Abstract
Vasectomy is a safe and effective method of contraception used by 42–60 million men worldwide. Approximately 3%–6% of men opt for a vasectomy reversal due to the death of a child or divorce and remarriage, change in financial situation, desire for more children within the same marriage, or to alleviate the dreaded postvasectomy pain syndrome. Unlike vasectomy, vasectomy reversal is a much more technically challenging procedure that is performed only by a minority of urologists and places a larger financial strain on the patient since it is usually not covered by insurance. Interest in this procedure has increased since the operating microscope became available in the 1970s, which consequently led to improved patency and pregnancy rates following the procedure. In this clinical update, we discuss patient evaluation, variables that may influence reversal success rates, factors to consider in choosing to perform vasovasostomy versus vasoepididymostomy, and the usefulness of vasectomy reversal to alleviate postvasectomy pain syndrome. We also review the use of robotics for vasectomy reversal and other novel techniques and instrumentation that have emerged in recent years to aid in the success of this surgery.
Keywords: epididymovasostomy, microsurgery, postvasectomy orchialgia, postvasectomy pain syndrome, vas deferens, vasectomy, vasectomy reversal, vasoepididymostomy, vasovasostomy
INTRODUCTION
Vasectomy is the contraception of choice for 6%–8% of married couples worldwide, involving 42–60 million men.1 Asia as a whole has a vasectomy prevalence of around 3%. Within Asia, Bhutan has the highest proportion, with almost 40% of couples relying on vasectomy. Due to their population, China and India together account for 20 million users.2 Changes in life circumstances such as the death of a child or divorce and remarriage lead many vasectomized patients to desire fertility again. Their options include undergoing either a vasectomy reversal (VR), or in vitro fertilization with intracytoplasmic sperm injection (IVF/ICSI). For those 3%–6% of men undergoing VR, desire for fertility and relief from postvasectomy pain syndrome (PVPS) are the top reasons.3,4
VR is accomplished by one of two techniques: vasovasostomy (VV) or vasoepididymostomy (VE). Martin described the first human vasal repair in 1902, by performing a VE that led to the birth of a full-term infant.5 Almost two decades later, Quinby and his associate O’Conor performed the first VV in 1919.5 Since then, increasing surgeon experience with these procedures has led to innovations in both instrumentation and technique, with improving outcomes. The aim is to review the literature in a clinically oriented approach and discuss some of the latest advances within the field.
INDICATIONS AND PATIENT EVALUATION
Vasectomized men who wish to conceive traditionally only had one option, VR, until the development of IVF/ICSI in 1992.6 Several factors should be considered by the couple when choosing between these two options, such as time to pregnancy, number of desired children, time commitment, cost, and maternal age. VR is typically more cost-effective and is the favored approach for patients desiring multiple pregnancies with unimpaired female partner fertility. In general, any man who has had a vasectomy is a candidate for VR.
Patient history
A thorough medical history is the cornerstone of a preoperative evaluation for patients interested in VR. Particular emphasis is placed on the patient and his partner's prevasectomy fertility. If the patient had difficulty conceiving before the vasectomy, it is highly likely that he will also struggle after a VR.7 Attention should be paid to the duration of time since the vasectomy, the female partner's age, parity, and any medical conditions that would make it difficult to conceive naturally. A history of pelvic surgery, inguinal surgery (hernia repairs), or any postvasectomy complications, including bleeding and infection should be noted, and the patient should be counseled regarding the more challenging nature of the repair. Due to the increased use of testosterone supplement therapy (TST) in the population, patients should be inquired about their use of supplemental testosterone, and their medication list should be examined. Review of the literature reveals that the majority of men on TST will have impaired spermatogenesis.8 Failure to discontinue TST before VR may decrease the likelihood of finding sperm on vasal fluid analysis, complicating intra-operative decision-making. Failure to identify sperm may guide the surgeon to perform the more difficult VE, potentially negatively impacting patency and pregnancy rates. Coward et al. recently shared their experience with performing VR on men with a history of TST and advocated preoperative medical testicular salvage therapy with clomiphene citrate or hCG to improve the accuracy of vasal fluid analysis at the time of VR, potentially avoiding this pitfall.9
Physical examination
Careful physical examination should be performed in a warm examination room to assess signs of hypogonadism, the size of the testicles, a palpable vasal defect, the presence of a sperm granuloma, and if able, the length of the testicular vasal segment. Since the seminiferous tubules make up the majority of the testicular volume, a small or soft testis suggests impaired sperm production. Noticing the presence of a varicocele is also essential since varicocelectomy alongside VR can be performed in selected cases.10,11
Blood tests
No particular set of blood tests are critical to obtain before VR, but serum FSH and testosterone levels should be obtained in any man with small testes, history of abnormal semen analysis, or impaired sexual function. We do not recommend routinely obtaining serum antisperm antibodies (ASA) since they can be detected in most men who have had a vasectomy, and their presence does not provide any prognostic value for VR.1,12
Imaging
Routine preoperative imaging is not needed before VR. If vasal obstruction at a site other than the vasectomy is suspected, vasography may be useful; however, since scarring can result from vasography, it should only be performed when a formal reconstruction is immediately possible.13 McCammack et al. recently published a pilot study investigating the utility of MRI in preoperative planning.14 Specifically, they correlated increase in epididymal T1 signal intensity (compared with the ipsilateral testicular parenchyma) above 19% with a >90% chance of performing a VE rather than a VV during VR. Although only 10 patients participated and the authors failed to mention the surgical criteria for performing a VE rather than a VV, it is an intriguing avenue of research that with further validation may be a tool to counsel patients preoperatively on the probability of needing a VE, as a surrogate for patency and pregnancy rates.
PREOPERATIVE PROGNOSTIC FACTORS
Many factors have the potential to influence the success rate of a VR. Identifying these factors and their importance in predicting VR outcomes has been an active area of research. It is important for the urologist to be aware of this data to clarify postsurgical expectations for the patient and his partner.
