Abstract
BACKGROUND:
For patients with chronic respiratory failure (CRF) who are treated with noninvasive positive pressure ventilation (NPPV), a little is known regarding the effects of low-intensity NPPV (LI-NPPV) on the clinical course of CRF and the frequency of adjustments in these patients.
OBJECTIVES:
This study investigated the effects of LI-NPPV on the clinical course of patients with CRF as compared with patients who were treated with conventional NPPV (C-NPPV) and determined how frequently NPPV was adjusted during therapy.
METHODS:
Clinical data from 21 patients who received long-term NPPV were retrospectively analyzed. Patients were categorized into two groups based on the level of initial pressure support (PS): C-NPPV group (PS ≥ 10 cm H2O) and LI-NPPV group (PS < 10 cm H2O).
RESULTS:
Patients in the LI-NPPV group had significantly more exacerbations of CRF (P < 0.05). There was no significant difference in the number of patients who required adjustments of NPPV settings between the two groups. There was no significant difference in PaCO2 levels 1 month after the start of NPPV between the two groups; however, PaCO2 levels were significantly lower after 1 year in the C-group (P < 0.001). Seventy-one percent of LI-NPPV patients and 43% of C-NPPV patients needed NPPV adjustments.
CONCLUSIONS:
Attention should be paid to CRF patients who are initially administered LI-NPPV; they should be carefully observed because they can develop more exacerbations of CRF than patients undergoing C-NPPV. If possible, higher initial PS should be administered to prevent CRF exacerbations.
Keywords: Adjustment, chronic respiratory failure, exacerbation, low-intensity noninvasive positive pressure ventilation, noninvasive positive pressure ventilation
The efficacy of long-term, noninvasive positive pressure ventilation (NPPV) has been established for patients with chronic respiratory failure (CRF).[1,2] In general, NPPV relieves respiratory muscle fatigue, improves respiratory system compliance by reversing microatelectasis of the lung, and lowers the respiratory center set point for CO2 by ameliorating chronic hypoventilation.[1] Previous studies of patients with chronic hypercapnic respiratory insufficiency due to restrictive thoracic disease (RTD), including pulmonary tuberculosis sequelae (PTS) and kyphoscoliosis (KS), have shown that long-term NPPV improved the hypoventilation symptoms,[3,4] arterial blood gases (ABG),[2,5,6,7,8] survival and quality of life,[1] and also reduced hospital admissions due to respiratory complications.[3,7]
In patients with CRF due to RTD, PaCO2 levels ≥50 mm Hg at 1 month after the start of home ventilation are an independent predictor of mortality.[9] Moreover, PaCO2 levels at 3–6 months were associated with rates of NPPV continuation.[10] To show such an efficacy of ventilatory support, certain levels of pressure support (PS) are needed.[3,10,11] According to the protocols for initiating NPPV,[1] the initial settings should provide low inspiratory positive airway pressures (IPAPs) of 8–10 cm H2O and should be gradually increased to the patient's tolerance level. Actually, the majority of published randomized controlled trials have used PS (IPAP-expiratory positive airway pressure [EPAP] [≥10 cm H2O]).[10,12] Because of the open-circuit design of NPPV, however, success depends largely on patient cooperation and acceptance.[1] Some patients cannot tolerate the ideal PS at the start of NPPV, so with reluctance, clinicians must begin with a lower PS because continuity of treatment with NPPV is a priority. The prognostic value of such low PS after starting NPPV is unknown. This preliminary study retrospectively investigated the effects of low-intensity NPPV (LI-NPPV) on the clinical course of patients with CRF as compared with patients who were treated with conventional NPPV (C-NPPV).
A previous study has shown that 36% of patients with CRF treated by NPPV required adjustments of a face mask or ventilator settings to maintain optimal gas exchange and compliance with their therapies during a follow-up period of 6 months.[13] However, a little is known regarding the effects of NPPV adjustments on the long-term clinical course of CRF. Therefore, we also determined the frequency of the need to adjust NPPV during the course of therapy.
