Abstract
Post-vasectomy pain syndrome remains one of the more challenging urological problems to manage. This can be a frustrating process for both the patient and clinician as there is no well-recognized diagnostic regimen or reliable effective treatment. Many of these patients will end up seeing physicians across many disciplines, further frustrating them. The etiology of post-vasectomy pain syndrome is not clearly delineated. Postulations include damage to the scrotal and spermatic cord nerve structures via inflammatory effects of the immune system, back pressure effects in the obstructed vas and epididymis, vascular stasis, nerve impingement, or perineural fibrosis. Post-vasectomy pain syndrome is defined as at least 3 months of chronic or intermittent scrotal content pain. This article reviews the current understanding of post-vasectomy pain syndrome, theories behind its pathophysiology, evaluation pathways, and treatment options.
Keywords: epididymectomy, microdenervation, orchalgia, post-vasectomy pain management, post-vasectomy pain syndrome, testicular pain, vasectomy reversal, vaso-vasostomy
INTRODUCTION
Vasectomies are one of the most common urological procedures performed by urologists worldwide. It is the most effective male contraceptive method. It is estimated that 500,000 vasectomies are performed in the United States per annum.1 This procedure involves dividing the vas deferens and is often performed under local anesthesia in an outpatient setting. Traditionally, the procedure involves making small bilateral scrotal incisions to expose and visualize the vas deferens, excising at least 1 cm of the vas deferens, followed by electrocautery fulguration of the ends of the vas deferens, placing sutures or clips on each end and interposing tissue between the two cut ends to further prevent recanalization. The success rate of the procedure is estimated to be between 98% and 99%.2,3 The most common complications include bleeding, development of a hematoma and infection of the scrotal incision sites.
Although rare, patients may experience chronic scrotal content pain following a vasectomy. The 2012 American Urological Association (AUA) guideline for vasectomy which was updated in 2015 states that 1–2% of men who undergo a vasectomy will develop chronic scrotal pain that is severe enough to interfere with their quality of life and require medical attention.4 This syndrome has been coined by many terms including testialgia, chronic orchialgia, chronic scrotal content pain, post-vasectomy orchialgia, congestive epididymitis, and chronic testicular pain. At present, the syndrome is widely accepted as post-vasectomy pain syndrome (PVPS).5 In this article, we aim to review the therapeutic intervention for this perplexing problem.
METHODOLOGY
Search strategy
We conducted a computerized bibliographic search of the PubMed, Medline, Embase, and Cochrane databases for all reports pertaining to PVPS using the Mesh Words “Post-vasectomy Pain Syndrome,” “Post Vasectomy Pain Syndrome,” “Microdenervation of Spermatic Cord,” “Epididymectomy,” “Vasectomy Reversal,” and “Orchiectomy” through October 31, 2015.
Eligibility criteria and patients
We specifically reviewed all articles pertaining to PVPS and microdenervation of the spermatic cord, epididymectomy, vasectomy reversal or orchiectomy. We only included articles in the English literature.
BACKGROUND
PVPS is different from acute post procedure pain. Acute post procedure pain typically resolves 2–4 weeks postoperatively whereas PVPS continues to persist or may occur months to years after the vasectomy. PVPS can be an extremely frustrating problem to treat for both the patient and the clinician, as there remains no widely accepted protocol for evaluation and treatment of the problem. PVPS is defined as constant or intermittent testicular pain for 3 months or longer with a severity that interferes with daily activities prompting the patient to seek medical treatment.6 The exact incidence of PVPS is unknown but was estimated to be very low (<1%) in the past.4,5,6,7 However surveys in recent years have found that almost 15% of men suffer from PVPS, with 2% of men experiencing pain intense enough to impact their quality of life.8,9 One review reported that 1 in 1000 men who undergo a vasectomy will sustain long-term pain requiring surgical intervention.10
ETIOLOGY
The pathophysiology of PVPS remains unclear, but speculations regarding the mechanism leading to pain include damage to the scrotal and spermatic cord nerve structures via inflammatory effects of the immune system, back pressure effects in the obstructed vas and epididymis, vascular stasis, nerve impingement, or perineural fibrosis.11 Histological findings within the proximal segment of the vas following vasectomy include spermatid degeneration, thickened basement membranes, and increased phagocytosis by Sertoli cells, which are responsible for maintaining normal epididymal pressure.12 Pressure build-up that is too great for compensatory mechanisms eventually occur, leading to the formation of sperm granulomas, epididymal blowout, or vasitis nodosa.4 Studies have reported that in vasectomized men, testicular histology show dilation of the seminiferous tubules, reduction in seminiferous cell population and interstitial fibrosis using quantitative morphometric analysis.13
Another possible explanation of PVPS is that the epididymis is trapped between two opposing forces when ejaculation occurs. The epididymal duct and vas deferens are lined with smooth muscle cells which contract rhythmically during ejaculation to facilitate sperm movement through the duct. However, in the setting of a vasectomized patient, this contraction results in testicular fluid being discharged into the caudal epididymis leading to increase pressure, causing epididymal fibrosis, and blowout within the epididymis.14
In vasectomized patients, the blood-testes barrier is also disrupted, causing detectable levels of serum antisperm antibodies in 60%–80% of men.8 About 7%–30% of vasectomized patients will also have antisperm antibodies within the epididymis.15,16 Animal studies have found that these antibodies can result in agglutination of sperm and activation of the complement cascade, with immune complex formation and deposition of these complexes in the basement membrane.17 All the above mechanisms together or individually may result in PVPS.