Surgical skill
The vas deferens (VD) has a luminal diameter of 0.3–0.5 mm, and the epididymal tubule has even a smaller diameter of 0.15–0.2 mm.15 Performing microsurgery on such a delicate tissue is certainly challenging, and as with any procedure, outcomes improve with experience. Several studies have shown a correlation between the number of procedures performed annually by the surgeon and the success rate of VR. One study found that VV performed by surgeons with >15 operations annually resulted in higher patency rates than those performing <6 operations per year (87% vs 56%, respectively).16 Crain et al. surveyed fellowship-trained, academic, and community urologists performing VRs, and found they all had nonsignificant differences in patency rates (79%, 69%, and 71%, respectively). However, all surgeons performed an average of 10 operations yearly.17 Even training in a laboratory setting has an impact on patency rates. Nagler and Jung found that surgeons who practiced their microsurgical skills in a laboratory before the actual VV had higher patency rates than those performing microsurgical VV without recent practice (89% vs 53%, respectively).18
Most surgeons consider VE to be more technically challenging than VV due to the added difficulty of isolating a smaller segment of the epididymis and working with the disparity in the size of an epididymal tubule and the VD. As a result, some surgeons only offer VV to their patients, regardless of intra-operative findings. Chawla and colleagues examined 22 cases of repeat VR after failed VV.19 On exploration, they found that 48% of the men had epididymal obstruction as the etiology for their initial failure, indicating that these patients would have benefitted from a VE rather than the VV. Their findings highlight the need for surgeons offering VR to have adequate skills in performing both VV as well as VE.
Fenig et al. have provided a nomogram for clinicians to predict preoperatively the need for VE during VR.20
Obstructive interval (OI)
Silber studied the impact of increasing OI (time from vasectomy to VR) on the success of VR and reported that there was a precipitous decrease in success 10 years after vasectomy.21 Dohle and Smit also reported a higher patency rate with interval <5 years as compared to that >10 years (89% vs 75%).22 In contrast, the Vasovasostomy Study Group (VVSG) discovered a gradual downward trend in patency rates rather than a steep decline.23
Unlike the previous studies, Boorjian and colleagues demonstrated no change in patency rates (88%–91%), even >15 years after vasectomy.24 The pregnancy rates, on the other hand, declined quickly 15 years after the vasectomy, from 82%–89% to 44%; however, before 15 years they stayed the same, even when a more complicated VE was performed. Similarly, Magheli et al. found no difference in patency rates in men with OI between 11 and 15 years, and >15 years (95.3% and 97.1%, respectively).25 Increasing OI is associated with an increased incidence of epididymal obstruction and the consequent need for VE. In the previous study by Magheli and colleagues, 52% of their patients with an OI >15 years required VE on at least 1 side.25 Fuchs and Burt similarly found 62% of patients with an OI >15 years needed a VE.26
As the trends indicate, more contemporary studies show that patency can be achieved irrespective of the OI, but VR does become more technically challenging as the OI increases due to the higher likelihood of needing a VE.
Partner characteristics
Partner characteristics are just as important as the patient factors for the success of VR, and likely contribute to the discordance between patency and pregnancy rates. Some of the important factors are discussed below.
Previous partner fertility
A history of proven fertility is expected in a patient with a history of vasectomy, and by itself does not provide much prognostic value when undergoing VR. Prior fertility in the female partner does, however, provide useful information. This was studied by the VVSG, and results showed a difference in pregnancy rates after VR for patients whose current partner was previously pregnant (57%) and those whose current spouse had not been previously pregnant (49%).23
Same partner
Moreover, if the patient has had a conception with the current partner in the past, the outcomes are better than if he is trying to conceive with a new partner. In the VVSG report, the indication for VR in 21 men was death of a child (same partner) and their pregnancy rate was 76%. When the indication was divorce (new partner), the pregnancy rate in the 612 men was only 50%.23 The results of this study were validated more than a decade later by Kolettis et al. who analyzed 34 men undergoing VR with same partner, reporting a patency rate of 93% and a pregnancy rate of 60%.27 Similarly, Chan and Goldstein found a patency rate of 100% and a pregnancy rate of 86% in a subgroup of 27 men undergoing VR with same partners.28 No scientific explanation has been found, but possible reasons for higher success rates in same partners include proven fecundity as a couple, shorter OI, and stronger emotional dedication.
Partner age
Perhaps the most important factor in transitioning from patency to pregnancy is the age of the female partner. A retrospective analysis of 212 patients undergoing VR showed female age >40 years to be an independent predictor of a lower pregnancy rate with similar patency rates (pregnancy rate was 73% if partner <40-year-old, but only 42% if >40-year-old).29 However, these pregnancy rates still compared favorably to IVF/ICSI.29 Table 1 shows patency and pregnancy rates found by Gerrard et al.30 The patency rates were similar ranging from 83% to 90%, but the pregnancy rate for patients with female partners >40-year-old was significantly lower (14%) than for those with female partners <40-year-old (56%). The evidence thus shows a swift drop in pregnancy rates when the female partner is >40-year-old.
Table 1.
Patency and pregnancy rates related to female partner age
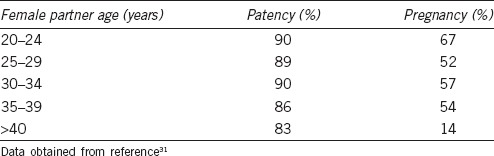
Unlike the lifelong production of spermatozoa in males, women are born with approximately 2 million oocytes, of which only 500 are ovulated, and 0 remain at menopause.31 Decrease in fertility with advancing age is likely related to decreasing number of available oocytes (and chromosomal abnormalities in those remaining). This “ovarian reserve” can inferred by measuring FSH and estradiol levels on day 3 of the ovulatory cycle, and by counting the number of preantral follicles (2–8 mm in diameter) using a transvaginal ultrasound. The favorable reserve is indicated by FSH <10 IU ml−1, estradiol <50 pg ml−1, and at least 10 follicles bilaterally.31 As the couple are considering partner age and ovarian reserve, they should also be counseled that it takes an average of 12 months for conception to occur after VR, which may influence their decision to proceed with IVF/ICSI or VR.23
Prior vasectomy reversal
Many authors have commented on the favorable outcomes of a repeat VR after failed initial reversal. Early studies showed a patency rate of 67%–85% and pregnancy rates of 25%–44% with repeat VR.32,33 Hollingsworth et al. analyzed 49 men undergoing repeat VR. Average OI was 10.5 years for the original VR, and 2.7 years for repeat VR, with a 41% pregnancy rate. Thirty-four percent of patients required, at least, a unilateral VE if they had a VV as their first procedure. OI, reconstruction type, anastomotic site, patient age, and postoperative semen parameters have been shown not to influence repeat VR outcomes.34 Hernandez and Sabanegh reviewed 41 men undergoing repeat VR and found up to 73% of patients required at least a unilateral VE compared to 4% in the initial VR.35 The only significant predictor for pregnancy was a history of a child with the same partner. In another study, analysis of repeat VE in 18 men revealed patency rates varied according to the level of anastomosis: 66.7% in the caput, 62.5% in the corpus, and 100% in the cauda.36 The collective experience with VR shows that patients with a history of VR failure are still great candidates for repeat reconstructive procedures.