Methods
Study subjects
Data were collected from 21 patients with CRF associated with RTD (PTS or KS) who were prescribed chronic NPPV therapy at our institution. The clinical data from these patients, who had been followed as outpatients, were retrospectively analyzed. The ethics committee of the hospital approved to access patient records. All patients met the criteria for hypercapnic respiratory failure (PaCO2≥45 mm Hg on room air).[14] PTS was defined as severe restrictive pulmonary dysfunction, with or without cor pulmonale, as a result of previous Mycobacterium tuberculosis infection involving the chest wall, lung parenchyma, and/or pleura.[15] KS was defined as a Cobb angle of >50°.[16]
All patients met the selection guidelines for long-term NPPV for RTD, as previously reported.[1] Briefly, all patients showed typical symptoms such as a morning headache, daytime hypersomnolence, energy loss, impaired gas exchange attributable to chronic daytime, and sustained nocturnal hypoventilation.[1] Before beginning NPPV therapy, all study patients were in clinically stable condition (no exacerbation of CRF or hospital admission for at least 1 month prior to the study). No patient had rapidly progressive neuromuscular disease or obesity hypoventilation syndrome. Arterial blood samples were obtained immediately before and 1 month after the start of NPPV therapy and obtained every 6 months or 1 year during follow-up.
Measurements
The evaluated parameters included age at the start of NPPV, sex, body mass index (BMI), spirometry, ABGs, whether or not they had received long-term oxygen therapy (LTOT), type of ventilator at the start of NPPV, and ventilator settings. All data and information on the clinical course of each patient were collected from their clinical records.
Noninvasive positive pressure ventilation
Bilevel NPPV was delivered via nasal or oronasal mask using one of the following ventilators: (1) VPAP ST series (VPAP II ST, VPAP II ST-A, or VPAP III ST-A; ResMed, Sydney, Australia), (2) VS SERENA (Res Med, Sydney, Australia), or (3) BiPAP Synchrony series (BiPAP Synchrony or BiPAP Harmony; Respironics, Inc., Murrysville, PA). The commercial masks were sized appropriately to each patient's nose or face. In all cases, the ventilator was initially set to spontaneous/timed (S/T) mode. We start NPPV with low inspiratory pressures (8–10 cm H2O) and gradually titrate upward as tolerated by the patient as previously reported[1] with an EPAP in the range of 2–5 cm H2O, with a backup respiratory frequency (fR) below the rate of awake spontaneous breathing. We did not set up any target minute ventilation or tidal volume on the initiation of NPPV. Oxygen was supplied from the mask's side port at a flow rate needed to achieve a target SpO2 of 90%. Heated humidifiers were used for all patients.
Comparisons of patients according to the initial pressure support
Patients were categorized into the following two groups based on the level of initial PS: C-NPPV (PS ≥ 10 cm H2O, n = 7) and LI-NPPV (PS < 10 cm H2O, n = 14).
Noninvasive positive pressure ventilation adjustments
The level of support could be increased if ABG tensions did not improve or after exacerbation of CRF, as previously reported.[17] IPAP was adjusted with step-by-step increases to the level of patient tolerance.[1,12,17] In addition, if the patient desired an elevation of IPAP, the IPAP was adjusted to tolerance. If optimal improvement in ABG was not achieved despite increased PS, the fR was increased. The fR was set slightly below the spontaneous breathing rate without NPPV,[1] and the EPAP was set as low as possible for ease of expiration. However, for patients with concomitant obstructive ventilatory disorder and/or upper airway obstruction, EPAP was increased to improve inspiratory triggering.
Nasal masks were often used on the initial setting; however, if an air leak from the mouth was problematic, the nasal mask was changed to an oronasal mask. An oronasal mask was exchanged for a nasal mask to achieve patient satisfaction. Oxygen supplementation was appropriately increased to achieve a target SpO2 of 90%. If it was impossible to improve gas exchange by the adjustment of the IPAP (PS) or backup respiratory rate, the S/T mode was changed to the timed (T) mode.
