Abstract
The use of testosterone replacement therapy (TRT) for hypogonadism continues to rise, particularly in younger men who may wish to remain fertile. Concurrently, awareness of a more pervasive use of anabolic-androgenic steroids (AAS) within the general population has been appreciated. Both TRT and AAS can suppress the hypothalamic-pituitary-gonadal (HPG) axis resulting in diminution of spermatogenesis. Therefore, it is important that clinicians recognize previous TRT or AAS use in patients presenting for infertility treatment. Cessation of TRT or AAS use may result in spontaneous recovery of normal spermatogenesis in a reasonable number of patients if allowed sufficient time for recovery. However, some patients may not recover normal spermatogenesis or tolerate waiting for spontaneous recovery. In such cases, clinicians must be aware of the pathophysiologic derangements of the HPG axis related to TRT or AAS use and the pharmacologic agents available to reverse them. The available agents include injectable gonadotropins, selective estrogen receptor modulators, and aromatase inhibitors, but their off-label use is poorly described in the literature, potentially creating a knowledge gap for the clinician. Reviewing their use clinically for the treatment of hypogonadotropic hypogonadism and other HPG axis abnormalities can familiarize the clinician with the manner in which they can be used to recover spermatogenesis after TRT or AAS use.
Keywords: anabolic steroids, hypogonadism, infertility, spermatogenesis, testosterone, testosterone replacement therapy, vasectomy reversal
INTRODUCTION
In recent years, mass marketing has led to a greater public awareness of the age-related decline in serum testosterone levels and the association of hypogonadism with many already common medical comorbidities.1,2 This in part has fueled the growth of testosterone replacement therapy (TRT) for hypogonadism, which experienced a 12-fold increase in sales worldwide from 2000 to 2011.3 The same trend occurred in the United States where the greatest increase was observed in younger men aged 40–49 years by 4-fold, resulting in an age group-specific prevalence of 2.3% in 2011.4 This is not surprising since approximately 7% of men less than 40 years and 38% of men older than 45 years demonstrate biochemical hypogonadism when defined as <300 ng dl-1.1,5 As such, younger men are seeking treatment for hypogonadism with as many as 12.4% of all testosterone prescriptions occurring in men <39 years of age.6
Similar to TRT, there has also been an increase in the availability and use of anabolic-androgenic steroids (AAS). It is estimated that up to 3 million people use AAS in the Unites States alone, including up to 3% of high school age adolescents, 14% of collegiate athletes, and 30% of community weight trainers; however, many of these estimates are based upon older data.7,8 A more recent review revealed that AAS use is a common cause of profound hypogonadism with up to one of five men seeking treatment for hypogonadism reporting prior AAS use.9 Interestingly, much of the increase in amateur athletic use has been attributed to cosmetic instead of athletic improvements.10 These numbers indicate a concerning shift in use to beyond the realm of professional athletics. In addition, many “dietary supplements” used for athletic or cosmetic enhancement also discretely contain AAS, with contamination rates as high as 15%.11 Unfortunately, up to 50% of previous AAS users choose not to disclose their previous AAS use with physicians, potentially masking a clinician's overall impression of the burden of AAS abuse.12
Both TRT and AAS use can lead to suppression of the hypothalamic-pituitary-gonadal (HPG) axis, resulting in a diminution of spermatogenesis and potential infertility. Spontaneous recovery of spermatogenesis after cessation of TRT or AAS is possible but may take several months to several years, and in some cases may be permanent.13,14,15,16 Taken together, the rising use of TRT and AAS in young- to middle-aged men, in conjunction with a societal shift toward greater paternal age,17 is creating an environment where clinicians are increasingly likely to encounter men seeking treatment for infertility related to prior TRT and/or AAS use or treatment for hypogonadism with interest in preserving their fertility. Meanwhile, men present to infertility specialists for vasectomy reversal (VR) at an average age of 41 (n = 1300), some of whom may also suffer from hypogonadism and report current or previous TRT use.18 Therefore, clinicians need to be keenly aware of the effects of TRT and AAS on spermatogenesis and what treatment options are available to reverse these effects to restore spermatogenesis.
NORMAL SPERMATOGENESIS
Normal spermatogenesis is dependent on appropriate signaling from the HPG axis. This signaling initially consists of a pulsatile release of gonadotropin-releasing hormone (GnRH) from the hypothalamus via the portal system to the pituitary gland where stimulation results in gonadotropin release. Luteinizing hormone (LH) from the pituitary stimulates Leydig cells in the testis to produce testosterone and leads to intratesticular production of insulin-like growth factor 1 (IGF-1), which plays an integral role in Leydig cell LH receptor upregulation, steroidogenesis, and maturation.19,20 Follicle-stimulating hormone (FSH) from the pituitary stimulates Sertoli cells in the testis, which supports spermatogonial differentiation and maturation. Both FSH and maintenance of high intratesticular testosterone (ITT) levels (50–100 fold higher than serum) in response to LH are critical for normal spermatogenesis to occur.21,22,23,24 Historically, Sertoli cell-produced androgen-binding protein was thought to be responsible for such high ITT levels, but recent data suggest that other factors are also involved.25 Interestingly, animal studies have demonstrated that the absence of FSH signaling results in impaired spermatogenesis whereas loss of sufficiently high ITT levels results in the absence of spermatogenesis.26
Regulation of the HPG axis occurs via feedback inhibition. Endogenous testosterone directly inhibits GnRH and LH release at the hypothalamus and pituitary levels, respectively, leading to downstream attenuation of testosterone production. Testosterone also indirectly regulates gonadotropin secretion via estrogen, derived from testosterone conversion peripherally by aromatase enzyme. Estrogen exhibits a greater effect on LH secretion than FSH although additional FSH feedback inhibition occurs with inhibin B secreted from Sertoli cells. Inhibin B levels have been considered a surrogate for spermatogenesis; for example, men with spermatogenetic defects express lower inhibin B levels.27 Additional autocrine, paracrine, and endocrine factors within the hypothalamus, pituitary, and testis can function to further modulate the HPG axis in complex ways including endocannabinoids, GnRH, kisspeptin, norepinephrine, growth hormone, interleukins, and TGF-β.28 Therefore, the HPG axis represents a dynamic, but tightly regulated, system at multiple levels resulting in spermatogenesis, among other things.
INFLUENCE OF EXOGENOUS ANDROGENS ON SPERMATOGENESIS
The use of exogenous androgens can influence the HPG axis by similar mechanisms as endogenous testosterone by exerting negative feedback in a dose- and duration-dependent fashion, resulting in reductions in ITT, blunting of FSH production, and ultimately decrease or complete cessation of spermatogenesis.29 Data specifically describing the natural history of unassisted spermatogenesis recovery after long-term TRT are lacking, but such information can be extrapolated from the male contraceptive literature.16 Multiple and international trials using various testosterone preparations have been performed and demonstrate a median time to spermatogenesis suppression to <1 × 106 ml-1 sperm within 3.5 months. Alternatively, the same data demonstrate a median time to recovery of 20 × 106 ml-1 sperm ranging from 3 to 6 months, with probability estimates suggesting recovery in 67%, 90%, 96%, and 100% of men at 6, 12, 16, and 24 months, respectively, after discontinuation of testosterone exposure.13 These data also suggest that a longer exposure to exogenous testosterone, Asian ethnicity, and older age may result in a prolonged recovery time after treatment cessation.13,30,31,32 Importantly, one must consider that these data are carefully collected in men within the tightly controlled, clinical trial environment, and may not be generalizable. Certainly, men with a prior, multiple year history of TRT or AAS use may not expect the same rate of recovery.