Antisperm antibodies
In the late 1980s and early 1990s, many authors were interested in investigating the presence of ASA in postvasectomy patients, suspecting that their presence may have an adverse effect on fertility after VR.37,38,39 Studies have largely shown that ASA form in the majority of patients after vasectomy, yet majority of men also conceive after VR.38 Carbone et al. reviewed 14 patients with partial obstruction (epididymal fullness) with positive ASA who had previously undergone VR.40 After repeat VR without treatment of the ASA, the pregnancy rate was 50%, suggesting that ASA likely did not contribute to the lack of fertility, but rather the problem was technical. Since the majority of men conceive after patency is established, the presence or absence of ASA neither provides prognostic value nor changes management. Most authors thus recommend against routinely obtaining ASA before VR.
INTRA-OPERATIVE DECISION-MAKING AND TECHNIQUE
VRs under both general and local anesthesia have been described. If performing a VE is anticipated, general anesthesia is recommended due to the longer duration of the procedure and the need for prolonged patient immobility to aide in precise reconstruction.
Traditionally, bilateral high vertical incisions or a small midline raphe incision have been described. In 1999, Costabile et al. described a minimally invasive technique.41 In 2008, Jarvi and colleagues revisited the technique by applying the no-scalpel vasectomy principles to VR, calling it a mini-incision VR.42,43 The authors compared the mini-incision technique with the traditional technique and found patency rates to be similar (96% vs 91%, respectively). Patients undergoing the mini-incision had significantly less pain 2 days following the procedure, and they returned to work 2 days earlier.42,43 It is important to note that the traditional incision is still indicated if the location of the vasectomy occlusion site is uncertain, the vasal gap is wide, large sperm granuloma is present, or if the patient requires a VE or a redo VR.
Once the vasal defect is exposed, the vas on either side of the defect is stabilized using a slotted nerve holder, and the defect is excised using smooth perpendicular cuts. The abdominal end is then examined for patency by cannulating and irrigating the lumen with a 24 Fr angiocath filled with saline; or alternatively, 0 prolene suture can be passed via the lumen and the area of obstruction can be identified.
The decision to perform a VV or a VE depends on the microscopic and macroscopic characteristics of fluid expressed from the testicular end of the cut vasal stump. Macroscopic examination includes fluid opacity and viscosity, and microscopic examination looks for the quantity and quality of the sperm, including motility, sperm parts, and any deformity.
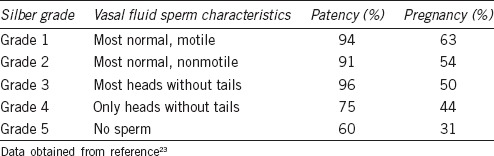
Many studies have correlated patency and pregnancy outcomes with intra-operative vasal fluid characteristics and the subsequent decision to perform a VV or a more challenging VE. Widely accepted scenarios include performing a VV if clear fluid with whole sperm is found, and performing a VE if pasty fluid without any sperm is found. In the VVSG, of patients with motile intravasal sperm, 94% were patent, compared with 60% of those with no sperm in the vasal fluid. The pregnancy rates for these two groups were 63% and 31%, respectively. To aid in intra-operative decision-making, some authors have relied on the Silber scale; Table 2 provides a description of the scale and its correlation with clinical outcomes. With clear fluid present, most authors proceed with VV for Grades 1, 2 and 3. Since the VVSG report found lower patency and pregnancy rates with Grade 4 sperm were present, historically surgeons have performed VE with less than whole sperm, especially paired with nonclear intravasal fluid.
Table 2.
Correlating patency and pregnancy rates with Silber grading
To investigate the matter further, Smith et al. reported results from performing a VV in 14 men with finding of only sperm heads and/or short tails, and majority with poor intravasal fluid quality (creamy or pasty).44 Surprisingly, VV was successful in 90.9% of patients, surpassing the expected patency rate for a VE. They encouraged surgeons to perform VV as long as any sperm fragments are found, regardless of fluid quality. Scovell et al. recently conducted a meta-analysis of studies investigating the presence of sperm in the vasal fluid and postoperative patency, identifying 1293 patients from four case series and two retrospective cohort series.45 Their analysis showed that the odds ratio of patency following VR was 4 times higher if either whole sperm or sperm parts were present in the vasal fluid. These two studies provide encouragement to surgeons that in the absence of azoospermia, performing a VV instead of VE regardless of fluid quality can provide patency.
Since the decision to perform a VV or VE relies on the microscopic examination of the vasal fluid in addition to its gross appearance, the availability of a microscope is critical for a VR. Once the decision has been made to perform VV or VE, detailed descriptions of these anastomotic techniques have been published elsewhere.5,13,46,47 A brief review is discussed here.
Vasovasostomy
Contemporary anastomotic options for VV include a modified one-layer or a multi-layer technique. In the former, a full-thickness suture (usually a 9-0 Nylon) is placed through all layers of the vas to bring the two ends together, followed by an additional layer of interrupted seromuscular sutures in between the full-thickness sutures. During a multi-layer technique, an inner layer of sutures is placed to only approximate the mucosa, followed by an outer layer(s) of sutures closing the seromuscular layers. The VVSG compared the outcomes of these two techniques, and no differences were found.23 Goldstein described a modification to the multi-layer technique in 1998 that allows for temporally separating planning of the sutures from the actual placement.48 Using a microtip marking pen, six “microdots” are placed around each of the lumens of the vas. The dots serve as needle exit points in the innermost mucosal to mucosal apposition with a 10-0 Nylon, followed by an approximation of the deep muscularis layer with a 9-0 Nylon and lastly the adventitia with another 9-0 layer. Goldstein used this technique in 194 consecutive patients and reported an impressive 99.5% patency rate.
Vasoepididymostomy
Due to the discrepancy in size of the epididymal tubule and VD, performing VE is more challenging than VV. The first step involves delivering the testis into the field and locating the area of obstruction, which typically lies in the cauda epididymis. Once the area is identified, a dilated tubule proximal to the obstruction is identified.