Chronic respiratory failure exacerbation
A CRF exacerbation was defined as an event during the natural course of the disease characterized by a change in the patient's baseline dyspnea, cough, and/or sputum production that warranted a change in management, plus uncompensated respiratory acidosis (pH < 7.30).[18]
Statistical analysis
Results are given as median values with ranges in parentheses. Differences in baseline characteristics, lung function, and ABG values between the LI-NPPV and the C-NPPV patients were analyzed by the Mann–Whitney U test. Differences between the ABG values before NPPV therapy and those 1 month and 1 year after NPPV therapy were analyzed by the Wilcoxon signed-rank test. Statistical significance was accepted at P < 0.05. Statistical analysis was performed using StatMate (version 4.01, ATMS Co. Ltd., Tokyo, Japan).
Results
Patient characteristics
Baseline characteristics of the study patients are shown in Table 1. The median age of the patients (12 men and 9 women) was 71 years (54–86 years). Eighteen patients had PTS and 3 had KS. Patients were categorized into two groups based on the degree of clinical breathlessness, according to the Fletcher-Hugh-Jones classification:[19] grade III (n = 6) and grade IV (n = 15). A total of 16 of 21 patients (76%) had received LTOT before NPPV therapy. ABG results and lung function indices obtained before NPPV are shown in Table 2.
Table 1.
Baseline characteristics of study patients (n=21)

Table 2.
Pulmonary function test results before NPPV

Initial noninvasive positive pressure ventilation settings
Ventilators for all patients were set to S/T modes. Table 3 shows the initial NPPV settings, including IPAP, EPAP, PS, backup fR, and supplemental O2 dose. Table 4 indicates that the initial IPAP and PS values were significantly different between the two groups of patients (P < 0.001). There were no statistically significant differences for fR and doses of supplemental O2 between the two groups (data not shown).
Table 3.
Initial NPPV settings in all patients

Table 4.
Comparisons of baseline characteristics, pulmonary function results, treatment periods, initial ventilator settings, and numbers of patients who suffered from CRF exacerbations between the two study groups
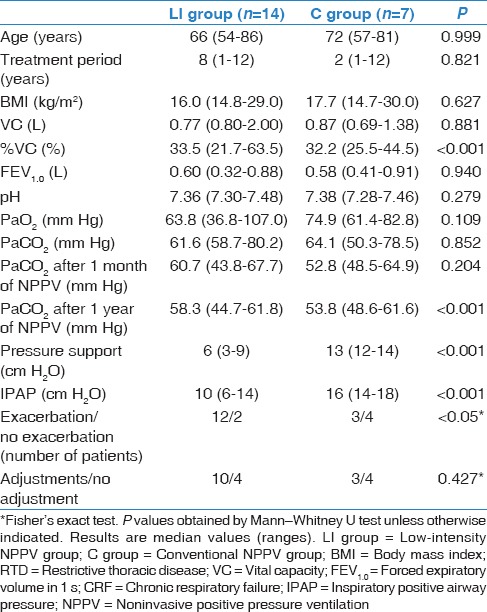
Comparisons between the low-intensity noninvasive positive pressure ventilation and the conventional noninvasive positive pressure ventilation groups
Except for the initial %VC, there were no significant differences in baseline characteristics between the two groups, including age, treatment period, BMI, lung function, and ABG values [Table 4]. There were no significant differences in PaCO2 levels 1 month after the start of NPPV between the two groups; however, PaCO2 levels were significantly lower 1 year after in the C group (P < 0.001). The LI-NPPV patients experienced significantly more exacerbations of CRF than the C-NPPV patients (P < 0.05). In addition, two patients of C-NPPV patients experienced the total 23 episodes of CRF exacerbations, and 10 of 23 episodes (43%) were due to airway infection whereas 12 patients of LI-NPPV patients experienced total 45 episodes of CRF exacerbations, and 16 of 45 episodes (36%) were due to airway infection.