CLINICAL PRESENTATION
The mean time of the onset of PVPS is reported to be 7–24 months.9,18 Demographics (age, socioeconomic status, race) and operative techniques have not been shown to have a correlation to the development of PVPS.13 Signs and symptoms of PVPS include a tender vas deferens and/or epididymis, fullness of the vas deferens, orchialgia, dyspareunia, pain with ejaculation, premature ejaculation, and pain with straining. Scrotal ultrasound may show engorgement or thickening of the epididymis.
EVALUATION
The evaluation includes a thorough history and physical examination. The history should include the duration and nature of the pain, severity (on a 0–10 scale), location, radiation, aggravating factors, associated symptoms, and previous therapeutic maneuvers. The physician should also determine if voiding, bowel movements, sexual or physical activities or prolonged sitting aggravate the pain. Previous surgery to the spine, inguinal, scrotal, pelvic, and retroperitoneal space should also be recorded. Psychosocial questions to rule out depression, history of sexual abuse, Munchausen syndrome or other somatoform disorders should also be included.19
Physical examination focusing on the genitalia is essential. The patient should be examined while standing and supine, beginning on the normal/less painful side. A thorough examination of the testes, epididymides, vas deferens and a 360° rectal exam is also recommended to evaluate abnormalities of the prostate and hypertonicity or tenderness of the pelvic floor structures. A neurological examination of the lower limbs and genitals should also be performed to rule out radicular pain syndromes and neurosensory deficits. Laboratory investigations include a urinalysis, urine and semen culture to rule out infection when indicated.4 Should microscopic hematuria be identified, computed tomography (CT) scan of the abdomen and pelvis is indicated as stones in the ureter can cause scrotal pain. All men with chronic orchialgia should also undergo a high-resolution scrotal ultrasound with color-flow Doppler to evaluate the contents of the scrotum to rule out any pathological processes such as testicular tumor, varicocele or infection.4 Magnetic resonance imaging (MRI) scan of the spine or hips is suggested when there is a history of back or hip pain to rule out nerve impingement. A spermatic cord block should also be considered to determine if the pain is being generated from within the scrotum. We recommend that this block should be performed by injecting 20 ml of 0.25% bupivacaine/ropivacaine without epinephrine, into the spermatic cord at the level of the pubic tubercle.19 If the pain is conducted via the spermatic cord nerves, the pain should be temporarily relieved after performing the cord block. A saline control to exclude malingering remains controversial as it has not been found to be a reliable tool to rule out malingering behavior.20
Differential diagnoses for PVPS include varicocele, hydrocele, infection, tumor, intermittent testicular torsion, inguinal hernia, trauma, pelvic floor myalgia, referred pain, and psychogenic causes.20 PVPS is a diagnosis of exclusion and the diagnosis should only be made after all these investigative studies have been performed.
TREATMENT
Currently, there are no published data with good evidence regarding non-surgical intervention for PVPS. However, pharmacotherapy should be considered the first line followed by a series of spermatic cord blocks. Pelvic floor physical therapy, acupuncture, and a psychological evaluation may also be beneficial. Failing non-surgical treatment, repeating the vasectomy with wide excision of the severed ends, microdenervation of the spermatic cord, epididymectomy, vasectomy reversal or orchiectomy should be considered (Figure 1).
Figure 1.
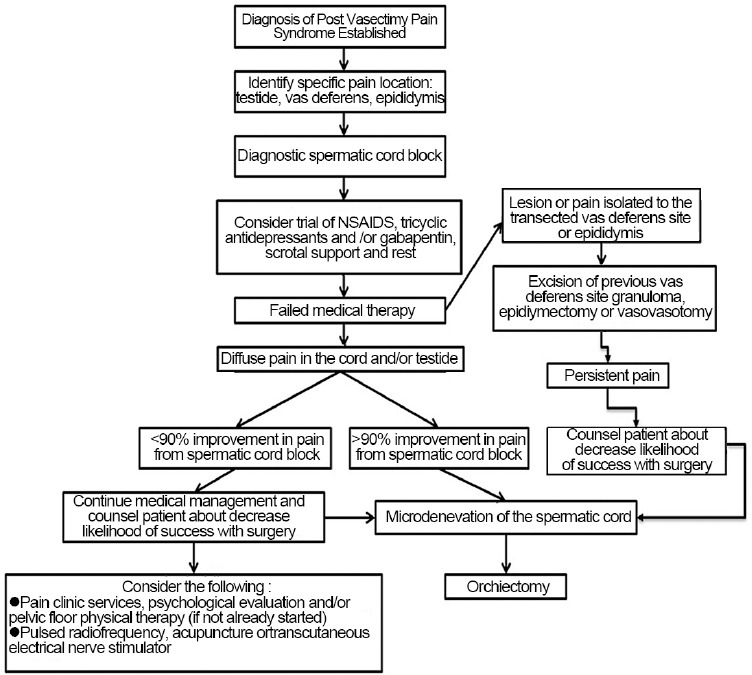
Treatment algorithm for patient with postvasectomy pain syndrome.59
Pharmacological treatment
Antibiotics should be initiated if the patient shows any signs of orchitis or epididymitis. Trimethoprim/sulfamethoxazole or quinolone antibiotics for 2–4 weeks are preferred due to their lipophilic nature which penetrates the testis and epididymis well. It would be inappropriate to empirically treat men for an infection in the absence of any signs or symptoms of an infection, which may in fact be more harmful than good.