Martin initially described the side-to-side vasoepididymal fistula method in 1902, which had poor outcomes, but still prevailed until Silber reported the new microsurgical end-to-end technique in 1978.49 Thomas then popularized the end-to-side method in 1987, and most recently, Drs. Chan, Li, and Goldstein developed the longitudinal intussusception vasoepididymostomy (LIVE) in 2001.5,49 Similar to performing a VV, the optimal technique is the one the surgeon feels the most comfortable performing. However, several fundamental tenets must be kept in mind for any anastomosis, no matter the technique: the anastomosis must be tension-free, waterproof, must have accurate mucosal-to-mucosal apposition, adequate blood supply, and be carried out in an atraumatic fashion. If the anastomosis is not watertight, sperm extravasation can lead to granuloma formation, which may distract the anastomosis and lead to secondary azoospermia through the stenosis.
Difficult situations
Removal or cauterization of a large segment of the vas during vasectomy necessitates extended mobilization of the vasal stumps to perform the VR. The testicular end of the vas is usually more easily manipulated than the abdominal end. In severe cases, the testicle can be mobilized, rotated, and/or pexied higher in the scrotum to allow for a tension-free anastomosis. If additional length is needed, some authors have described transecting the vas from the internal inguinal ring, straightening it by mobilizing it medially, and pulling it out of the pelvis through the external ring to meet the testicular end. This can be accomplished by making a Gibson-type or Pfannenstiel incisions, or through a laparoscopic approach.50,51,52
Another challenging scenario arises when lack of patency is discovered by the inability to flush the abdominal end of the cut vas. In this case, the location of the obstruction can be estimated by passing a 2-0 prolene suture through the vas and measuring how much suture is able to be advanced. Alternatively, contrast media can be injected through the vas if intra-operative fluoroscopy is available. The location of obstruction is typically in the inguinal canal (many of these patients have a history of ipsilateral hernia repair). The aforementioned technique of mobilizing the testicular end of the vas or re-routing the abdominal end can be used in this scenario as well. It is important to note that two simultaneous VVs should not be performed on the same vas due to potential disruption of the vasal vessels at both locations. Alternatively, if whole sperm and/or parts are visualized on the obstructed side, and the contralateral side is azoospermic, a VV can be performed by rerouting the testicular end of the problematic vas through the dartos to the contralateral side and anastomosing is to the contralateral nonobstructed abdominal vas (to avoid performing a more technically challenging VE on this side).
OUTCOMES
In the past, most VVs were performed by a macroscopic technique with the use of an indwelling stent.53 The introduction of the operating microscope in 1975 greatly advanced the field of infertility by allowing greater patency and fertility rates and continues to be the standard of care in performing VR.54 There are still centers where the macroscopic technique with the use of loupes is still utilized, especially when a microscope is unavailable. Jee and Hong published results comparing microsurgical versus macroscopic loupe-assisted VV in 50 patients (25 in each group), with the one-layered approach.55 The loupe-assisted group had a 72% patency and a 28% pregnancy rates, whereas the microsurgical group had a 96% patency and a 40% pregnancy rates. The higher patency and pregnancy rates of the microsurgical group were attributed to a lower postprocedural stricture rate.
Several authors have a look specifically at VE outcomes. Patency rates for the end-to-end and end-to-side techniques of VE range from 31% to 85%,56,57 with mean pregnancy rate of 35%.58,59 Using the newer LIVE technique, patency rates have been reported to be more favorable, ranging from 80% to 92%, with limited reporting of the pregnancy data.
Until recently, the most comprehensive study on VR was the VVSG report, published in 1991. The major findings included decreased patency with increasing OI, and no difference in patency between one-layer and two-layer anastomoses.74 Since then, changes have occurred in the anastomotic techniques and instrumentation, which have affected the patency and pregnancy rates. Herrel et al. conducted a meta-analysis of 31 studies from 1980 to 2014 encompassing 6633 patients.60 Key findings include a patency rate of 89% (range 69%–98%) and a pregnancy rate of 73% (range 37%–93%) across all studies (though authors mention the difficulty in interpreting these numbers due to varied definitions across studies). No difference in single versus Multi-Layered anastomoses were found, and patients with OI <10 years had higher patency (95% CI: 1.09–1.25) and pregnancy (95% CI: 1.12–1.38) rates. They did, however, exclude any studies with >30% patients undergoing VE, which left out two major studies.
Hsiao et al. analyzed 548 patients undergoing VR (including both VV and VE) at a tertiary referral center with 30 years of experience. They constructed two nomograms, one preoperative and postoperative, to predict the likelihood of success, defined as a sperm concentration >0.1 × 106 ml−1, with motile sperm, no evidence of late failure (secondary azoospermia), and no need for additional procedures.61 The preoperative nomogram was constructed using the clinical predictors of duration of obstruction, presence of sperm granuloma, history of previously attempted VR, type of reconstruction performed, testicular volume and age at surgery. The postoperative nomogram added gross characteristics of vasal fluid and the presence of sperm on microscopy at the time of reconstruction. Interesting facets of this report include that average testicular volume (20–25 ml), and OI had the largest effects in the modeling while the factor with the least effect was the presence of a sperm granuloma. The preoperative nomogram may be helpful to patients in choosing between VR and IVF/ICSI whereas the postoperative nomogram may guide the decision to cryopreserve the sperm after the return of sperm to ejaculate.
RECENT ADVANCES
Robotic microsurgery
The feasibility of robotic-assisted VR in an ex vivo rat model was first studied by Schoor et al. in 2003,62 followed shortly in an ex vivo human model in 2004.63 Parekattil et al. published the first series of robotic VR cases in 2010, comparing the outcome results of 20 patients who underwent robotic VV and seven men who underwent microsurgical VV using a three-layer technique.64,65 Vasal patency was 100% in both groups and the length of operating time actually favored the robotic approach (109 min vs 128 min). The same group again reported a larger series of 155 patients with patency rates of 96% in the robotic group and 80% in the microsurgical group.70 Kavoussi recently published his series of 27 microsurgical and 25 robotic-assisted VR cases, again finding that there was no difference in overall patency rates (89% vs 92%), 6-week mean sperm concentrations (28 × 106 ml−1 vs 26 × 106 ml−1), or mean operative time (141 min vs 150 min).66
These studies highlight some advantages of robotic surgery over microscopic surgery, including decreased operative time, increased patency rates (limited data), and decreased learning curve compared to traditional microsurgery. Other theoretical advantages include the increased ease of placing the needle and counteracting surgeon tremor. Disadvantages of the robotic approach include higher cost, the necessity of a specialized surgical team, low availability of microsurgical instruments, and the inferior magnification (×10–15) compared to a microscope (×20–30). The increased magnification is particularly valuable when performing the more challenging VE, and may be a reason why the current literature only describes VV. Unlike robotic surgery, surgeons have had decades of collective experience with microsurgery, and high level of skill and efficiency has been reached. As robotic surgery also matures, it remains to be seen whether surgical quality and efficacy can be improved.