Noninvasive positive pressure ventilation adjustments
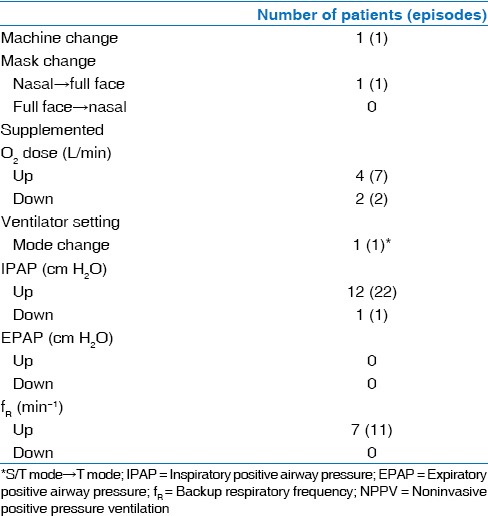
Consequently, 13 of 21 patients (62%) required NPPV adjustments: 71% of LI-NPPV and 43% of C-NPPV patients. There was no significant difference in the number of patients who required adjustments of NPPV settings between the two groups [Table 4]. Table 5 summarizes the types of NPPV adjustments. Twelve patients required IPAP elevations (22 episodes), 7 patients required increases in fR (11 episodes), and 4 patients required increases in supplemental O2 doses (7 episodes). Decreases in the initial settings of pressures or supplemental O2 doses were infrequent (2 patients, 2 episodes). One patient each required a machine change, a mask change, or a mode change. Adjustments were often performed after an acute exacerbation of CRF or at the request of the patient.
Table 5.
Details for NPPV adjustments
Discussion
The main finding of the present study was that patients in the LI-NPPV group developed significantly more exacerbations of CRF, and a total of 62% of the study patients required NPPV adjustments.
It is of great interest that in the LI-NPPV group, significantly more patients experienced CRF exacerbations. The causes of CRF exacerbation seemed to be similar between the two groups, so the reasons why LI-NPPV patients had more exacerbations were probably associated with the insufficient treatment effect by the extremely LI-NPPV. In the C group patients, the levels of PaCO2 were lowered over 10 mmHg after 1 month of NPPV versus about 1 mm Hg in the LI group patients, which was not statistically different. In the C group, lowered PaCO2 levels were maintained for 1 year. According to the protocols for initiating NPPV,[1] the initial settings should provide a low IPAP (8–10 cm H2O), which should be gradually increased to the level of patient tolerance. Recent studies for RTD have used mean IPAP settings of 12.7–16.5 cm H2O.[10,12] In the present study, the median initial pressure parameters in the C-NPPV patients were as follows: IPAP = 16 (14–18) cm H2O and PS = 13 (12–14) cm H2O. These settings, which were similar to the settings reported in literature, showed various clinical benefits.[1,2,3,4,5,6,7,8] However, in the LI-NPPV group, the initial IPAP and PS settings were significantly lower than those used for the C-NPPV group: IPAP = 10 (6–14) cm H2O and PS = 6 (3–9) cm H2O. LI-NPPV patients could not tolerate conventional IPAP levels when NPPV was started. Therefore, because long-term treatment was a priority, settings providing lower IPAPs were used, although the importance of higher PS to achieve stabilized gas exchange was recognized. The decreased ventilatory support was not able to compensate for the alveolar nocturnal hypoventilation. Therefore, most LI-NPPV patients required adjustments after their exacerbation of CRF to achieve improved gas exchange. Increased IPAP or PS settings may improve the prognoses of CRF patients who receive NPPV. Careful follow-up and proper NPPV adjustments are very important for patients who are initially treated with extremely low IPAP or PS settings.
If NPPV is initiated with low PS, more frequent adjustments may be needed. In the present study, 71% of LI-NPPV patients and 43% of C-NPPV patients needed NPPV adjustments.