Initial pharmacological therapy should include non-steroidal anti-inflammatory drugs (NSAIDs) over a period of 2–4 weeks. In our experience, NSAIDs typically work best in patients who experience PVPS <1 year from their vasectomy. Failing NSAIDs therapy, we recommend using a tricyclic antidepressant (TCA). There has been no clinical trial showing any efficacy in using a TCA for PVPS. Sinclair et al. found that 66.6% of patients with idiopathic testicular pain had improvement of pain in a trial of six patients after 3 months with nortriptyline therapy.21 However, a subgroup analysis of patients with PVPS did not show the same improvement.21 Limiting factors of this study include a small sample size and its retrospective nature. However, TCA has been shown to treat nerve pain in patients with diabetic neuropathy as well as postherpetic neuralgia.22,23 TCA works by inhibiting the reuptake of norepinephrine and serotonin in the brain. It also inhibits sodium channel blockers and L-type calcium channels that are thought to be responsible for its analgesic effect by modulating first order neuron synapses with second order neuron synapses in the dorsal horn of the spinal cord. Tertiary amines (amitriptyline and clomipramine) are reported to be more effective for neuropathic pain compared to secondary amines (desipramine and nortriptyline).24,25 However, tertiary amines are also associated with more sedation and postural hypotension.26 TCA may take 2–3 weeks from initiation of therapy to be effective.
Anticonvulsants have also been shown to work for neuropathic pain. The two mainstays of anticonvulsants used for neuropathic pain are gabapentin and pregabalin due to the paucity of side effects in the older generation anticonvulsants. There has been no clinical trial showing any efficacy in using anticonvulsants for PVPS. Sinclair et al. found that 61.5% of patients with idiopathic testicular pain had improvement of pain in a trial of 13 patients after 3 months with gabapentin therapy.21 However, a subgroup analysis of patients with PVPS also did not show any improvement of pain.21 Limiting factors of this study have been discussed above and include a small sample size with only 13 patients on gabapentin having complete data and an even smaller PVPS group of four patients. However, gabapentin has also been shown in large, randomized, placebo-controlled trials to relieve pain in patients with diabetic polyneuropathy, postherpetic neuralgia, and other types of neuralgia.27,28,29 The proposed mechanism of gabapentin as an analgesic is that it modulates the α-2-d subunit of N-type calcium channels which affects the afferent pain fibers.
Long-term treatment with narcotic agents is not recommended as this does not address the underlying pathological condition and carries the risk of addiction. We occasionally offer a short duration of narcotics for temporary relief of PVPS.
Nonsurgical treatment
Originating around 100 BC in China, acupuncture is regarded as the earliest form of neuromodulation. It is considered a form of alternative medicine and continues to remain a key component of traditional Chinese medicine. This modality may be recommended for patients with chronic genitourinary pain. There are no published trials on acupuncture for PVPS.
Pelvic floor therapy may also benefit patients with pelvic floor dysfunction. This is particularly beneficial in patients who have muscle dysfunction or myofascial trigger points. In our practice, we routinely recommend specialized pelvic floor physical therapy to patients with PVPS if a positive 360° digital rectal exam is identified.
A series of spermatic cord blocks with local anesthetic agents with or without steroids to disrupt the afferent pain pathway may also relieve testicular pain. Studies have demonstrated that this technique rarely provides long-term relief and usually only lasts the duration of the local anesthetic.19 The block is performed by isolating the spermatic cord at the inguinal-scrotal junction. A 25–27-gauge needle is then introduced into the spermatic cord at the level of the pubic tubercle. We typically use 20 cc of 0.25% Bupivacaine Hydrochloride for our initial cord block. The patient is instructed to call the office in 24 hours to report the duration and level of relief if any from the block. Should he experience >90% temporary pain relief, we offer a series of cord blocks every 2 weeks for 4–5 blocks using 9 cc of 0.75% Bupivacaine Hydrochloride combined with 1 cc (10 mg) of triamcinolone acetonide. If there is no alleviation of pain with a well-placed injection, we do not recommend repeating this treatment. In our experience, this technique is rarely successful with long-term pain relief, especially when the duration of chronic pain exceeds 6 months.
Other nonsurgical techniques include pulsed radiofrequency of the spermatic cord and genital branch of the genitofemoral nerve for PVPS if the patient receives temporary relief from a spermatic cord block.30,31 This technique has only been reported in small non-randomized trials.
Surgical treatment
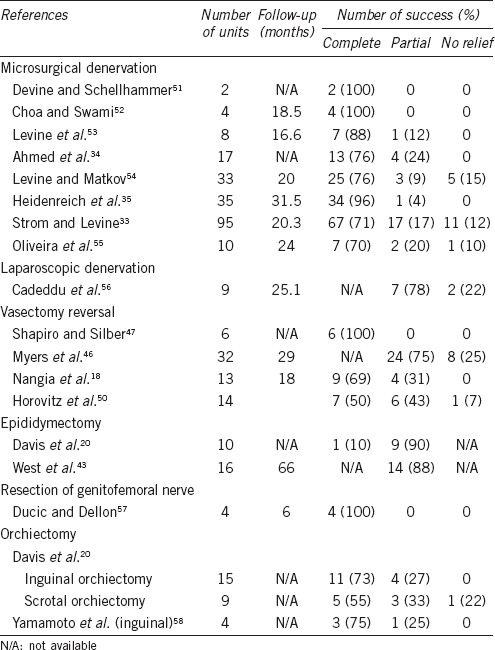
Patients who fail medical therapy should be considered for surgical intervention. Surgical intervention includes excision of sperm granuloma, microdenervation of the spermatic cord (MDSC), epididymectomy, vasectomy reversal or orchiectomy. The success rates of these procedures remain unclear due to the availability of only small case series of men undergoing surgical treatment for PVPS. Table 1 depicts the published success rates of surgical treatments for men with chronic orchialgia. No clear predictors of success for any procedure have been reported except as listed below.