Novel instrumentation
Crosnoe et al. described a unique method of excising the vasectomy defect; instead of cutting the testicular and abdominal vasal ends perpendicularly, they used vas-cutting forceps angled at 15°.67 In contrast with the straight-cut technique, angled cutting has the advantage of an increase in vasal surface area for re-anastomosis, which in turn may lead to increased neovascularity and decreased fibrosis. Similar patency rates were observed, but the average sperm concentration was 11 × 106 ml−1 higher in the angled-cut group. Although an innovative early study, a 15° angle of the cut only results in a 3.5% increased surface area by the authors’ own calculations. Angled cutting may also make it more difficult for the surgeon to align properly the two cut edges in a waterproof fashion. Although the use of a vas cutter is only one of the many variables influencing successful outcomes after a VV, the authors demonstrate an intriguing concept that remains to be tested by other centers.
Moon described another innovation, a double-ringed instrument designed to facilitate handling and dissecting the vas away from peri-vasal tissue in an atraumatic fashion.68 The Moon's clamp has two rings, separated by two ridges that allow transfer of tissue from one ring to another. The advantage of this clamp lies in the ability of the surgeon to gently dissect the peri-vasal tissue (without transection) and to secure it temporarily away from the vas. Preservation of the tissue may prevent interruption of peri-anastomotic blood supply that may cause secondary azoospermia in the form of a stricture. Moon reported favorable outcomes with this technique in 263 patients, with a 97% patency rate. However, since the primary benefit of this instrument seems to be in preventing long-term strictures, a longer follow-up is needed to assess its utility.
Adhesives
Since the first report by Silverstein and Mellinger in 1991 of using a fibrin sealant to supplant the sutured VV in a rat model, various adhesives have been evaluated in rats with generally favorable patency rates.69 Most early studies used one or two sutures to secure the anastomosis, followed by application of the glue. Vankemmel and colleagues performed VV with the conventional one-layer closure versus three transmural sutures followed by fibrin sealant in rats, and found shortened operative time with the use of the glue and comparable patency rates (85% with conventional, and 92% with glue).70 For the more complicated VE, Shekarriz compared the traditional VE technique versus anastomosis with only two sutures augmented by fibrin glue, and found similar patency rates but significantly decreased time needed to complete the anastomosis (15 min vs 33 min) in the fibrin glue cohort.71 Hakky et al. performed a trial of surgical glue in humans in 2014.72 Four patients underwent microsurgical VV with four sutures placed at the 12, 3, 6, and 9 o’clock positions, followed by application of a cyanoacrylate surgical sealant. One patient was lost to follow-up, but the remaining three patients had sperm present on semen analysis at 3 months. They noted a significantly decreased operative time of 63 min, as compared to the average time of 155 min for one-layered and 320 min for a two-layered anastomosis at their institution. We await further studies exploring this technology in patients, as this reduced dependence on sutures would decrease the time and complexity of a VR.
POSTVASECTOMY PAIN SYNDROME
Some level of long-term scrotal pain or discomfort seems to be surprisingly common after a vasectomy. In 1992, McMahon and colleagues found 33% of men had chronic testicular pain 4 years after the vasectomy, with 15% rating it as troublesome but only 1.7% proceeding with surgical therapy.73 A prospective study in 2007 used the no-scalpel technique for the majority of patients and found 15% of men had an average 3.4/10 pain 7 months after vasectomy, and 0.9% of men reported pain “quite severe and noticeably affecting their quality of life.”74 PVPS refers to such new-onset pain or dull ache in the epididymis or testis, discomfort with sexual intercourse or after vigorous activity, or pain during or after ejaculation that lasts longer than 3 months. Most patients respond to conservative treatment, and the pain rarely lasts longer than 1 year. But for those with persistent pain, VR may be able to treat two proposed mechanisms for PVPS: by relieving obstruction and removing sperm from exposure to the immune system, thus decreasing peri-vasal inflammation.
Several authors have described their experience with VR as a means of relieving PVPS. The numbers of patients in these studies range from 4 to 32, with 66%–100% pain-free rate.75 Lee et al. explored the efficacy of VR according to patency rate in conjunction with pain-free rates in 32 patients and observed a significant difference between the patency and no patency groups in terms of pain reduction and the degree of patient procedural satisfaction.76 Another retrospective study from the Canadian health system found overall high success rates with lasting resolution of the PVPS during follow-up of 40.5 months. 11/14 patients had improvement in pain after VR (2 out of the 3 patients who did not improve were noted to be azoospermic on semen analysis). Interestingly, although 14 of 14 patients stated that they would not have a vasectomy again, 13 of 14 reported that they would have VR again due to its success in diminishing or complete relieving the pain.77 Despite the success of VR in treating PVPS, insurance may not cover VV for PVPS; as such the cost of the procedure can be a barrier for some patients. These studies highlight an important tool in helping patients overcome PVPS when conservative therapies fail.
CONCLUSIONS
VR has become a main-stay of treatment for patients desiring fertility after vasectomy, and for those with refractory PVPS. Major advances have been made in the technique and instrumentation since the procedure was pioneered in the early 20th century. Numerous patient and partner factors have been reviewed to optimize the outcomes of this technically challenging procedure, culminating in the development of nomograms to provide the best preoperative counseling to patients. Careful patient and partner evaluation, intra-operative analysis of vasal fluid, and utilization of the operative microscope has shown to yield excellent technical and satisfactory reproductive outcomes. Although a high level of excellence has been reached today, pioneers continue to innovate and strive to achieve better outcomes for our patients.
COMPETING INTERESTS
None of the authors declare competing financial interests.