AAS are synthetic derivatives of testosterone with chemical modifications intended to mimic the anabolic more than the androgenic effects of testosterone. Many abusers use “stacking” regimens with multiple, high-dose AAS agents to maximize muscle mass and weight gain, which are often “cycled” to minimize side effects. Nevertheless, AAS can still bind the androgen receptor within target cells and exert the same negative feedback effects as endogenous testosterone, often resulting in anabolic steroid-induced hypogonadism (ASIH) and associated reductions in serum gonadotropin levels and ITT.9,15,21,33 With abnormally low ITT and FSH, these patients often exhibit azoospermia or oligospermia with reduced motility and/or morphology on semen analysis.15
Such effects on the HPG axis are potentially reversible with cessation of AAS use, but the time to recovery is highly variable and influenced by the dose and extent of stacking multiple AAS agents, duration of AAS use, and patient age.8,34 Data specifically looking at recovery of spermatogenesis after cessation of AAS are scant, but case reports suggest that recovery is feasible within 4–12 months although some patients may require up to 24–30 months to return to sperm concentrations of >20 × 106 ml-1.14,15,35,36,37 It cannot be understated that given the inherent variability in patient characteristics and AAS agent(s) used, a uniform recovery of the HPG axis cannot be expected in all patients.
PHARMACOLOGIC AGENTS TO RESTORE OR MAINTAIN SPERMATOGENESIS
Gonadotropins: hCG and FSH
Human chorionic gonadotropin (hCG) is a naturally occurring protein produced by the human placenta with a serum half-life of approximately 36 h. Structurally, hCG shares an identical α-subunit with LH and FSH. However, hCG has a unique β-subunit that is virtually identical to the LH β-subunit except that it has an additional 24 amino acid tail at the amino terminus of the protein, which is highly glycosylated and leads to both a longer circulating half-life of hCG (~36 h) versus LH (~30 min) and increased receptor activity. The increased LH receptor activity, along with its longer half-life, makes it a clinically useful LH analog. Initially extracted from the urine of pregnant females, naturally occurring hCG has demonstrated efficacy at restoring spermatogenesis.38 Newer, recombinant hCG has emerged and is considered equivalent to urinary sources pharmacologically although further study is warranted to confirm its equivalency to urinary forms in restoring spermatogenesis.39 Similarly, FSH has traditionally been derived from the urine of postmenopausal women in the form of human menopausal gonadotropin (HMG). A large proportion of naturally occurring HMG consists of copurified urinary proteins inactive at the FSH receptor, with a lesser proportion containing a blend of FSH, LH, and hCG.40 Therefore, similar to hCG, refinements have led to production of highly purified urinary HMG, and more recently recombinant FSH (rFSH), to achieve higher specificity for the FSH receptor. To date, direct comparisons between the two have not occurred for use at inducing spermatogenesis in men, but data from use in women suggest that rFSH is equivalent to urinary preparations and can avoid the theoretical risk of Creutzfeld–Jakob disease;38,40 therefore, rFSH is the preferred method of pharmacologic delivery of FSH in men.
Clinically, hCG has proven successful at inducing and/or maintaining spermatogenesis alone or in combination with FSH in patients with hypogonadotropic hypogonadism (HH). HH is an uncommon but treatable cause of male factor infertility classically considered secondary to pathology of the hypothalamus or pituitary gland as seen with Kallman's syndrome, Prader–Willi syndrome, panhypopituitarism from prolactinomas, tumors, infection or radiation, or idiopathic causes.21 Recently, recognition that the increasingly common use of exogenous TRT and/or AAS can also induce HH, also known as ASIH, with associated diminished spermatogenesis. Therefore, men with azoospermia or severe spermatogenic defects due to classic HH serves as a useful context in whom to appreciate the effect of gonadotropins upon spermatogenesis clinically. However, due to the uncommon prevalence of HH, high-quality data are lacking and most are limited to case reports and retrospective series.41
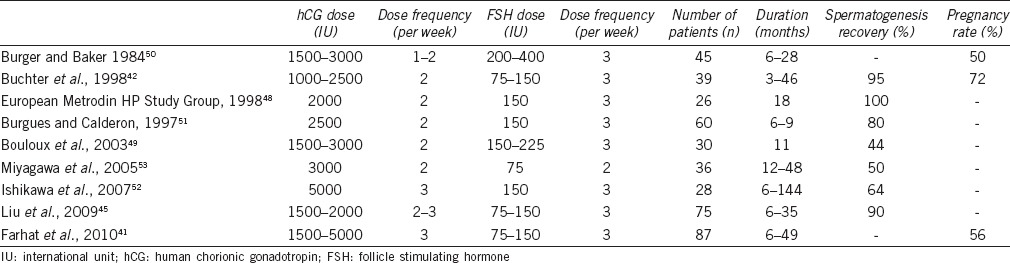
Historically, treatment approaches for HH have focused upon physiologic, pulsatile GnRH therapy to induce secondary sex characteristics and spermatogenesis with reported pregnancy rates as high as 80%.42,43 However, widespread use of pulsatile GnRH is inherently limited due to the need for an external pump for periodic hormone release, cost, and requirement of a functionally intact pituitary gland to appropriately respond to hypothalamic signals.44 Alternatively, treatment with injectable gonadotropin regimens has demonstrated equivalent clinical efficacy compared with GnRH for triggering spermatogenesis based upon a recent meta-analysis.44 Therefore, gonadotropins offer patients an efficacious and more convenient treatment approach.45 FSH given alone or in combination with testosterone has proven unsuccessful at inducing spermatogenesis or maintaining spermatogenesis in those previously induced with hCG/FSH (hCG 1500 IU and HMG 150 IU both subcutaneous and 3 times per week), confirming the need for maintenance of elevated ITT.46 However, long-term use of hCG alone can induce spermatogenesis in up to 70% of patients, with a greater effect seen in men with initial testis length >4 cm, but further improvement is appreciated with the addition of FSH (HMG) suggesting a timelier recovery with both gonadotropins.47 The success of inducing spermatogenesis with a combination of hCG and FSH is supported by several studies (Table 1).41,42,45,48,49,50,51,52,53 In these data, most begin by stimulating endogenous testosterone production with trial of hCG alone with doses ranging from 1500 to 5000 IU 2–3 times per week titrated according to serum testosterone levels. Most experts treat with hCG alone for 3–6 months after which a certain number of cases will result in spermatogenesis induction. In those without adequate spermatogenesis induction, treatment proceeds with the addition of FSH with doses ranging from 75 to 400 IU 2–3 times per week titrated according to semen analysis results. Success defined as induction of spermatogenesis with >1–1.5 × 106 ml-1 sperm was reported to occur in 44%–100% of patients treated for 6–144 months.52 Pregnancy rates, when reported, were observed in 40%–75% of patients usually at sperm concentration levels below “normal.”42,51,54 Factors predicting success include larger baseline testis volume, previous natural gonadotropin exposure (normal puberty), and repeated treatment cycles whereas previous exogenous testosterone exposure and cryptorchidism portend a slower response although these findings are variable.42,55 It is important to consider these data are in men with HH due to classic causes and not patients with previous TRT/AAS use in whom better outcomes can theoretically be expected given the likelihood of normal pubertal development and HPG axis function at some point before TRT/AAS exposure.