Criner et al. reported that 36% of CRF patients treated by NPPV required face mask or ventilator setting adjustments to maintain optimal gas exchange and compliance with therapy.[13] IPAP elevation, increased fR, and increased doses of oxygen supplementation were common. These adjustments improved the ABG findings in all patients. The questions we must ask here regarding NPPV adjustments for CRF patients are when and how should we adjust NPPV? In fact, NPPV adjustments are not more common in patients with CRF than in patients with acute respiratory failure because long-term NPPV is usually performed at home. A recent study showed the importance of stabilizing PaCO2 throughout long-term NPPV for NPPV continuance.[20] Thus, timely and appropriate NPPV adjustments are important because some patients who receive NPPV inevitably experience a downward clinical course in spite of long-term NPPV. Treatment failure in NPPV for CRF patients may be a result of the progression of the original disease, acute exacerbations, complications from other diseases that affect cardiopulmonary status, or complications from NPPV itself. Another cause of treatment failure may be an inadequate initial NPPV setting. We previously reported a patient with CRF and secondary pulmonary hypertension associated with asbestos pleurisy and PTS.[21] We adjusted the ventilator settings 6 months after the initiation of NPPV. As in the present study, the IPAP and EPAP settings were increased to obtain improvement in nocturnal hypoventilation. NPPV adjustments provided improvements in both hypoxemia and hypercapnia, which were followed by reduced pulmonary hypertension. Periodic evaluations, using ABG analysis and nocturnal SpO2 monitoring, and medical interviews are needed for each patient to determine if NPPV adjustments are needed. The British Thoracic Society NPPV guidelines for acute respiratory failure provide some of the recommended NPPV adjustments for the cases of treatment failure.[22] These provisions could also be applied to the adjustments used for patients with CRF.
This study has limitations. First, it was a small retrospective study. However, the results of the present study indicate that there are ethical problems with conducting a randomized prospective study to elucidate the clinical effects of LI-NPPV. Second, we initiated NPPV in the S/T mode for all patients. A recent study has shown that patients with CRF associated with PTS who were treated with only the controlled mode (T mode) had significantly higher continuation and survival rates than those treated with an assisted mode.[12] Third, the treatment period varied from 1 to 12 years.
Despite the limitations of this study, the results suggest that patients with CRF who initially are administered LI-NPPV should be followed carefully because they could develop more CRF exacerbations than if treated with C-NPPV. If possible, higher PS should be used initially to prevent CRF exacerbations.
Financial support and sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Mehta S, Hill NS. Noninvasive ventilation. Am J Respir Crit Care Med. 2001;163:540–77. doi: 10.1164/ajrccm.163.2.9906116. [DOI] [PubMed] [Google Scholar]
- 2.Buyse B, Meersseman W, Demedts M. Treatment of chronic respiratory failure in kyphoscoliosis: Oxygen or ventilation? Eur Respir J. 2003;22:525–8. doi: 10.1183/09031936.03.00076103. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez C, Ferris G, Diaz J, Fontana I, Nuñez J, Marín J. Kyphoscoliotic ventilatory insufficiency: Effects of long-term intermittent positive-pressure ventilation. Chest. 2003;124:857–62. doi: 10.1378/chest.124.3.857. [DOI] [PubMed] [Google Scholar]
- 4.Hill NS, Eveloff SE, Carlisle CC, Goff SG. Efficacy of nocturnal nasal ventilation in patients with restrictive thoracic disease. Am Rev Respir Dis. 1992;145(2 Pt 1):365–71. doi: 10.1164/ajrccm/145.2_Pt_1.365. [DOI] [PubMed] [Google Scholar]