REFERENCES
- 1.Pile JM, Barone MA. Demographics of vasectomy – USA and international. Urol Clin North Am. 2009;36:295–305. doi: 10.1016/j.ucl.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberg ML, Lipshultz LI. Estimating the number of vasectomies performed annually in the United States: data from the national survey of family growth. J Urol. 2010;184:2068–72. doi: 10.1016/j.juro.2010.06.117. [DOI] [PubMed] [Google Scholar]
- 3.Potts JM, Pasqualotto FF, Nelson D, Thomas AJ, Jr, Agarwal A. Patient characteristics associated with vasectomy reversal. J Urol. 1999;161:1835–9. [PubMed] [Google Scholar]
- 4.Engelmann UH, Schramek P, Tomamichel G, Deindl F, Senge T. Vasectomy reversal in central Europe: results of questionnaire of urologists in Austria, Germany and Switzerland. J Urol. 1990;143:64–7. doi: 10.1016/s0022-5347(17)39867-1. [DOI] [PubMed] [Google Scholar]
- 5.Dickey RM, Pastuszak AW, Hakky TS, Chandrashekar A, Ramasamy R, et al. The evolution of vasectomy reversal. Curr Urol Rep. 2015;16:40. doi: 10.1007/s11934-015-0511-0. [DOI] [PubMed] [Google Scholar]
- 6.Palermo GD, Neri QV, Takeuchi T, Rosenwaks Z. ICSI: where we have been and where we are going. Semin Reprod Med. 2009;27:191–201. doi: 10.1055/s-0029-1202309. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein M. Vasectomy reversal. Compr Ther. 1993;19:37–41. [PubMed] [Google Scholar]
- 8.Moss JL, Crosnoe LE, Kim ED. Effect of rejuvenation hormones on spermatogenesis. Fertil Steril. 2013;99:1814–20. doi: 10.1016/j.fertnstert.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Coward RM, Mata DA, Smith RP, Kovac JR, Lipshultz LI. Vasectomy reversal outcomes in men previously on testosterone supplementation therapy. Urology. 2014;84:1335–40. doi: 10.1016/j.urology.2014.06.081. [DOI] [PubMed] [Google Scholar]
- 10.Loughlin KR. Microsurgical vasectomy reversal and varicocele ligation. Urology. 1999;53:239–40. [PubMed] [Google Scholar]
- 11.Ninz S. Effect of obesity on sex hormone levels, antisperm antibodies, and fertility after vasectomy reversal. Urology. 2010;76:851–6. doi: 10.1016/j.urology.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 12.Carbone DR., Jr Partial obstruction, not antisperm antibodies, causing infertility after vasovasostomy. J Urol. 1998;159:827–30. [PubMed] [Google Scholar]
- 13.Schwarzer JU, Steinfatt H. Current status of vasectomy reversal. Nat Rev Urol. 2013;10:195–205. doi: 10.1038/nrurol.2013.14. [DOI] [PubMed] [Google Scholar]
- 14.McCammack KC, Aganovic L, Hsieh TC, Guo Y, Welch CS, et al. MRI of the epididymis: can the outcome of vasectomy reversal be predicted preoperatively? Am J Roentgenol. 2014;203:91–8. doi: 10.2214/AJR.13.11619. [DOI] [PubMed] [Google Scholar]
- 15.Esteves SC, Hamada A, Agarwal A. Surgical management of male infertility. In: Dubey AK, editor. Infertility Diagnosis, Management and IVF. New Delhi: JP Medical Publishers; 2012. p. 98. [Google Scholar]
- 16.Wood S, Montazeri N, Sajjad Y, Troup S, Kingsland CR, et al. Current practice in the management of vasectomy reversal and unobstructive azoospermia in Merseyside and North Wales: a questionnaire-based survey. BJU Int. 2003;91:839–44. doi: 10.1046/j.1464-410x.2003.04227.x. [DOI] [PubMed] [Google Scholar]
- 17.Crain DS, Roberts JL, Amling CL. Practice patterns in vasectomy reversal surgery: results of a questionnaire study among practicing urologists. J Urol. 2004;171:311–5. doi: 10.1097/01.ju.0000100801.40282.b0. [DOI] [PubMed] [Google Scholar]
- 18.Nagler HM, Jung H. Factors predicting successful microsurgical vasectomy reversal. Urol Clin North Am. 2009;36:383–90. doi: 10.1016/j.ucl.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Chawla A, O’Brien J, Lisi M, Zini A, Jarvi K. Should all urologists performing vasectomy reversals be able to perform vasoepididymostomies if required? J Urol. 2004;172:1048–50. doi: 10.1097/01.ju.0000135118.43383.b1. [DOI] [PubMed] [Google Scholar]
- 20.Fenig DM, Kattan MW, Mills JN, Gisbert M, Yu C, et al. Nomogram to preoperatively predict the probability of requiring epididymovasostomy during vasectomy reversal. J Urol. 2012;187:215–8. doi: 10.1016/j.juro.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 21.Silber SJ. Pregnancy after vasovasostomy for vasectomy reversal: a study of factors affecting long-term return of fertility in 282 patients followed for 10 years. Hum Reprod. 1989;4:318–22. doi: 10.1093/oxfordjournals.humrep.a136896. [DOI] [PubMed] [Google Scholar]
- 22.Dohle GR, Smit M. Microsurgical vasovasostomy at the Erasmus MC, 1998-2002: results and predictive factors. Ned Tijdschr Geneeskd. 2005;149:2743–7. [PubMed] [Google Scholar]
- 23.Belker AM, Thomas AJ, Jr, Fuchs EF, Konnak JW, Sharlip ID. Results of 1,469 microsurgical vasectomy reversals by the Vasovasostomy study group. J Urol. 1991;145:505–11. doi: 10.1016/s0022-5347(17)38381-7. [DOI] [PubMed] [Google Scholar]
- 24.Boorjian S, Lipkin M, Goldstein M. The impact of obstructive interval and sperm granuloma on outcome of vasectomy reversal. J Urol. 2004;171:304–6. doi: 10.1097/01.ju.0000098652.35575.85. [DOI] [PubMed] [Google Scholar]
- 25.Magheli A, Rais-Bahrami S, Kempkensteflfen C, Weiske WH, Miller K, et al. Impact of obstructive interval and sperm granuloma on patency and pregnancy after vasectomy reversal. Int J Androl. 2010;33:730–5. doi: 10.1111/j.1365-2605.2009.01007.x. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs EF, Burt RA. Vasectomy reversal performed 15 years or more after vasectomy: correlation of pregnancy outcome with partner age and with pregnancy results of in vitro fertilization with intracytoplasmic sperm injection. Fertil Steril. 2002;77:516–9. doi: 10.1016/s0015-0282(01)03219-8. [DOI] [PubMed] [Google Scholar]