Table 1.
Gonadotropins for recovery of spermatogenesis in classic hypogonadotropic hypogonadism
Data specifically evaluating induction or maintenance of spermatogenesis in men with HH and azoospermia specifically due to previous TRT and/or AAS use is scarce (Table 2). In a study of normal men treated with TRT and randomized to concurrent administration of placebo or low-dose hCG (125, 150 or 500 IU) every other day, ITT levels were maintained in all hCG groups with levels closest to baseline normal in the 250 and 500 IU dose groups, thereby suggesting preservation of spermatogenesis.56 These data are supported by the finding from Depenbusch and colleagues that preservation of spermatogenesis is possible with hCG alone (500–2500 IU twice weekly based upon serum testosterone levels) in men with HH and azoospermia in whom spermatogenesis was previously initiated with hCG/FSH.57 However, in this study, spermatogenesis was only maintained “qualitatively,” in that mean sperm concentrations with hCG alone were 43% of levels previously achieved with spermatogenesis induction using a combination of hCG and FSH, suggesting both are needed for “quantitatively” normal spermatogenesis. Alternatively, a series of hypogonadal men wishing to preserve fertility while initiating TRT with different agents (transdermal gels and injections) demonstrated that low-dose hCG (500 IU every other day) preserves all aspects of analyzed semen parameters despite improvement in serum testosterone levels, and with no differences observed between different types of TRT agent used.58 A more recent multi-institutional series of men previously treated with TRT and established to be azoospermic or severely oligospermic (<1 × 106 ml-1) were given hCG 3000 IU every other day supplemented with either FSH, clomiphene citrate (CC), tamoxifen, or anastrozole. This series demonstrated a mean recovery of spermatogenesis to a density of 22 × 106 ml-1 in 4 months.59 Similarly, a series of hypogonadal men on TRT seeking VR underwent “testicular salvage” with CC and hCG (3000 IU every other day) before VR, resulting in normalization of HPG parameters and successful VR in 83%.60 Finally, even less data are available to support gonadotropin use for restoration of spermatogenesis in azoospermic men with ASIH after AAS use. A few case reports indicate that hCG alone at variable doses (2000 IU 3 times per week to 10 000 IU once weekly)15,36,61 or both hCG (10 000 IU weekly) and FSH (75 IU daily) in combination62 can restore spermatogenesis and in some cases lead to conception. Collectively, these data demonstrate that restoration and maintenance of spermatogenesis using gonadotropins is a successful strategy in men with prior TRT and/or AAS use, with results similar to those observed with gonadotropin use in men with classic HH.
Table 2.
Commonly used pharmacologic agents for maintenance or restoration of spermatogenesis after anabolic-androgenic steroid or exogenous testosterone use

Selective estrogen receptor modulators (SERMs)
SERMs are a group of medications that function to disrupt binding of estrogen at estrogen receptors in the hypothalamus through competitive antagonism. In men, normal binding of estrogen at these receptors functions as an indirect negative feedback mechanism of endogenous testosterone production to downregulate GnRH and subsequently pituitary gonadotropin production. Therefore, SERMs function to block estrogen feedback thereby increasing GnRH and gonadotropin production and ultimately increasing ITT levels in men without evidence of primary hypogonadism.16,63,64 Clinically, tamoxifen and CC are two of the most commonly used SERMs, with the former popularized by use in breast cancer treatment protocols and the latter popularized by its initial development for triggering ovulation in women. CC exists as a racemic mixture of shorter acting enclomiphene (purely anti-estrogenic effects) and longer acting zuclomiphene (both estrogen agonist and antagonist effects) and exhibits a serum half-life of approximately 5 days.65
CC use in men was first reported in 1966 for treatment of subfertile males to improve pregnancy rates based upon the theoretical benefit from its mechanism of action.66 Since then, it has been used off-label to treat various subpopulations of infertile men with reported dosing schedules ranging from 25 to 50 mg administered daily, every other day or cyclically with intermittent “off periods,” all of which may be titrated based upon serum testosterone levels.67,68,69 Several studies looking at CC use in men with secondary hypogonadism demonstrate clear improvement in serum testosterone levels, hypogonadal symptoms, and testosterone: estrogen ratios indicative of CC's positive therapeutic effects on the HPG axis.69,70,71 Similarly, the use of CC in men with idiopathic oligospermia or azoospermia with or without hypogonadism has demonstrated favorable changes in hormone profiles and semen analyses, but data evaluating pregnancy rates have yielded conflicting results.72,73,74 Nonetheless, CC continues to be used clinically for the treatment of idiopathic male infertility and for the treatment of hypogonadal symptoms in men wishing to preserve spermatogenesis in the absence of randomized controlled data. Overall, CC is well tolerated and considered safe in men who tend to experience much fewer side effects than seen with CC use in women.75,76 However, isolated case reports have demonstrated the possibility of developing azoospermia with CC use in oligospermic men that is reversible with CC cessation.77 For this reason, it is necessary to inform patients of its potentially unpredictable results, and follow-up serum laboratory studies and semen analyses are important during treatment with CC.
Literature assessing CC use to restore spermatogenesis in oligospermic and azoospermic men with HH after TRT and/or AAS use is very limited (Table 2). Case reports of CC use at higher doses (100 mg daily) in young men with ASIH resulted in normalization of the HPG axis within 2–3 months, but spermatogenesis was not evaluated.78,79 A small and retrospective case series looking at two men with idiopathic, acquired HH with oligospermia and azoospermia, and one man with ASIH and azoospermia who were each given CC 50 mg 3 times per week found 100% recovery of serum gonadotropins, testosterone, and spermatogenesis within 3 months and a 66% pregnancy rate.80 More recently, a larger retrospective series of 63 men given a combination of hCG 3000 IU 3 times per week and CC or tamoxifen demonstrated recovery of spermatogenesis to >1 × 106 ml-1 sperm in 98% of men in 4–5 months, with a mean initial sperm concentration of 22.6 × 106 ml-1.59 Similarly, a testicular salvage regimen of CC 25 mg daily or combination with hCG 3000 IU every other day for six men with a history of TRT presenting for VR resulted in normalization of the HPG axis and successful VR in 83% of patients.60
CC consists of a racemic mixture of zuclomiphene and enclomiphene with the latter exhibiting purely anti-estrogenic effects with a relatively shorter half-life (10.5 h) than CC.81 Therefore, more promising than CC are the recently reported results using enclomiphene citrate (EC) in men. Several positive clinical trials have been performed, but official FDA approval is still pending. A phase IIB clinical trial followed 12 men previously treated with TRT for >6 months and documented HH with oligospermia or azoospermia were randomized to EC 25 mg daily versus topical testosterone gel for 6 months. The study demonstrated equivalent responses in serum testosterone levels in both arms, and statistically significant improvements in semen parameters were appreciated in the EC group (P = 0.004).82 Similarly, another phase II trial looking at EC use in men with secondary hypogonadism demonstrated increases in serum testosterone and gonadotropin levels within 2 weeks of treatment.83 Finally, the most recent phase IIB trial studied 73 hypogonadal men with normal spermatogenesis who were randomized to EC 12.5 mg or 25 mg daily, topical TRT, or placebo. Equivalent increases in serum testosterone, estradiol, and LH levels among TRT and EC groups were demonstrated, but an increase in oligospermia and azoospermia was observed in the TRT group whereas spermatogenesis was preserved in the EC and placebo groups.84 Therefore, EC represents an exciting new treatment on the horizon for restoration and preservation of spermatogenesis in hypogonadal men, which will hopefully obtain future FDA approval pending results from upcoming phase III studies.