Table 1.
Surgical treatment of orchialgia in the literature33
Microdenervation of the spermatic cord (MDSC)
MDSC is a relatively new surgical option which became more popular over the last two decades. The goal of the procedure involves transecting all the nerves in the spermatic cord while preserving all the arteries (testicular, cremasteric, and deferential) along with several lymphatic channels to reduce the likelihood of developing a hydrocele (Figure 2).32 Informed consent is imperative as the pain may persist, and in some situations worsen following surgery.33 This may be due to accessory fibers from the pudendal nerve, incomplete cord denervation, central nervous system sensitization or malingering. Other risks include the development of a hydrocele and testicular atrophy. Patients with bilateral pain are advised to undergo surgery for the more painful side first as the contralateral site may occasionally resolve following MDSC.
Figure 2.
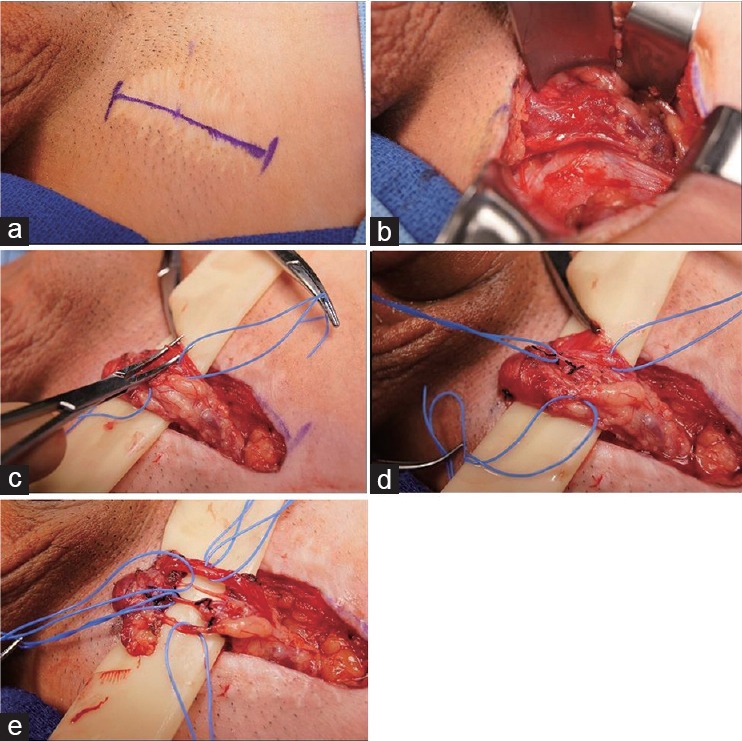
Microdenervation of the spermatic cord. (a) Marking of inguinal site. (b) Dissection to expose spermatic cord. (c) Spermatic cord supported by 5/8 inch Penrose drain with cord fascia opened. (d) Arteries secured by blue Vessel loop. (e) After completion of dissection, only the cremasteric artery, testicular artery, deferential artery, lymphatics remain (top to bottom).22,43
There has only been one study to date that has specifically evaluated the success rate of MDSC for PVPS. Ahmed et al. conducted a retrospective survey of 560 post-vasectomy patients to which 396 patients replied. A total of 17 patients underwent MDSC for PVPS with 13 (76.5%) reporting complete relief of pain at their first follow-up visit. The other four patients had significant improvement in terms of pain and were satisfied with the results.34 Multiple studies have shown good success with MDSC for chronic testicular pain due to a variety of etiologies including idiopathic pain (Table 1). A total of 152 out of 191 men (81.2%) based on all cases of MDSC in the English literature had complete resolution of scrotal content pain (Table 1). Heidenreich et al. reported a series of 35 patients with 96% of patients experiencing complete resolution of pain following MDSC.35 Strom and Levine reported that durable relief was noted in 71% of men following MDSC. A total of 17% of patients reported partial relief, whereas 12% reported no change in pain but no patient reported worsening pain.33 Men with PVPS were included in these studies but were not specifically addressed.
Larsen et al. also reported our institution's results on MDSC, showing that patients who had not undergone a prior attempt at surgical correction for scrotal content pain had a mean post-MDSC visual analog pain scale (VAPS) score of 2 (range 0–10) with an average pain decrease of 79%.36 Whereas, patients who failed prior surgical procedure including epididymectomy, varicocelectomy, and vas reversal who then underwent MDSC reported a mean postoperative VAPS score of 3 (range 0–10) with an average decrease in pain of 67%.36 There was a complete response in 64% of patients in the surgery naïve group compared to 50% in patients whom prior surgical correction for pain had failed. In a separate study, we also found that a positive response to a spermatic cord block defined as at least a 50% temporary reduction of pain was an independent predictor of a successful MDSC.37
Please refer to the Supplementary Appendix (39.4KB, pdf) section on our technique for performing MDSC.
Epididymectomy
Epididymectomy continues to remain a more popular approach compared to MDSC especially in Europe. A survey among Swiss urologists in 2005 concluded that 74% of urologists would perform an epididymectomy, 7% would perform an inguinal orchiectomy and 6% would perform a MDSC for PVPS.38 The reported success rates with epididymectomy range from 50% to 92% and have been reported to produce a better result in relieving pain if a structural abnormality (cyst, granuloma or mass) was noted in the epididymis on examination or with ultrasonography.39,40,41,42 When diffuse pain in the cord and/or testicle is noted during physical examination, this should lead to a MDSC being performed rather than epididymectomy.
