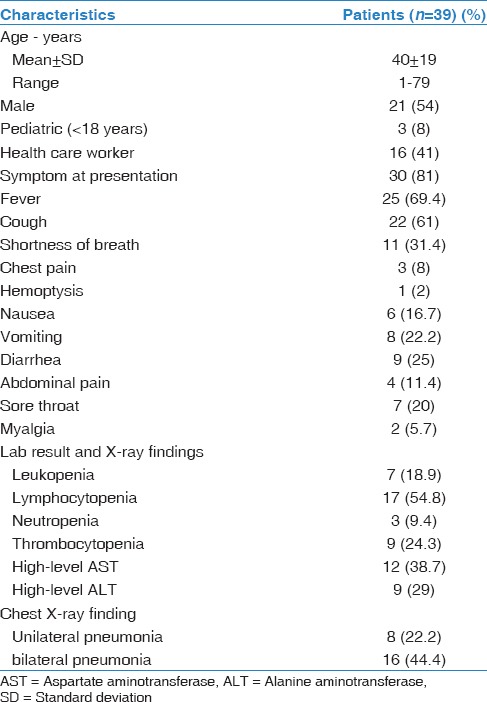
Table 2.
Comorbidities in Middle East respiratory syndrome coronavirus patients
A majority of patients were symptomatic (81%). Fever was the most common presentation (69%), followed by cough (61%) and shortness of breath (36%). Lymphopenia was the most common laboratory abnormality with 54% of patients had lymphopenia at time of presentation. Twenty-four patients (62%) developed abnormal chest radiograph during their illness [Table 1].
Severity of illness and mortality
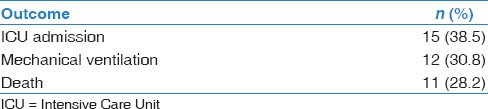
During this outbreak, 15 patients (38.5%) required admission to ICU and 12 patients (30.8%) required mechanical ventilation [Table 3]. Eight patients (21%) required renal replacement therapy. None of the patients required extracorporeal membrane oxygenation. Most of the patients received empiric antibiotics. Of the 39 patients with confirmed MERS-CoV infection, 11 patients (28%) died. Nine of 15 patients required ICU died (60%). Median time from onset of symptoms to death was 23 days. All deceased patients reported at least one comorbidity at baseline. The highest mortality was among patients who reported chronic kidney disease (CKD) at baseline (71%) [Table 2].
Table 3.
Outcome of patients with Middle East respiratory syndrome coronavirus infection
Risk factors for mortality
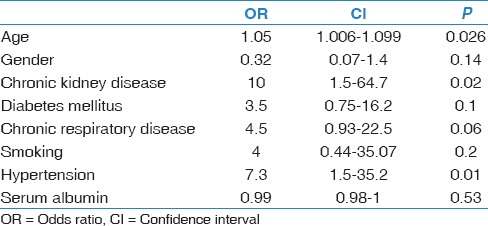
In univariate analysis for mortality outcome, age, CKD, and hypertension were significantly associated with mortality – odds ratio (OR): 1.05, 95% confidence interval (CI) (1.01–1.1); OR: 10, 95% CI (1.5–64.7); OR: 7.3, 95% CI (1.5–35.2), respectively. There was a trend toward increased risk of death in patients with diabetes, history of respiratory disease, or history of smoking. However, these associations did not reach statistical significance [Table 4].
Table 4.
Univariate analysis of mortality
Discussion
This hospital outbreak was the second largest hospital cluster that occurred in Jeddah during April–May 1014. Clinical presentations were variable, ranging from asymptomatic infection to severe disease requiring mechanical ventilation and ICU care. The case fatality rate was 28%. The mortality in our cohort was less than previously reported in literature; however, we identified several asymptomatic and mild cases through contact tracing. Age, hypertension, and CKD at baseline were associated with death in univariate analysis. We did not perform multivariable analysis given the small number of our patients. Studies with a larger sample size are required to confirm if these risk factors are independently associated with mortality.
Clinical presentation of MERS-CoV disease in this cluster is consistent with previous reports.[8,9] However, 19% of patients were asymptomatic with majority being HCWs identified during contact tracing.