Aromatase inhibitors (AIs)
AIs are a class of medications FDA approved for the treatment of early- and late-stage breast cancer and historically include nonselective steroidal, and highly selective nonsteroidal agents, including anastrozole and letrozole. AIs function by inhibiting the aromatase enzyme, which is a cytochrome P450 converter of testosterone-to-estrogen within the testes, liver, brain, and adipose tissues.16 Estrogen is an indirect mediator of testosterone feedback inhibition of the HPG axis. Therefore, aromatase inhibition in men can result in decreased estrogen levels and ultimately increased gonadotropin production. Their use clinically in men is off-label and has focused upon improving male infertility and symptoms of hypogonadism, particularly in obese men or in those with a serum testosterone-to-estrogen (T/E) ratios <10 where improvements of approximately 77% have been observed.64 In addition, AIs can be prescribed for use with exogenous testosterone or hCG to mitigate side effects of hyperestrogenemia such as gynecomastia.
Studies evaluating the use of older AI agents in oligospermic men have suggested improvement in T/E ratios and spermatogenesis but randomized controlled data using testolactone failed to support these findings.85 However, recent data by Raman and Schlegel compared testolactone (500–1000 mg twice daily) to a more selective AI, anastrozole (1 mg daily), in subfertile men. Patients had abnormal T/E ratios along with idiopathic oligospermia or azoospermia, and the study demonstrated a statistically significant improvement in T/E ratios and spermatogenic parameters with anastrozole in those with oligospermia, but not those with azoospermia.86 Similar success adding anastrozole (1 mg daily) to existing treatment in men with idiopathic oligospermia and abnormally low T/E ratios unresponsive to 3 months of treatment with tamoxifen alone demonstrates improvement in sperm concentration and motility.87 The previous suggestion of AI failure at improving semen parameters in men with nonobstructive azoospermia by Raman and Schlegel has been challenged by recent data showing improved success rates with microsurgical testicular sperm extraction in men with nonmosaic Klinefelter's syndrome and normalized serum testosterone levels after treatment with anastrozole preoperatively.88 The use of letrozole (2.5 mg daily), a newer and more selective AI, has demonstrated improvements in T/E ratio and spermatogenic parameters similar to anastrozole with up to 20% spontaneous pregnancy rate for oligospermic men and 24% return of sperm to the ejaculate of previously azoospermic men.89
The use of AIs in infertile men with previous TRT and/or AAS use has not been reported in prospective trials. The aforementioned retrospective series from Wenker et al. evaluating hCG used concurrently with SERMs, AIs, and FSH, in men with previous TRT use and severe oligospermia or azoospermia demonstrated an overall 98% success rate at recovering spermatogenesis and 38% spontaneous pregnancy rate, with no differences between the type of supplemental therapy given with hCG or type of TRT used (Table 2).59 More recently, a study of 26 hypogonadal, infertile, nonazoospermic men randomized to CC 25 mg daily or anastrozole 1 mg daily for 6 weeks was performed and demonstrated improvements in both overall testosterone levels and T/E ratios, but CC had a greater impact on testosterone levels and anastrozole had a greater impact on T/E ratio.90 Therefore, in hypogonadal men with T/E ratios <10, CC and AIs may be used concurrently to achieve the best result; however, this theoretically beneficial pharmacologic combination has not been reported in prospective studies, but anecdotally may prove useful.
Overall, the use of AIs is generally well tolerated in women, but elevated liver enzymes have been reported in up to 17% of the patients suggesting caution in those with a history of hepatic dysfunction. A series evaluating anastrozole for treatment of secondary hypogonadism in 69 older men followed for 1 year confirmed a generally low rate of adverse events although one instance each of new diagnosis hepatitis, pulmonary embolism, and embolic stroke was reported.91 A similar observation of pulmonary embolism incidence was confirmed in a more recent series after only 12 weeks of treatment.90 In addition, some data have suggested potentially worse skeletal bone health with anastrozole use in older men, presumably based upon lower estrogen levels.92 Taken collectively, AIs may result in a positive influence on the HPG axis after TRT or AAS use, but in the absence of more robust clinical data and an uncertain side effect profile with long-term use, AI use may be limited to an adjunctive role only in those who have abnormally low T/E ratios.
CLINICAL SCENARIOS
Men with infertility related to previous TRT and/or AAS use can present clinically in a number of scenarios that can be challenging to navigate as a clinician. In this review, we have provided the pathophysiology of TRT and AAS effects on normal spermatogenesis and the pharmacologic tools available to potentially reverse these effects. Certain clinical scenarios are commonly encountered, and a brief discussion of these authors’ recommendations for treatment in each scenario follows.
Infertile male with a recent history or current use of TRT and/or AAS
A patient who presents for treatment of male factor infertility, indicated by oligospermia or nonobstructive azoospermia, who either reports a recent history or current use of TRT and/or AAS is a common scenario faced by a male fertility specialist. Several options could be discussed depending on the severity of his hypogonadal symptoms, timing in which he and his partner wish to achieve pregnancy, and assuming there is no clinical evidence of primary hypogonadism.
If the patient and his partner are willing to wait and his hypogonadal symptoms are manageable without TRT or AAS, the patient could simply discontinue the use of TRT or AAS to allow spontaneous recovery. Data from the male contraception literature indicate a reasonable probability of recovery in 67%, 90%, 96%, and 100% of men at 6, 12, 16, and 24 months, respectively, with a median time to recovery of 20 × 106 ml-1 sperm in 3–6 months.13,30,31 Yet, many men will not tolerate discontinuation either due to severe hypogonadal symptoms, uncertainty of recovery, and/or timing issues, and these men may require some form of alternate androgen supplementation. Therefore, one could administer gonadotropin analogs similar to those implemented in patients with HH. Assuming there is no major component of primary hypogonadism, this option is safe, would treat hypogonadal symptoms, and would hasten the time to recovery. It is reasonable to start with hCG 3000 IU subcutaneous injection 3 times weekly for 3 months with additional titration pending interim serum testosterone levels although the optimal hCG dose has not been clearly established. If at 3 months seminal parameters have not improved, one could add FSH. A typical starting dose is rFSH 75 IU subcutaneous injection 3 times weekly.
During gonadotropin therapy, adjunctive treatments with AIs or SERMs are typically implemented. Such an approach has demonstrated excellent results on average within 4–5 months.59 CC 25 mg daily or 50 mg every other day, titrated up to 50 mg daily, may demonstrate improvement in seminal parameters in as little as 3 months for men with HH. CC is cost effective and has been more effective as a combined therapy in this setting, with less extensive data to support it as a monotherapy.80 If the patient exhibits a low T/E ratio, an AI could be prescribed, with anastrozole 1 mg oral twice weekly is a reasonable starting dose that may be titrated up or down according to the response.
Maintenance of spermatogenesis before beginning or during TRT or AAS use
A second scenario is a patient who wishes to preserve existing spermatogenesis before beginning TRT or AAS use. Maintenance of normal ITT levels is critically necessary to maintain spermatogenesis. hCG has proven to maintain ITT levels with doses as low as 500 IU every other day.56,57 Clinical data evaluating higher doses of hCG given as monotherapy (500–2500 IU twice weekly), or low-dose hCG (500 IU every other day) in combination with TRT, have demonstrated satisfactory results for maintaining spermatogenesis,57,58 and either would be a good choice as recommended by these authors.