There are multiple small series in the literature on epididymectomy for PVPS. West et al. reviewed 16 patients that underwent epididymectomy for pain after vasectomy (3 bilateral, 13 unilateral). A total of 14 patients had excellent initial symptomatic benefit following epididymectomy. Ten patients were followed up for at least 3 years and 90% of patients had sustained improvement in their scrotal pain.43 Lee et al. reviewed 22 patients who underwent epididymectomy and 16 who underwent vasectomy reversal (VR) for PVPS. The difference in the mean preoperative and postoperative VAPS scores was 6.00 ± 1.34 (range 3–8) in the epididymectomy group and 5.50 ± 1.03 (range 4–8) in the VR group. There was no significant difference in pain reduction or patient satisfaction between epididymectomy and vasectomy reversals; hence selection of the procedure should be determined based on physician and patient preference.44
Chung et al. published a multicenter, randomized controlled, single-blind study in 2013 where 21 patients underwent epididymectomy alone and 22 patients underwent epididymectomy with concurrent administration of hyaluronic acid and carboxymethyl cellulose to inhibit adhesion and fibrosis for PVPS.45 At postoperative week 24, 15.8% of patients from the epididymectomy only group were pain free and 57.1% of patients from the epididymectomy with concurrent administration of hyaluronic acid and carboxymethyl cellulose group were pain free. A total of 31.6% from the epididymectomy only group exhibited limited pain relief and 9.5% of patients from the epididymectomy with concurrent administration of hyaluronic acid and carboxymethyl cellulose group, exhibited limited pain relief.
Please refer to the Supplementary Appendix (39.4KB, pdf) section on our technique for performing epididymectomy.
Vasectomy reversal
Vaso-vasostomy appears to be an intuitive solution to PVPS. The goal of the procedure is to relieve the pressure from the obstruction, hence decreasing pain levels. Only data from small single-center studies are available. However, these studies show that up to 100% of patients experience some improvement in pain scores, and complete resolution of pain have been reported to be as high as 50%–69%.18,46,47 The benefits of this approach are the potential resolution of pain and preservation of all intrascrotal structures. However, this contradicts the purpose of the vasectomy, may be costly and may not be covered by health insurance. Reasons that this approach may not succeed include any nonobstructive etiologies such as nerve entrapment.
Polackwich et al. identified 26 patients who underwent a vaso-vasostomy and seven patients who underwent an epididymo-vasostomy for PVPS. A total of 34% of patients had complete resolution of pain and 59% of patients reported improvements in pain scores.48 Lee et al. identified 32 patients who underwent a vasectomy reversal for PVPS and noted that the improvement in the mean preoperative and postoperative VAPS was 6.00 ± 1.25 (4–8) in the patency group (sperm in the ejaculate) and 4.43 ± 0.98 (3–6) in the no patency group (P = 0.011).49 The authors concluded that there was a significant difference in pain reduction in patients who had patency following vasectomy reversal compared to patients who remain obstructed. However, patients who remain obstructed had a decrease in VAPS suggesting that the mechanism of action to which PVPS may not be due to the obstructed vas deferens alone.
Horovitz et al. also published a series of 14 patients who underwent vasectomy reversal for PVPS.50 Fifty percent of patients were rendered pain-free whereas 93% of patients had improvement in pain. Myers et al. reviewed the records of 32 patients undergoing vasectomy reversal for PVPS and found that 24 patients had relief of symptoms after the initial procedure. Of the eight men with recurrent pain, six underwent the second reversal where 50% of these men subsequently had relief of symptoms.46
Please refer to the Supplementary Appendix (39.4KB, pdf) section on our technique for performing vasectomy reversal.
Orchiectomy
Orchiectomy is considered the last resort in patients who do not respond to other means of therapy. Davis et al. reviewed 24 patients with chronic unilateral or bilateral orchialgia not necessarily for PVPS who underwent inguinal orchiectomy.20 A total of 15 patients underwent inguinal orchiectomy where 11 (73%) reported complete relief of pain while 4 had partial relief. Of the nine patients who underwent scrotal orchiectomy, 5 (55%) reported complete relief of pain, 3 (33%) had partial relief and 1 (11%) denied improvement.20 On the basis of these results, the authors recommended inguinal orchiectomy as the procedure of choice for the management of chronic testicular pain when other management is unsuccessful.
Please refer to the Supplementary Appendix (39.4KB, pdf) section on our technique for performing orchiectomy.
OUR PROTOCOL
Once a diagnosis of PVPS has been established, our protocol involves a trial of oral ibuprofen 600–800 mg every 4–6 hours or oral celecoxib 200 mg daily for 10–14 days. Failing NSAIDs therapy, we recommend 10–20 mg of amitriptyline nightly. After 1 month of TCA therapy without success, we recommend adding pregabalin 75 mg 3 times a day. A patient is considered to have failed pharmacology therapy should pain persist after initiating pregabalin for 4 weeks.
Our next step is to perform a spermatic cord block with local long-acting anesthetic agents (bupivacaine or ropivacaine) with or without steroids which aims to disrupt the pain cycle in men with PVPS. This is both therapeutic and diagnostic as patients who respond to a cord block are more likely to respond to MDSC. Should the patient experience >90% relief, we offer a series of cord blocks every 2 weeks for 4–5 blocks using 9 cc of 0.75% Bupivacaine Hydrochloride injection combined with 1 cc (10 mg) of triamcinolone acetonide. If there is no reduction of pain with a well-placed injection, we do not repeat the cord block.