MERS-CoV resulted in respiratory failure and death in a significant number of patients. Risk factors for these complications remain unclear. Previous report from the largest cohort of MERS-CoV patients found that age >65 was an independent predictor of mortality, and concomitant infections and low serum albumin were predictors for ICU admission.[9] In another cohort recently published, older age, renal failure, and diabetes were associated with mortality.[14] However, these studies are limited by small sample size. Further studies with larger number of patients are needed to identify predictors for worse outcome. This is will be vital in the future to identify candidates for potential future vaccines and therapy.
This investigation is subject to several limitations. The contact tracing of HCW was not universal. Several HCWs might have been infected and remained asymptomatic. As a result, the extent of transmission and number of asymptomatic patients might be under reported. Second, this was a single-center study with a unique population so the finding from our report might not reflect the whole spectrum of the disease. Third, the number of patients was small precluding further analysis to identify independent predictors for severe disease.
Conclusions
MERS-CoV presentation varies from asymptomatic infections to severe respiratory disease causing death; fever and abnormal chest X-ray were the most common presentations of MERS-CoV disease. Future studies to identify risk factors for severe outcome and death are needed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–20. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 2.de Groot RJ, Baker SC, Baric RS, Brown CS, Drosten C, Enjuanes L, et al. Middle east respiratory syndrome coronavirus (MERS-CoV): Announcement of the Coronavirus Study Group. J Virol. 2013;87:7790–2. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) 2015. [Last cited on 2015 May 19]. Available from: http://www.who.int/csr/don/18-may-2015-mers-are/en/
- 4.WHO. Middle East Respiratory Syndrome Coronavirus (MERS-CoV): Summary of Current Situation, Literature Update and Risk Assessment – As of 5 February, 2015. 2015. [Last updated on 2015 Feb 05; Last accessed on 2015 Feb 12]. Available from: http://www.who.int/csr/disease/coronavirus_infections/mers-5-february-2015.pdf?ua=1 .
- 5.Al-Tawfiq JA, Hinedi K, Ghandour J, Khairalla H, Musleh S, Ujayli A, Memish ZA. Middle East respiratory syndrome coronavirus: A case-control study of hospitalized patients. Clin Infect Dis. 2014;59:160–5. doi: 10.1093/cid/ciu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DA, et al. Hospital outbreak of middle east respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–16. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Abdallat MM, Payne DC, Alqasrawi S, Rha B, Tohme RA, Abedi GR, et al. Hospital-associated outbreak of middle east respiratory syndrome coronavirus: A serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014;59:1225–33. doi: 10.1093/cid/ciu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of middle east respiratory syndrome coronavirus disease from Saudi Arabia: A descriptive study. Lancet Infect Dis. 2013;13:752–61. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saad M, Omrani AS, Baig K, Bahloul A, Elzein F, Matin MA, et al. Clinical aspects and outcomes of 70 patients with middle east respiratory syndrome coronavirus infection: A single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–6. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) – Saudi Arabia. 2014. [Last updated on 2014 Dec 17; Last accessed on 2014 Dec 24]. Available from: http://www.who.int/csr/don/17-december-2014-mers/en/
- 11.Gulland A. WHO voices concern over rising numbers of MERS-CoV cases. BMJ. 2014;348:g2968. doi: 10.1136/bmj.g2968. [DOI] [PubMed] [Google Scholar]
- 12.Oboho IK, Tomczyk SM, Al-Asmari AM, Banjar AA, Al-Mugti H, Aloraini MS, et al. 2014 MERS-CoV outbreak in Jeddah – A link to health care facilities. N Engl J Med. 2015;372:846–54. doi: 10.1056/NEJMoa1408636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corman VM, Müller MA, Costabel U, Timm J, Binger T, Meyer B, et al. Assays for laboratory confirmation of novel human coronavirus (hCoV-EMC) infections. Euro Surveill. 2012;17 doi: 10.2807/ese.17.49.20334-en. pii: 20334. [DOI] [PubMed] [Google Scholar]
- 14.Shalhoub S, Farahat F, Al-Jiffri A, Simhairi R, Shamma O, Siddiqi N, et al. IFN-α2a or IFN-β1a in combination with ribavirin to treat middle east respiratory syndrome coronavirus pneumonia: A retrospective study. J Antimicrob Chemother. 2015;70:2129–32. doi: 10.1093/jac/dkv085. [DOI] [PMC free article] [PubMed] [Google Scholar]