Alternatively, CC is commonly used as an alternative to TRT to treat hypogonadism in men wishing to preserve spermatogenesis. The ability to take an oral medicine that is relatively inexpensive and has good long-term safety data and is clinically efficacious at ameliorating hypogonadal symptoms is clearly advantageous.69,71 However, data are currently not available specifically evaluating CC in this manner, and randomized controlled trials are needed. The newer SERM on the horizon, EC, has been studied in the phase II clinical trial setting specifically demonstrating preservation of spermatogenesis on semen analysis while satisfactorily improving hypogonadal symptoms and serum testosterone levels, and phase III data is pending.82,84 Finally, AIs such as anastrozole or letrozole may be helpful in this clinical scenario for patients who are obese and/or exhibit a low T/E ratio <10:1.
Patient presenting for vasectomy reversal with a history of secondary hypogonadism or previously treated with TRT
A final scenario is a patient who presents for VR for which he is otherwise a good candidate, who has a history of hypogonadism currently or previously treated with TRT. Such a scenario is difficult because the current status of his spermatogenesis may be deduced only by careful history, testis volume on clinical exam, and serum hormone testing of the HPG axis. Spermatogenesis cannot be definitively evaluated without testis biopsy in the setting of a vasectomized patient. Likewise, the risk of proceeding with VR under the assumption of normal spermatogenesis based upon physical exam poses an increased risk of intraoperative difficulty evaluating vasal fluid for sperm and ultimately a higher risk of failure. Data on this topic are limited, but a recent small, retrospective series was published of six men with median age of 39 using a preoperative testicular salvage regimen of CC 25 mg daily with or without hCG 3000 IU every other day for 3 months after discontinuation of TRT. After testicular salvage therapy, the authors demonstrated an 83% overall success rate with VR, which was 100% if at least one vasovasostomy was performed.60 In that case series, if improvement in testicular volume on physical exam and increase in gonadotropin levels was appreciated, VR was performed; however, testicular sperm aspiration was performed in two of the six men to confirm spermatogenesis before VR due to insufficient testis volume improvement and/or lack of improvement of the HPG axis on serum hormone testing. Such an approach for the vasectomized patient before VR has otherwise not been previously described, nor has the use of hCG and CC outside of another retrospective study,59 but the results appear promising. These authors would consider treatment with CC 25 mg daily with hCG 3000 IU every other day for 3 months, with a reassessment of the HPG axis and physical exam to ensure improvement before VR.
CONCLUSIONS
In the era of rising testosterone use and greater awareness of AAS use in younger men, clinicians need to be aware of the detrimental effects of these agents on spermatogenesis. As the body of evidence grows in support of restorative therapies for recovery of spermatogenesis in this patient population, it is important to be familiar with the various treatment options, their effects on the HPG axis, and when to use them. A historical perspective on gonadotropin use for HH is helpful to interpret the current use of gonadotropins for restoration or maintenance of spermatogenesis. Likewise, understanding the clinical use and effectiveness of CC and other SERMs helps lay the foundation for implementation of newer agents such as EC. Despite off-label use of each restorative agent discussed herein, a definite lack of high quality data, and the general understanding of male reproductive endocrinology still in its infancy, the field of male infertility is rapidly advancing in this area as the importance of restoring and maintaining spermatogenesis in men before, during, and after TRT is becoming fully realized.
AUTHOR CONTRIBUTIONS
JAM is responsible for the design, literature search, and creation of the manuscript. RMC made significant contributions to guidance during the design and editing process. All authors read and approved the final manuscript.
COMPETING INTERESTS
None of the authors declared competing financial interests.
REFERENCES
- 1.Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60:762–9. doi: 10.1111/j.1742-1241.2006.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 3.Handelsman DJ. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199:548–51. doi: 10.5694/mja13.10111. [DOI] [PubMed] [Google Scholar]
- 4.Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465–6. doi: 10.1001/jamainternmed.2013.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rohrmann S, Platz EA, Selvin E, Shiels MS, Joshu CE, et al. The prevalence of low sex steroid hormone concentrations in men in the Third National Health and Nutrition Examination Survey (NHANES III) Clin Endocrinol. 2011;75:232–9. doi: 10.1111/j.1365-2265.2011.04043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Layton JB, Li D, Meier CR, Sharpless JL, Sturmer T, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99:835–42. doi: 10.1210/jc.2013-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sjoqvist F, Garle M, Rane A. Use of doping agents, particularly anabolic steroids, in sports and society. Lancet. 2008;371:1872–82. doi: 10.1016/S0140-6736(08)60801-6. [DOI] [PubMed] [Google Scholar]
- 8.Evans NA. Current concepts in anabolic-androgenic steroids. Am J Sports Med. 2004;32:534–42. doi: 10.1177/0363546503262202. [DOI] [PubMed] [Google Scholar]
- 9.Coward RM, Rajanahally S, Kovac JR, Smith RP, Pastuszak AW, et al. Anabolic steroid induced hypogonadism in young men. J Urol. 2013;190:2200–5. doi: 10.1016/j.juro.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Evans NA. Gym and tonic: a profile of 100 male steroid users. Br J Sports Med. 1997;31:54–8. doi: 10.1136/bjsm.31.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parr MK, Flenker U, Schanzer W. Sports-related issues and biochemistry of natural and synthetic anabolic substances. Endocrinol Metab Clin North Am. 2010;39:45–57. doi: 10.1016/j.ecl.2009.11.004. viii. [DOI] [PubMed] [Google Scholar]
- 12.Pope HG, Kanayama G, Ionescu-Pioggia M, Hudson JI. Anabolic steroid users’ attitudes towards physicians. Addiction. 2004;99:1189–94. doi: 10.1111/j.1360-0443.2004.00781.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu PY, Swerdloff RS, Christenson PD, Handelsman DJ, Wang C. Rate, extent, and modifiers of spermatogenic recovery after hormonal male contraception: an integrated analysis. Lancet. 2006;367:1412–20. doi: 10.1016/S0140-6736(06)68614-5. [DOI] [PubMed] [Google Scholar]