Failing pharmacotherapy, the next step is to consider excision of granuloma, MDSC, epididymectomy or a vasectomy reversal. We recommend surgical excision of a granuloma if a tender mass is palpable at the site of the transected vas deferens. We typically perform MDSC in patients with diffuse pain involving the cord, epididymis, and/or testicle. An epididymectomy is beneficial in patients with pain isolated solely to the epididymis especially in those with structural abnormalities noted on examination or ultrasound. Epididymectomy is rarely performed in our practice as most patients present with more diffuse pain rather than just the epididymis. Should MDSC fail to relieve the pain and the testicle is still sensate on examination, we recommend orchiectomy via an inguinal approach particularly if a cord block results in temporary relief of pain. Vasectomy reversal is rarely offered except in circumstances when the pain is localized to the vasectomy site, and/or epididymis and the patient understands the risk of failure and restoration of fertility (Figure 1).
CONCLUSIONS
PVPS remains a challenge to clinicians due to its poorly understood pathophysiology. Large multicenter, well-constructed trials are essential in hopes of establishing level one evidence to facilitate a standardized algorithm to approach this disease. Our evaluation and treatment algorithm for patient with PVPS is listed in Figure 1. A multidisciplinary approach including pain clinic services, psychologist/psychiatrist and pelvic floor physical therapist along with the urologist is warranted before considering surgery. When nonsurgical treatments fail, MDSC remains a valuable approach with high success rates and should be considered for PVPS that are refractory to medical therapy. MDSC appears to have the most success for patients who experience a temporary relief from a cord block, and can significantly improve the patient's quality of life and ability to return to daily activities.
AUTHOR CONTRIBUTIONS
WPT drafted the manuscript and LAL reviewed and edited the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declare that they have no competing interests.
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Barone MA, Hutchinson PL, Johnson CH, Hsia J, Wheeler J. Vasectomy in the United States, 2002. J Urol. 2006;176:232–6. doi: 10.1016/S0022-5347(06)00507-6. [DOI] [PubMed] [Google Scholar]
- 2.Schwingl PJ, Guess HA. Safety and effectiveness of vasectomy. Fertil Steril. 2000;73:923–36. doi: 10.1016/s0015-0282(00)00482-9. [DOI] [PubMed] [Google Scholar]
- 3.Jamieson DJ, Costello C, Trussell J, Hillis SD, Marchbanks PA, et al. US collaborative review of sterilization working group. The risk of pregnancy after vasectomy. Obstet Gynecol. 2004;103:848–50. doi: 10.1097/01.AOG.0000123246.11511.e4. [DOI] [PubMed] [Google Scholar]
- 4.Sharlip ID, Belker AM, Honig S, Labrecque M, Marmar JL, et al. American urological association. Vasectomy: AUA guideline. J Urol. 2012;188:2482–91. doi: 10.1016/j.juro.2012.09.080. [DOI] [PubMed] [Google Scholar]
- 5.Christiansen CG, Sandlow JI. Testicular pain following vasectomy: a review of postvasectomy pain syndrome. J Androl. 2003;24:293–8. doi: 10.1002/j.1939-4640.2003.tb02675.x. [DOI] [PubMed] [Google Scholar]
- 6.Valencic M, Granitsiotis P, Kirk D. Chronic testicular pain: an overview. Eur Urol. 2004;45:430–6. doi: 10.1016/j.eururo.2003.11.004. Eur Urol 2005; 47: 720. [DOI] [PubMed] [Google Scholar]
- 7.McMahon A, Buckley J, Taylor A, Lloyd S, Deane R, et al. Chronic testicular pain following vasectomy. Br J Urol. 1992;69:188–91. doi: 10.1111/j.1464-410x.1992.tb15494.x. [DOI] [PubMed] [Google Scholar]
- 8.Manikandan R, Srirangam SJ, Pearson E, Collins GN. Early and late morbidity after vasectomy: a comparison of chronic scrotal pain at 1 and 10 years. BJU Int. 2004;93:571–4. doi: 10.1111/j.1464-410x.2003.04663.x. [DOI] [PubMed] [Google Scholar]
- 9.Leslie TA, Illing RO, Cranston DW, Guillebaud J. The incidence of chronic scrotal pain after vasectomy: a prospective audit. BJU Int. 2007;100:1330–3. doi: 10.1111/j.1464-410X.2007.07128.x. [DOI] [PubMed] [Google Scholar]
- 10.Sandlow JI, Winfield HN, Goldstein M. Surgery of the scrotum and seminal vesicles. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, editors. Campbell-Walsh Urology. 9th ed. Philadelphia: WB Saunders; 2006. pp. 1103–9. [Google Scholar]
- 11.Scherger JE, Ellis AR. Post-Vasectomy Pain Syndrome: Common But Hidden. 2012. [Last accessed on 2015 Oct 25]. pp. 1–8. Available from: http://www.vasectomy-information.com/moreinfo/pvps.htm .