- 27.Kolettis PN, Woo L, Sandlow JI. Outcomes of vasectomy reversal performed for men with the same female partners. Urology. 2003;61:1221–3. doi: 10.1016/s0090-4295(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 28.Chan PT, Goldstein M. Superior outcomes of microsurgical vasectomy reversal in men with the same female partners. Fertil Steril. 2004;81:1371–4. doi: 10.1016/j.fertnstert.2003.09.066. [DOI] [PubMed] [Google Scholar]
- 29.Hinz S, Rais-Bahrami S, Kempkensteffen C, Weiske WH, Schrader M, et al. Fertility rates following vasectomy reversal: importance of age of the female partner. Urol Int. 2008;81:416–20. doi: 10.1159/000167839. [DOI] [PubMed] [Google Scholar]
- 30.Gerrard ER, Jr, Sandlow JI, Oster RA, Burns JR, Box LC, et al. Effect of female partner age on pregnancy rates after vasectomy reversal. Fertil Steril. 2007;87:1340–4. doi: 10.1016/j.fertnstert.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 31.Rinaudo P, Strauss JF., III Endocrine function of the postmenopausal ovary. Endocrinol Metab Clin North Am. 2004;33:661. doi: 10.1016/j.ecl.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Donovan JF, Jr, DiBaise M, Sparks AE, Kessler J, Sandlow JI. Comparison of microscopic epididymal sperm aspiration and intracytoplasmic sperm injection/in-vitro fertilization with repeat microscopic reconstruction following vasectomy: is second attempt vas reversal worth the effort? Hum Reprod. 1998;13:387. doi: 10.1093/humrep/13.2.387. [DOI] [PubMed] [Google Scholar]
- 33.Matthews GJ, McGee KE, Goldstein M. Microsurgical reconstruction following failed vasectomy reversal. J Urol. 1997;157:844. [PubMed] [Google Scholar]
- 34.Hollingsworth MR, Sandlow JI, Schrepferman CG, Brannigan RE, Kolettis PN. Repeat vasectomy reversal yields high success rates. Fertil Steril. 2007;88:217–9. doi: 10.1016/j.fertnstert.2006.11.077. [DOI] [PubMed] [Google Scholar]
- 35.Hernandez J, Sabanegh ES. Repeat vasectomy reversal after initial failure: overall results and predictors for success. J Urol. 1999;161:1153. doi: 10.1016/s0022-5347(01)61616-1. [DOI] [PubMed] [Google Scholar]
- 36.Pasqualotto FF, Agarwal A, Srivastava M, Nelson DR, Thomas AJ. Fertility outcomes after repeat vasoepididymostomy. J Urol. 1999;162:1626. [PubMed] [Google Scholar]
- 37.Linnet L, Hjort T, Fogh-Andersen P. Association between failure to impregnate after vasovasostomy and sperm agglutinins in semen. Lancet. 1981;1:117–9. doi: 10.1016/s0140-6736(81)90708-x. [DOI] [PubMed] [Google Scholar]
- 38.Haas GG., Jr Antibody mediated causes of male infertility. Urol Clin North Am. 1987;14:539–50. [PubMed] [Google Scholar]
- 39.Meinertz H, Linnet L, Fogh-Andersen P, Hjort T. Antisperm antibodies and fertility after vasovasostomy: a followup study of 216 men. Fertil Steril. 1990;64:315–8. [PubMed] [Google Scholar]
- 40.Carbone DJ, Shah A, Thomas AJ, Jr, Agarwal A. Partial obstruction, not antisperm antibodies, causing infertility after vasovasostomy. J Urol. 1998;159:827–30. [PubMed] [Google Scholar]
- 41.Costabile RA, Goldstein M, Schlegel P, Belker AL, Lipschultz LI, et al. Vol. 18. Lesson 36. the American Urological Association; 1999. Technique of Vasovasostomy and Vasoepididymostomy: Shortening the learning curve. AUA Update Series. American Urological Association. [Google Scholar]
- 42.Jarvi K, Grober ED, Lo KC, Patry G. Mini-incision microsurgical vasectomy reversal using no-scalpel vasectomy principles and instruments. Urology. 2008;72:913–5. doi: 10.1016/j.urology.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 43.Grober ED, Jarvi K, Lo KC, Shin EJ. Mini-incision vasectomy reversal using no-scalpel vasectomy principles: efficacy and postoperative pain compared with traditional approaches to vasectomy reversal. Urology. 2011;77:602–6. doi: 10.1016/j.urology.2010.09.051. [DOI] [PubMed] [Google Scholar]
- 44.Smith RP, Khanna A, Kovac JR, Badhiwala N, Coward R, et al. The significance of sperm heads and tails within the vasal fluid during vasectomy reversal. Indian J Urol. 2014;30:164–8. doi: 10.4103/0970-1591.126898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scovell JM, Mata DA, Ramasamy R, Herrel LA, Hsiao W, et al. Association between the presence of sperm in the vasal fluid during vasectomy reversal and postoperative patency: a systematic review and meta-analysis. Urology. 2015;85:809–13. doi: 10.1016/j.urology.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lipshultz LI, Rumohr JA, Bennett RC. Techniques for vasectomy reversal. Urol Clin North Am. 2009;36:375–82. doi: 10.1016/j.ucl.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 47.Herrel L, Hsiao W. Microsurgical vasovasostomy. Asian J Androl. 2013;15:44–8. doi: 10.1038/aja.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldstein M, Li PS, Matthews GJ. Microsurgical vasovasostomy: the microdot technique of precision suture placement. J Urol. 1998;159:188–90. doi: 10.1016/s0022-5347(01)64053-9. [DOI] [PubMed] [Google Scholar]
- 49.Kim HH, Goldstein M. History of vasectomy reversal. Urol Clin North Am. 2009;36:359–73. doi: 10.1016/j.ucl.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Shaeer OK, Shaeer KZ. Pelviscrotal vasovasostomy: refining and troubleshooting. J Urol. 2005;174:1935–7. doi: 10.1097/01.ju.0000176738.55343.75. [DOI] [PubMed] [Google Scholar]
- 51.Nagler HM, Belletete BA, Gerber E, Dinlenc CZ. Laparoscopic retrieval of retroperitoneal vas deferens in vasovasostomy for postinguinal herniorrhaphy obstructive azoospermia. Fertil Steril. 2005;83:1842. doi: 10.1016/j.fertnstert.2004.11.083. [DOI] [PubMed] [Google Scholar]