- 14.Boregowda K, Joels L, Stephens JW, Price DE. Persistent primary hypogonadism associated with anabolic steroid abuse. Fertil Steril. 2011;96:e7–8. doi: 10.1016/j.fertnstert.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 15.Jarow JP, Lipshultz LI. Anabolic steroid-induced hypogonadotropic hypogonadism. Am J Sports Med. 1990;18:429–31. doi: 10.1177/036354659001800417. [DOI] [PubMed] [Google Scholar]
- 16.Moss JL, Crosnoe LE, Kim ED. Effect of rejuvenation hormones on spermatogenesis. Fertil Steril. 2013;99:1814–20. doi: 10.1016/j.fertnstert.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Kovac JR, Addai J, Smith RP, Coward RM, Lamb DJ, et al. The effects of advanced paternal age on fertility. Asian J Androl. 2013;15:723–8. doi: 10.1038/aja.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarzer JU. Vasectomy reversal using a microsurgical three-layer technique: one surgeon's experience over 18 years with 1300 patients. Int J Androl. 2012;35:706–13. doi: 10.1111/j.1365-2605.2012.01270.x. [DOI] [PubMed] [Google Scholar]
- 19.Baker J, Hardy MP, Zhou J, Bondy C, Lupu F, et al. Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol. 1996;10:903–18. doi: 10.1210/mend.10.7.8813730. [DOI] [PubMed] [Google Scholar]
- 20.Wang G, Hardy MP. Development of Leydig cells in the insulin-like growth factor-I (igf-I) knockout mouse: effects of igf-I replacement and gonadotropic stimulation. Biol Reprod. 2004;70:632–9. doi: 10.1095/biolreprod.103.022590. [DOI] [PubMed] [Google Scholar]
- 21.Dohle GR, Smit M, Weber RF. Androgens and male fertility. World J Urol. 2003;21:341–5. doi: 10.1007/s00345-003-0365-9. [DOI] [PubMed] [Google Scholar]
- 22.Anawalt BD, Bebb RA, Matsumoto AM, Groome NP, Illingworth PJ, et al. Serum inhibin B levels reflect Sertoli cell function in normal men and men with testicular dysfunction. J Clin Endocrinol Metab. 1996;81:3341–5. doi: 10.1210/jcem.81.9.8784094. [DOI] [PubMed] [Google Scholar]
- 23.Finkel DM, Phillips JL, Snyder PJ. Stimulation of spermatogenesis by gonadotropins in men with hypogonadotropic hypogonadism. N Engl J Med. 1985;313:651–5. doi: 10.1056/NEJM198509123131102. [DOI] [PubMed] [Google Scholar]
- 24.Nieschlag E, Simoni M, Gromoll J, Weinbauer GF. Role of FSH in the regulation of spermatogenesis: clinical aspects. Clin Endocrinol. 1999;51:139–46. doi: 10.1046/j.1365-2265.1999.00846.x. [DOI] [PubMed] [Google Scholar]
- 25.Jarow JP, Zirkin BR. The androgen microenvironment of the human testis and hormonal control of spermatogenesis. Ann N Y Acad Sci. 2005;1061:208–20. doi: 10.1196/annals.1336.023. [DOI] [PubMed] [Google Scholar]
- 26.Kumar TR. What have we learned about gonadotropin function from gonadotropin subunit and receptor knockout mice? Reproduction. 2005;130:293–302. doi: 10.1530/rep.1.00660. [DOI] [PubMed] [Google Scholar]
- 27.Marchetti C, Hamdane M, Mitchell V, Mayo K, Devisme L, et al. Immunolocalization of inhibin and activin alpha and betaB subunits and expression of corresponding messenger RNAs in the human adult testis. Biol Reprod. 2003;68:230–5. doi: 10.1095/biolreprod.102.004424. [DOI] [PubMed] [Google Scholar]
- 28.Ettore C. Male hypothalamic-pituitary-gonadal axis. In: Larry IL, Stuart SH, Craig SN, editors. Infertility in the Male. 4th ed. Cambridge, UK: Cambridge University Press; 2009. pp. 14–28. [Google Scholar]
- 29.MacIndoe JH, Perry PJ, Yates WR, Holman TL, Ellingrod VL, et al. Testosterone suppression of the HPT axis. J Investig Med. 1997;45:441–7. [PubMed] [Google Scholar]
- 30.Ly LP, Liu PY, Handelsman DJ. Rates of suppression and recovery of human sperm output in testosterone-based hormonal contraceptive regimens. Hum Reprod. 2005;20:1733–40. doi: 10.1093/humrep/deh834. [DOI] [PubMed] [Google Scholar]
- 31.Gu Y, Liang X, Wu W, Liu M, Song S, et al. Multicenter contraceptive efficacy trial of injectable testosterone undecanoate in Chinese men. J Clin Endocrinol Metab. 2009;94:1910–5. doi: 10.1210/jc.2008-1846. [DOI] [PubMed] [Google Scholar]
- 32.Manetti GJ, Honig SC. Update on male hormonal contraception: is the vasectomy in jeopardy? Int J Impot Res. 2010;22:159–70. doi: 10.1038/ijir.2010.2. [DOI] [PubMed] [Google Scholar]
- 33.de Souza GL, Hallak J. Anabolic steroids and male infertility: a comprehensive review. BJU Int. 2011;108:1860–5. doi: 10.1111/j.1464-410X.2011.10131.x. [DOI] [PubMed] [Google Scholar]
- 34.Rahnema CD, Lipshultz LI, Crosnoe LE, Kovac JR, Kim ED. Anabolic steroid-induced hypogonadism: diagnosis and treatment. Fertil Steril. 2014;101:1271–9. doi: 10.1016/j.fertnstert.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Boyadjiev NP, Georgieva KN, Massaldjieva RI, Gueorguiev SI. Reversible hypogonadism and azoospermia as a result of anabolic-androgenic steroid use in a bodybuilder with personality disorder. A case report. J Sports Med Phys Fitness. 2000;40:271–4. [PubMed] [Google Scholar]
- 36.Turek PJ, Williams RH, Gilbaugh JH, 3rd, Lipshultz LI. The reversibility of anabolic steroid-induced azoospermia. J Urol. 1995;153:1628–30. [PubMed] [Google Scholar]
- 37.Gazvani MR, Buckett W, Luckas MJ, Aird IA, Hipkin LJ, et al. Conservative management of azoospermia following steroid abuse. Hum Reprod. 1997;12:1706–8. doi: 10.1093/humrep/12.8.1706. [DOI] [PubMed] [Google Scholar]
- 38.Liu PY, Turner L, Rushford D, McDonald J, Baker HW, et al. Efficacy and safety of recombinant human follicle stimulating hormone (Gonal-F) with urinary human chorionic gonadotrophin for induction of spermatogenesis and fertility in gonadotrophin-deficient men. Hum Reprod. 1999;14:1540–5. doi: 10.1093/humrep/14.6.1540. [DOI] [PubMed] [Google Scholar]
- 39.Trinchard-Lugan I, Khan A, Porchet HC, Munafo A. Pharmacokinetics and pharmacodynamics of recombinant human chorionic gonadotrophin in healthy male and female volunteers. Reprod Biomed Online. 2002;4:106–15. doi: 10.1016/s1472-6483(10)61927-x. [DOI] [PubMed] [Google Scholar]
- 40.Lehert P, Schertz JC, Ezcurra D. Recombinant human follicle-stimulating hormone produces more oocytes with a lower total dose per cycle in assisted reproductive technologies compared with highly purified human menopausal gonadotrophin: a meta-analysis. Reprod Biol Endocrinol. 2010;8:112. doi: 10.1186/1477-7827-8-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farhat R, Al-zidjali F, Alzahrani AS. Outcome of gonadotropin therapy for male infertility due to hypogonadotrophic hypogonadism. Pituitary. 2010;13:105–10. doi: 10.1007/s11102-009-0203-1. [DOI] [PubMed] [Google Scholar]