- 12.Tandon S, Sabanegh E. Chronic pain after vasectomy: a diagnostic and treatment dilemma. BJU Int. 2008;102:166–9. doi: 10.1111/j.1464-410X.2008.07602.x. [DOI] [PubMed] [Google Scholar]
- 13.Sabanegh ES, Jr, Thomas A., Jr . Microsurgery in male infertility. In: Lipshultz L, Howards S, Niederberger C, editors. Infertility in the Male. 4th ed. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- 14.Chen TF, Ball RY. Epididymectomy for post-vasectomy pain: histological review. Br J Urol. 1991;68:407–13. doi: 10.1111/j.1464-410x.1991.tb15362.x. [DOI] [PubMed] [Google Scholar]
- 15.Linnet L, Hjort T. Sperm agglutinins in seminal plasma and serum after vasectomy: correlation between immunological and clinical findings. Clin Exp Immunol. 1977;30:413–20. [PMC free article] [PubMed] [Google Scholar]
- 16.Linnet L, Hjort T, Fogh-Anderson P. Association between failure to impregnate after vasovasostomy and sperm agglutinins in semen. Lancet. 1981;1:117–9. doi: 10.1016/s0140-6736(81)90708-x. [DOI] [PubMed] [Google Scholar]
- 17.Alexander NJ. Vasectomy: long-term effects in the rhesus monkey. J Reprod Fertil. 1972;31:399–406. doi: 10.1530/jrf.0.0310399. [DOI] [PubMed] [Google Scholar]
- 18.Nangia AK, Myles JL, Thomas AJ., Jr Vasectomy reversal for the post-vasectomy pain syndrome: a clinical and histological evaluation. J Urol. 2000;164:1939–42. [PubMed] [Google Scholar]
- 19.Levine L. Chronic orchialgia: evaluation and discussion of treatment options. Ther Adv Urol. 2010;2:209–14. doi: 10.1177/1756287210390409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis BE, Noble MJ, Weigel JW, Foret JD, Mebust WK. Analysis and management of chronic testicular pain. J Urol. 1990;143:936–9. doi: 10.1016/s0022-5347(17)40143-1. [DOI] [PubMed] [Google Scholar]
- 21.Sinclair AM, Miller B, Lee LK. Chronic orchialgia: consider gabapentin or nortriptyline before considering surgery. Int J Urol. 2007;14:622–5. doi: 10.1111/j.1442-2042.2007.01745.x. [DOI] [PubMed] [Google Scholar]
- 22.Dworkin RH, O’Connor AB, Audette J, Baron R, Gourlay GK, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85:S3–14. doi: 10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326:1250–6. doi: 10.1056/NEJM199205073261904. [DOI] [PubMed] [Google Scholar]
- 24.Jackson KC, St. Onge EL. Antidepressant pharmacotherapy: considerations for the pain clinician. Pain Pract. 2003;3:135–43. doi: 10.1046/j.1533-2500.2003.03020.x. [DOI] [PubMed] [Google Scholar]
- 25.Gallagher RM. Management of neuropathic pain: translating mechanistic advances and evidence-based research into clinical practice. Clin J Pain. 2006;22:S2–8. doi: 10.1097/01.ajp.0000193827.07453.d6. [DOI] [PubMed] [Google Scholar]
- 26.Sansone RA, Sansone LA. Pain, pain, go away: antidepressants and pain management. Psychiatry (Edgmont) 2008;5:16–9. [PMC free article] [PubMed] [Google Scholar]
- 27.Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998;280:1831–6. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- 28.Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280:1837–42. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- 29.Rice AS, Maton S Postherpetic Neuralgia Study Group. Gabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled study. Pain. 2001;94:215–24. doi: 10.1016/S0304-3959(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 30.Misra S, Ward S, Coker C. Pulsed radiofrequency for chronic testicular pain – A preliminary report. Pain Med. 2009;10:673–8. doi: 10.1111/j.1526-4637.2009.00581.x. [DOI] [PubMed] [Google Scholar]
- 31.Terkawi AS, Romdhane K. Ultrasound-guided pulsed radiofrequency ablation of the genital branch of the genitofemoral nerve for treatment of intractable orchalgia. Saudi J Anaesth. 2014;8:294–8. doi: 10.4103/1658-354X.130755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine LA. Microsurgical denervation of the spermatic cord. J Sex Med. 2008;5:526–9. doi: 10.1111/j.1743-6109.2007.00762.x. [DOI] [PubMed] [Google Scholar]
- 33.Strom KH, Levine LA. Microsurgical denervation of the spermatic cord for chronic orchialgia: long-term results from a single center. J Urol. 2008;180:949–53. doi: 10.1016/j.juro.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed I, Rasheed S, White C, Shaikh NA. The incidence of post-vasectomy testicular pain and the role of nerve stripping (denervation) of the spermatic cord in its management. Br J Urol. 1997;79:269–70. doi: 10.1046/j.1464-410x.1997.32221.x. [DOI] [PubMed] [Google Scholar]
- 35.Heidenreich A, Olbert P, Engelmann UH. Management of chronic testalgia by microsurgical testicular denervation. Eur Urol. 2002;41:392–7. doi: 10.1016/s0302-2838(02)00023-4. [DOI] [PubMed] [Google Scholar]
- 36.Larsen SM, Benson JS, Levine LA. Microdenervation of the spermatic cord for chronic scrotal content pain: single institution review analyzing success rate after prior attempts at surgical correction. J Urol. 2013;189:554–8. doi: 10.1016/j.juro.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 37.Benson JS, Abern MR, Larsen S, Levine LA. Does a positive response to spermatic cord block predict response to microdenervation of the spermatic cord for chronic scrotal content pain? J Sex Med. 2013;10:876–82. doi: 10.1111/j.1743-6109.2012.02937.x. [DOI] [PubMed] [Google Scholar]