- 5.Carroll N, Branthwaite MA. Control of nocturnal hypoventilation by nasal intermittent positive pressure ventilation. Thorax. 1988;43:349–53. doi: 10.1136/thx.43.5.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leger P, Bedicam JM, Cornette A, Reybet-Degat O, Langevin B, Polu JM, Jeannin L, Robert D. Nasal intermittent positive pressure ventilation. Long-term follow-up in patients with severe chronic respiratory insufficiency. Chest. 1994;105:100–5. doi: 10.1378/chest.105.1.100. [DOI] [PubMed] [Google Scholar]
- 7.Masa Jiménez JF, Sánchez de Cos Escuin J, Disdier Vicente C, Hernández Valle M, Fuentes Otero F. Nasal intermittent positive pressure ventilation. Analysis of its withdrawal. Chest. 1995;107:382–8. doi: 10.1378/chest.107.2.382. [DOI] [PubMed] [Google Scholar]
- 8.Simonds AK, Elliott MW. Outcome of domiciliary nasal intermittent positive pressure ventilation in restrictive and obstructive disorders. Thorax. 1995;50:604–9. doi: 10.1136/thx.50.6.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martí S, Pallero M, Ferrer J, Ríos J, Rodríguez E, Morell F, et al. Predictors of mortality in chest wall disease treated with noninvasive home mechanical ventilation. Respir Med. 2010;104:1843–9. doi: 10.1016/j.rmed.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Tsuboi T, Ohi M, Oga T, Machida K, Chihara Y, Harada Y, et al. Importance of the PaCO (2) from 3 to 6 months after initiation of long-term non-invasive ventilation. Respir Med. 2010;104:1850–7. doi: 10.1016/j.rmed.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 11.Adigüzel N, Karakurt Z, Güngör G, Moçin O, Balci M, Saltürk C, et al. Management of kyphoscoliosis patients with respiratory failure in the intensive care unit and during long term follow up. Multidiscip Respir Med. 2012;7:30. doi: 10.1186/2049-6958-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuboi T, Oga T, Machida K, Chihara Y, Matsumoto H, Niimi A, et al. Importance of ventilator mode in long-term noninvasive positive pressure ventilation. Respir Med. 2009;103:1854–61. doi: 10.1016/j.rmed.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Criner GJ, Brennan K, Travaline JM, Kreimer D. Efficacy and compliance with noninvasive positive pressure ventilation in patients with chronic respiratory failure. Chest. 1999;116:667–75. doi: 10.1378/chest.116.3.667. [DOI] [PubMed] [Google Scholar]
- 14.Grippi MA. Respiratory failure: An overview. In: Fishman AP, Elias JA, Fishman JA, Grippi MA, Kaiser LR, Senior RM, editors. Fishman's Pulmonary Disease and Disorders. 3rd ed. Vol. 2. New York: McGraw-Hill; 1997. pp. 2509–21. [Google Scholar]
- 15.Bach JR. Noninvasive Mechanical Ventilation. Philadelphia: Hanley and Belfus; 2002. [Google Scholar]
- 16.Masa JF, Celli BR, Riesco JA, Sánchez de Cos J, Disdier C, Sojo A. Noninvasive positive pressure ventilation and not oxygen may prevent overt ventilatory failure in patients with chest wall diseases. Chest. 1997;112:207–13. doi: 10.1378/chest.112.1.207. [DOI] [PubMed] [Google Scholar]
- 17.Tuggey JM, Elliott MW. Titration of non-invasive positive pressure ventilation in chronic respiratory failure. Respir Med. 2006;100:1262–9. doi: 10.1016/j.rmed.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Kadowaki T, Hamada H, Yokoyama A, Abe M, Nishimura K, Kohno N, et al. Significance of serum uric acid in patients with chronic respiratory failure treated with non-invasive positive pressure ventilation. Intern Med. 2007;46:691–7. doi: 10.2169/internalmedicine.46.6120. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher CM. The clinical diagnosis of pulmonary emphysema; an experimental study. Proc R Soc Med. 1952;45:577–84. [PubMed] [Google Scholar]
- 20.Tsuboi T, Oga T, Sumi K, Machida K, Ohi M, Chin K. The importance of controlling PaCO2 throughout long-term noninvasive ventilation. Respir Care. 2014;59:1671–8. doi: 10.4187/respcare.02829. [DOI] [PubMed] [Google Scholar]
- 21.Kadowaki T, Hamada H, Hamaguchi N, Ito R, Katayama H, Sakai K, et al. Successful treatment of secondary pulmonary hypertension by long-term non-invasive positive pressure ventilation. Respir Med Extra. 2006;2:120–2. [Google Scholar]
- 22.British Thoracic Society Standards of Care Committee. Non-invasive ventilation in acute respiratory failure. Thorax. 2002;57:192–211. doi: 10.1136/thorax.57.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]