- 42.Buchter D, Behre HM, Kliesch S, Nieschlag E. Pulsatile GnRH or human chorionic gonadotropin/human menopausal gonadotropin as effective treatment for men with hypogonadotropic hypogonadism: a review of 42 cases. Eur J Endocrinol. 1998;139:298–303. doi: 10.1530/eje.0.1390298. [DOI] [PubMed] [Google Scholar]
- 43.Pitteloud N, Hayes FJ, Dwyer A, Boepple PA, Lee H, et al. Predictors of outcome of long-term GnRH therapy in men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002;87:4128–36. doi: 10.1210/jc.2002-020518. [DOI] [PubMed] [Google Scholar]
- 44.Rastrelli G, Corona G, Mannucci E, Maggi M. Factors affecting spermatogenesis upon gonadotropin-replacement therapy: a meta-analytic study. Andrology. 2014;2:794–808. doi: 10.1111/andr.262. [DOI] [PubMed] [Google Scholar]
- 45.Liu PY, Baker HW, Jayadev V, Zacharin M, Conway AJ, et al. Induction of spermatogenesis and fertility during gonadotropin treatment of gonadotropin-deficient infertile men: predictors of fertility outcome. J Clin Endocrinol Metab. 2009;94:801–8. doi: 10.1210/jc.2008-1648. [DOI] [PubMed] [Google Scholar]
- 46.Schaison G, Young J, Pholsena M, Nahoul K, Couzinet B. Failure of combined follicle-stimulating hormone-testosterone administration to initiate and/or maintain spermatogenesis in men with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 1993;77:1545–9. doi: 10.1210/jcem.77.6.8263139. [DOI] [PubMed] [Google Scholar]
- 47.Vicari E, Mongioi A, Calogero AE, Moncada ML, Sidoti G, et al. Therapy with human chorionic gonadotrophin alone induces spermatogenesis in men with isolated hypogonadotrophic hypogonadism – Long-term follow-up. Int J Androl. 1992;15:320–9. doi: 10.1111/j.1365-2605.1992.tb01131.x. [DOI] [PubMed] [Google Scholar]
- 48.Efficacy and safety of highly purified urinary follicle-stimulating hormone with human chorionic gonadotropin for treating men with isolated hypogonadotropic hypogonadism. European Metrodin HP Study Group. Fertil Steril. 1998;70:256–62. doi: 10.1016/s0015-0282(98)00156-3. [DOI] [PubMed] [Google Scholar]
- 49.Bouloux PM, Nieschlag E, Burger HG, Skakkebaek NE, Wu FC, et al. Induction of spermatogenesis by recombinant follicle-stimulating hormone (puregon) in hypogonadotropic azoospermic men who failed to respond to human chorionic gonadotropin alone. J Androl. 2003;24:604–11. doi: 10.1002/j.1939-4640.2003.tb02712.x. [DOI] [PubMed] [Google Scholar]
- 50.Burger HG, Baker HW. Therapeutic considerations and results of gonadotropin treatment in male hypogonadotropic hypogonadism. Ann N Y Acad Sci. 1984;438:447–53. doi: 10.1111/j.1749-6632.1984.tb38305.x. [DOI] [PubMed] [Google Scholar]
- 51.Burgues S, Calderon MD. Subcutaneous self-administration of highly purified follicle stimulating hormone and human chorionic gonadotrophin for the treatment of male hypogonadotrophic hypogonadism. Spanish Collaborative Group on Male Hypogonadotropic Hypogonadism. Hum Reprod. 1997;12:980–6. doi: 10.1093/humrep/12.5.980. [DOI] [PubMed] [Google Scholar]
- 52.Ishikawa T, Ooba T, Kondo Y, Yamaguchi K, Fujisawa M. Assessment of gonadotropin therapy in male hypogonadotropic hypogonadism. Fertil Steril. 2007;88:1697–9. doi: 10.1016/j.fertnstert.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 53.Miyagawa Y, Tsujimura A, Matsumiya K, Takao T, Tohda A, et al. Outcome of gonadotropin therapy for male hypogonadotropic hypogonadism at university affiliated male infertility centers: a 30-year retrospective study. J Urol. 2005;173:2072–5. doi: 10.1097/01.ju.0000158133.09197.f4. [DOI] [PubMed] [Google Scholar]
- 54.Burris AS, Clark RV, Vantman DJ, Sherins RJ. A low sperm concentration does not preclude fertility in men with isolated hypogonadotropic hypogonadism after gonadotropin therapy. Fertil Steril. 1988;50:343–7. doi: 10.1016/s0015-0282(16)60084-5. [DOI] [PubMed] [Google Scholar]
- 55.Liu PY, Gebski VJ, Turner L, Conway AJ, Wishart SM, et al. Predicting pregnancy and spermatogenesis by survival analysis during gonadotrophin treatment of gonadotrophin-deficient infertile men. Hum Reprod. 2002;17:625–33. doi: 10.1093/humrep/17.3.625. [DOI] [PubMed] [Google Scholar]
- 56.Coviello AD, Matsumoto AM, Bremner WJ, Herbst KL, Amory JK, et al. Low-dose human chorionic gonadotropin maintains intratesticular testosterone in normal men with testosterone-induced gonadotropin suppression. J Clin Endocrinol Metab. 2005;90:2595–602. doi: 10.1210/jc.2004-0802. [DOI] [PubMed] [Google Scholar]
- 57.Depenbusch M, von Eckardstein S, Simoni M, Nieschlag E. Maintenance of spermatogenesis in hypogonadotropic hypogonadal men with human chorionic gonadotropin alone. Eur J Endocrinol. 2002;147:617–24. doi: 10.1530/eje.0.1470617. [DOI] [PubMed] [Google Scholar]
- 58.Hsieh TC, Pastuszak AW, Hwang K, Lipshultz LI. Concomitant intramuscular human chorionic gonadotropin preserves spermatogenesis in men undergoing testosterone replacement therapy. J Urol. 2013;189:647–50. doi: 10.1016/j.juro.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 59.Wenker EP, Dupree JM, Langille GM, Kovac J, Ramasamy R, et al. The use of HCG-based combination therapy for recovery of spermatogenesis after testosterone use. J Sex Med. 2015;12:1334–7. doi: 10.1111/jsm.12890. [DOI] [PubMed] [Google Scholar]
- 60.Coward RM, Mata DA, Smith RP, Kovac JR, Lipshultz LI. Vasectomy reversal outcomes in men previously on testosterone supplementation therapy. Urology. 2014;84:1335–40. doi: 10.1016/j.urology.2014.06.081. [DOI] [PubMed] [Google Scholar]
- 61.Gill GV. Anabolic steroid induced hypogonadism treated with human chorionic gonadotropin. Postgrad Med J. 1998;74:45–6. doi: 10.1136/pgmj.74.867.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Menon DK. Successful treatment of anabolic steroid-induced azoospermia with human chorionic gonadotropin and human menopausal gonadotropin. Fertil Steril. 2003;79(Suppl 3):1659–61. doi: 10.1016/s0015-0282(03)00365-0. [DOI] [PubMed] [Google Scholar]
- 63.Goldstein SR, Siddhanti S, Ciaccia AV, Plouffe L., Jr A pharmacological review of selective oestrogen receptor modulators. Hum Reprod Update. 2000;6:212–24. doi: 10.1093/humupd/6.3.212. [DOI] [PubMed] [Google Scholar]
- 64.Kim ED, Crosnoe L, Bar-Chama N, Khera M, Lipshultz LI. The treatment of hypogonadism in men of reproductive age. Fertil Steril. 2013;99:718–24. doi: 10.1016/j.fertnstert.2012.10.052. [DOI] [PubMed] [Google Scholar]
- 65.Shelly W, Draper MW, Krishnan V, Wong M, Jaffe RB. Selective estrogen receptor modulators: an update on recent clinical findings. Obstet Gynecol Surv. 2008;63:163–81. doi: 10.1097/OGX.0b013e31816400d7. [DOI] [PubMed] [Google Scholar]