- 38.Strebel RT, Leippold T, Luginbuehl T, Muentener M, Praz V, et al. Chronic scrotal pain syndrome: management among urologists in Switzerland. Eur Urol. 2005;47:812–6. doi: 10.1016/j.eururo.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Kavoussi PK, Costabile RA. Orchialgia and the chronic pelvic pain syndrome. World J Urol. 2013;31:773–8. doi: 10.1007/s00345-013-1092-5. [DOI] [PubMed] [Google Scholar]
- 40.Granitsiotis P, Kirk D. Chronic testicular pain: an overview. Eur Urol. 2004;45:430–6. doi: 10.1016/j.eururo.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Sweeney CA, Oades GM, Fraser M, Palmer M. Does surgery have a role in management of chronic intrascrotal pain? Urology. 2008;71:1099–102. doi: 10.1016/j.urology.2008.02.036. [DOI] [PubMed] [Google Scholar]
- 42.Calleary JG, Masood J, Hill JT. Chronic epididymitis: is epididymectomy a valid surgical treatment? Int J Androl. 2009;32:468–72. doi: 10.1111/j.1365-2605.2008.00880.x. [DOI] [PubMed] [Google Scholar]
- 43.West AF, Leung HY, Powell PH. Epididymectomy is an effective treatment for scrotal pain after vasectomy. BJU Int. 2000;85:1097–9. doi: 10.1046/j.1464-410x.2000.00656.x. [DOI] [PubMed] [Google Scholar]
- 44.Lee JY, Cho KS, Lee SH, Cho HJ, Cho JM, et al. A comparison of epididymectomy with vasectomy reversal for the surgical treatment of postvasectomy pain syndrome. Int Urol Nephrol. 2014;46:531–7. doi: 10.1007/s11255-013-0517-9. [DOI] [PubMed] [Google Scholar]
- 45.Chung JH, Moon HS, Choi HY, Jeong TY, Ha US, et al. Inhibition of adhesion and fibrosis improves the outcome of epididymectomy as a treatment for chronic epididymitis: a multicenter, randomized controlled, single-blind study. J Urol. 2013;189:1730–4. doi: 10.1016/j.juro.2012.11.168. [DOI] [PubMed] [Google Scholar]
- 46.Myers SA, Mershon CE, Fuchs EF. Vasectomy reversal for treatment of the post-vasectomy pain syndrome. J Urol. 1997;157:518–20. [PubMed] [Google Scholar]
- 47.Shapiro EI, Silber SJ. Open-ended vasectomy, sperm granuloma, and postvasectomy orchialgia. Fertil Steril. 1979;32:546–50. doi: 10.1016/s0015-0282(16)44357-8. [DOI] [PubMed] [Google Scholar]
- 48.Polackwich AS, Tadros NN, Ostrowski KA, Kent J, Conlin MJ, et al. Vasectomy reversal for postvasectomy pain syndrome: a study and literature review. Urology. 2015;86:269–72. doi: 10.1016/j.urology.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 49.Lee JY, Chang JS, Lee SH, Ham WS, Cho HJ, et al. Efficacy of vasectomy reversal according to patency for the surgical treatment of postvasectomy pain syndrome. Int J Impot Res. 2012;24:202–5. doi: 10.1038/ijir.2012.17. [DOI] [PubMed] [Google Scholar]
- 50.Horovitz D, Tjong V, Domes T, Lo K, Grober ED, et al. Vasectomy reversal provides long-term pain relief for men with the post-vasectomy pain syndrome. J Urol. 2012;187:613–7. doi: 10.1016/j.juro.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 51.Devine CJ, Schellhammer PF. The use of microsurgical denervation of the spermatic cord for orchalgia. Trans Am Assoc Genitourin Surg. 1978;70:149–51. [PubMed] [Google Scholar]
- 52.Choa RG, Swami KS. Testicular denervation. A new surgical procedure for intractable testicular pain. Br J Urol. 1992;70:417–9. doi: 10.1111/j.1464-410x.1992.tb15800.x. [DOI] [PubMed] [Google Scholar]
- 53.Levine LA, Matkov TG, Lubenow TR. Microsurgical denervation of the spermatic cord: a surgical alternative in the treatment of chronic orchialgia. J Urol. 1996;155:1005–7. doi: 10.1016/s0022-5347(01)66369-9. [DOI] [PubMed] [Google Scholar]
- 54.Levine LA, Matkov TG. Microsurgical denervation of the spermatic cord as primary surgical treatment of chronic orchalgia. J Urol. 2001;165:1927–9. doi: 10.1097/00005392-200106000-00020. [DOI] [PubMed] [Google Scholar]
- 55.Oliveira RG, Camara C, Alves Jde M, Coelho RF, Lucon AM, et al. Microsurgical testicular denervation for the treatment of chronic testicular paininitial results. Clinics (Sao Paulo) 2009;64:393–6. doi: 10.1590/S1807-59322009000500004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cadeddu JA, Bishoff JT, Chan DY, Moore RG, Kavoussi LR, et al. Laparoscopic testicular denervation for chronic denervation. J Urol. 1999;162:733–6. doi: 10.1097/00005392-199909010-00028. [DOI] [PubMed] [Google Scholar]
- 57.Ducic I, Dellon AL. Testicular pain after inguinal hernia repair: an approach to resection of the genital branch of the genitofemoral nerve. J Am Coll Surg. 2004;198:181–4. doi: 10.1016/j.jamcollsurg.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto M, Katsuno S, Miyake K. Management of chronic orchialgia of unknown etiology. Int J Urol. 1995;2:47–9. doi: 10.1111/j.1442-2042.1995.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 59.Levine LA, Hoeh MP. Evaluation and management of chronic scrotal content pain. Curr Urol Rep. 2015;16:1–8. doi: 10.1007/s11934-015-0510-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