- 52.Kramer WC, Meacham RB. Vasal reconstruction above the internal inguinal ring: what are the options? J Androl. 2006;27:481–2. doi: 10.2164/jandrol.06031. [DOI] [PubMed] [Google Scholar]
- 53.Shessel FS, Lynne CM, Politano VA. Use of exteriorized stents in vasovasostomy. Urology. 1981;17:163–5. doi: 10.1016/0090-4295(81)90228-4. [DOI] [PubMed] [Google Scholar]
- 54.Silber SJ. Perfect anatomical reconstruction of vas deferens with a new microscopic surgical technique. Fertil Steril. 1977;28:72–7. [PubMed] [Google Scholar]
- 55.Jee SH, Hong YK. One-layer vasovasostomy: microsurgical versus loupe-assisted. Fertil Steril. 2010;94:2308–11. doi: 10.1016/j.fertnstert.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 56.Berger RE. Triangulation end-to-side vasoepididymostomy. J Urol. 1998;159:1951–3. doi: 10.1016/S0022-5347(01)63205-1. [DOI] [PubMed] [Google Scholar]
- 57.Marmar JL. Modified vasoepididymostomy with simultaneous double needle placement, tubulotomy and tubular invagination. J Urol. 2000;163:483–6. [PubMed] [Google Scholar]
- 58.Chan PT, Brandell RA, Goldstein M. Prospective analysis of outcomes after microsurgical intussusception vasoepididymostomy. BJU Int. 2005;96:598–601. doi: 10.1111/j.1464-410X.2005.05691.x. [DOI] [PubMed] [Google Scholar]
- 59.Schiff J, Chan P, Li PS, Finkelberg S, Goldstein M. Outcome and late failures compared in 4 techniques of microsurgical vasoepididymostomy in 153 consecutive men. J Urol. 2005;174:651–5. doi: 10.1097/01.ju.0000165573.53109.92. [DOI] [PubMed] [Google Scholar]
- 60.Herrel LA, Goodman M, Goldstein M, Hsiao W. Outcomes of microsurgical vasovasostomy for vasectomy reversal: a meta-analysis and systematic review. Urology. 2015;85:819–25. doi: 10.1016/j.urology.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 61.Hsiao W, Goldstein M, Rosoff JS, Piccorelli A, Kattan MW, et al. Nomograms to predict patency after microsurgical vasectomy reversal. J Urol. 2012;187:607–12. doi: 10.1016/j.juro.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 62.Schoor RA, Ross L, Niederberger C. Robotic assisted microsurgical vasal reconstruction in a model system. World J Urol. 2003;21:48–9. doi: 10.1007/s00345-003-0324-5. [DOI] [PubMed] [Google Scholar]
- 63.Kuang W, Shin PR, Matin S, Thomas AJ., Jr Initial evaluation of robotic technology for microsurgical vasovasostomy. J Urol. 2004;171:300–3. doi: 10.1097/01.ju.0000098364.94347.02. [DOI] [PubMed] [Google Scholar]
- 64.Parekattil SJ, Atalah HN, Cohen MS. Video technique for human robot-assisted microsurgical vasovasostomy. J Endourol. 2010;24:511–4. doi: 10.1089/end.2009.0235. [DOI] [PubMed] [Google Scholar]
- 65.Parekattil SJ, Gudeloglu A, Brahmbhatt J, Wharton J, Priola KB. Robotic assisted versus pure microsurgical vasectomy reversal: technique and prospective database control trial. J Reconstr Microsurg. 2012;28:435–44. doi: 10.1055/s-0032-1315788. [DOI] [PubMed] [Google Scholar]
- 66.Kavoussi PK. Validation of robot-assisted vasectomy reversal. Asian J Androl. 2015;17:245–7. doi: 10.4103/1008-682X.142141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crosnoe LE, Kim ED, Perkins AR, Marks MB, Burrows PJ, et al. Angled vas cutter for vasovasostomy: technique and results. Fertil Steril. 2014;101:636–639.e2. doi: 10.1016/j.fertnstert.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 68.Moon HJ. Minimally invasive vas surgery using a newly designed double-ringed clamp. World J Urol. 2010;28:205–8. doi: 10.1007/s00345-009-0437-6. [DOI] [PubMed] [Google Scholar]
- 69.Silverstein JI, Mellinger BC. Fibrin glue vasal anastomosis compared to conventional sutured vasovasostomy in the rat. J Urol. 1991;145:1288–91. doi: 10.1016/s0022-5347(17)38616-0. [DOI] [PubMed] [Google Scholar]
- 70.Vankemmel O, Rigot JM, Burnouf T, Mazeman E. Delayed vasovasostomy: experimental study using fibrin glue. Eur Urol. 1997;31:182–6. doi: 10.1159/000474447. [DOI] [PubMed] [Google Scholar]
- 71.Shekarriz BM, Thomas AJ, Jr, Sabanegh E, Kononov A, Levin HS. Fibrin-glue assisted vasoepididymostomy: a comparison to standard end-to-side microsurgical vasoepididymostomy in the rat model. J Urol. 1997;158:1602–5. doi: 10.1016/s0022-5347(01)64288-5. [DOI] [PubMed] [Google Scholar]
- 72.Hakky TS, Duboy AJ, Lipshultz LI, Carrion RE. Absorbable cyanoacrylate for use in microsurgical vasovasostomy: a novel method to reinforce the anastomosis. American Society of Andrology Meeting. 2014 Abstract. [Google Scholar]
- 73.McMahon AJ, Buckley J, Taylor A, Lloyd SN, Deane RF, et al. Chronic testicular pain following vasectomy. Br J Urol. 1992;69:188–91. doi: 10.1111/j.1464-410x.1992.tb15494.x. [DOI] [PubMed] [Google Scholar]
- 74.Leslie TA, Illing RO, Cranston DW, Guillebaud J. The incidence of chronic scrotal pain after vasectomy: a prospective audit. BJU Int. 2007;100:1330–3. doi: 10.1111/j.1464-410X.2007.07128.x. [DOI] [PubMed] [Google Scholar]
- 75.Polackwich AS, Tadros NN, Ostrowski KA, Kent J, Conlin MJ, et al. Vasectomy Reversal for Postvasectomy Pain Syndrome: a study and literature review. Urology. 2015;86:269–72. doi: 10.1016/j.urology.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 76.Lee JY, Chang JS, Lee SH, Ham WS, Cho HJ, et al. Efficacy of vasectomy reversal according to patency for the surgical treatment of postvasectomy pain syndrome. Int J Impot Res. 2012;24:202–5. doi: 10.1038/ijir.2012.17. [DOI] [PubMed] [Google Scholar]
- 77.Horovitz D, Tjong V, Domes T, Lo K, Grober ED, et al. Vasectomy reversal provides long-term pain relief for men with the post-vasectomy pain syndrome. J Urol. 2012;187:613–7. doi: 10.1016/j.juro.2011.10.023. [DOI] [PubMed] [Google Scholar]