- 66.Mellinger RC, Thompson RJ. The effect of clomiphene citrate in male infertility. Fertil Steril. 1966;17:94–103. doi: 10.1016/s0015-0282(16)35830-7. [DOI] [PubMed] [Google Scholar]
- 67.Micic S, Dotlic R. Evaluation of sperm parameters in clinical trial with clomiphene citrate of oligospermic men. J Urol. 1985;133:221–2. doi: 10.1016/s0022-5347(17)48889-6. [DOI] [PubMed] [Google Scholar]
- 68.Check JH, Chase JS, Nowroozi K, Wu CH, Adelson HG. Empirical therapy of the male with clomiphene in couples with unexplained infertility. Int J Fertil. 1989;34:120–2. [PubMed] [Google Scholar]
- 69.Katz DJ, Nabulsi O, Tal R, Mulhall JP. Outcomes of clomiphene citrate treatment in young hypogonadal men. BJU Int. 2012;110:573–8. doi: 10.1111/j.1464-410X.2011.10702.x. [DOI] [PubMed] [Google Scholar]
- 70.Shabsigh A, Kang Y, Shabsign R, Gonzalez M, Liberson G, et al. Clomiphene citrate effects on testosterone/estrogen ratio in male hypogonadism. J Sex Med. 2005;2:716–21. doi: 10.1111/j.1743-6109.2005.00075.x. [DOI] [PubMed] [Google Scholar]
- 71.Taylor F, Levine L. Clomiphene citrate and testosterone gel replacement therapy for male hypogonadism: efficacy and treatment cost. J Sex Med. 2010;7:269–76. doi: 10.1111/j.1743-6109.2009.01454.x. [DOI] [PubMed] [Google Scholar]
- 72.Ghanem H, Shaeer O, El-Segini A. Combination clomiphene citrate and antioxidant therapy for idiopathic male infertility: a randomized controlled trial. Fertil Steril. 2010;93:2232–5. doi: 10.1016/j.fertnstert.2009.01.117. [DOI] [PubMed] [Google Scholar]
- 73.Hussein A, Ozgok Y, Ross L, Niederberger C. Clomiphene administration for cases of nonobstructive azoospermia: a multicenter study. J Androl. 2005;26:787–91. doi: 10.2164/jandrol.04180. [DOI] [PubMed] [Google Scholar]
- 74.Liu PY, Handelsman DJ. The present and future state of hormonal treatment for male infertility. Hum Reprod Update. 2003;9:9–23. doi: 10.1093/humupd/dmg002. [DOI] [PubMed] [Google Scholar]
- 75.Kaminetsky J, Hemani ML. Clomiphene citrate and enclomiphene for the treatment of hypogonadal androgen deficiency. Expert Opin Investig Drugs. 2009;18:1947–55. doi: 10.1517/13543780903405608. [DOI] [PubMed] [Google Scholar]
- 76.Moskovic DJ, Katz DJ, Akhavan A, Park K, Mulhall JP. Clomiphene citrate is safe and effective for long-term management of hypogonadism. BJU Int. 2012;110:1524–8. doi: 10.1111/j.1464-410X.2012.10968.x. [DOI] [PubMed] [Google Scholar]
- 77.Pasqualotto FF, Fonseca GP, Pasqualotto EB. Azoospermia after treatment with clomiphene citrate in patients with oligospermia. Fertil Steril. 2008;90:2014.e11–2. doi: 10.1016/j.fertnstert.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 78.Bickelman C, Ferries L, Eaton RP. Impotence related to anabolic steroid use in a body builder. Response to clomiphene citrate. West J Med. 1995;162:158–60. [PMC free article] [PubMed] [Google Scholar]
- 79.Tan RS, Vasudevan D. Use of clomiphene citrate to reverse premature andropause secondary to steroid abuse. Fertil Steril. 2003;79:203–5. doi: 10.1016/s0015-0282(02)04550-8. [DOI] [PubMed] [Google Scholar]
- 80.Whitten SJ, Nangia AK, Kolettis PN. Select patients with hypogonadotropic hypogonadism may respond to treatment with clomiphene citrate. Fertil Steril. 2006;86:1664–8. doi: 10.1016/j.fertnstert.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 81.Hill S, Arutchelvam V, Quinton R. Enclomiphene, an estrogen receptor antagonist for the treatment of testosterone deficiency in men. IDrugs. 2009;12:109–19. [PubMed] [Google Scholar]
- 82.Kaminetsky J, Werner M, Fontenot G, Wiehle RD. Oral enclomiphene citrate stimulates the endogenous production of testosterone and sperm counts in men with low testosterone: comparison with testosterone gel. J Sex Med. 2013;10:1628–35. doi: 10.1111/jsm.12116. [DOI] [PubMed] [Google Scholar]
- 83.Wiehle R, Cunningham GR, Pitteloud N, Wike J, Hsu K, et al. Testosterone restoration by enclomiphene citrate in men with secondary hypogonadism: pharmacodynamics and pharmacokinetics. BJU Int. 2013 doi: 10.1111/bju.12363. doi: 10.1111/bju.12363, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wiehle RD, Fontenot GK, Wike J, Hsu K, Nydell J, et al. Enclomiphene citrate stimulates testosterone production while preventing oligospermia: a randomized phase II clinical trial comparing topical testosterone. Fertil Steril. 2014;102:720–7. doi: 10.1016/j.fertnstert.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 85.Clark RV, Sherins RJ. Treatment of men with idiopathic oligozoospermic infertility using the aromatase inhibitor, testolactone. Results of a double-blinded, randomized, placebo-controlled trial with crossover. J Androl. 1989;10:240–7. doi: 10.1002/j.1939-4640.1989.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 86.Raman JD, Schlegel PN. Aromatase inhibitors for male infertility. J Urol. 2002;167:624–9. doi: 10.1016/S0022-5347(01)69099-2. [DOI] [PubMed] [Google Scholar]
- 87.Cakan M, Aldemir M, Topcuoglu M, Altug U. Role of testosterone/estradiol ratio in predicting the efficacy of tamoxifen citrate treatment in idiopathic oligoasthenoteratozoospermic men. Urol Int. 2009;83:446–51. doi: 10.1159/000251186. [DOI] [PubMed] [Google Scholar]
- 88.Ramasamy R, Ricci JA, Palermo GD, Gosden LV, Rosenwaks Z, et al. Successful fertility treatment for Klinefelter's syndrome. J Urol. 2009;182:1108–13. doi: 10.1016/j.juro.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 89.Saylam B, Efesoy O, Cayan S. The effect of aromatase inhibitor letrozole on body mass index, serum hormones, and sperm parameters in infertile men. Fertil Steril. 2011;95:809–11. doi: 10.1016/j.fertnstert.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 90.Helo S, Ellen J, Mechlin C, Feustel P, Grossman M, et al. A randomized prospective double-blind comparison trial of clomiphene citrate and anastrozole in raising testosterone in hypogonadal infertile men. J Sex Med. 2015;12:1761–9. doi: 10.1111/jsm.12944. [DOI] [PubMed] [Google Scholar]
- 91.Burnett-Bowie SA, Roupenian KC, Dere ME, Lee H, Leder BZ. Effects of aromatase inhibition in hypogonadal older men: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol. 2009;70:116–23. doi: 10.1111/j.1365-2265.2008.03327.x. [DOI] [PubMed] [Google Scholar]
- 92.Burnett-Bowie SA, McKay EA, Lee H, Leder BZ. Effects of aromatase inhibition on bone mineral density and bone turnover in older men with low testosterone levels. J Clin Endocrinol Metab. 2009;94:4785–92. doi: 10.1210/jc.2009-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]


