Abstract
As couples are increasingly delaying parenthood, the effect of the aging men and women on reproductive outcomes has been an area of increased interest. Advanced paternal age has been shown to independently affect the entire spectrum of male fertility as assessed by reductions in sperm quality and fertilization (both assisted and unassisted). Moreover, epidemiological data suggest that paternal age can lead to higher rates of adverse birth outcomes and congenital anomalies. Mounting evidence also suggests increased risk of specific pediatric and adult disease states ranging from cancer to behavioral traits. While disease states associated with advancing paternal age have been well described, consensus recommendations for neonatal screening have not been as widely implemented as have been with advanced maternal age.
Keywords: fertilization, paternal aging, semen quality
REPRODUCTIVE POTENTIAL
While the relation between female age and fecundity is well established, the effect of male age on reproductive potential is uncertain. Eskenazi et al. looked at a convenience sample of nearly 100 men from a national government laboratory and found a significant trend toward higher rate of abnormal semen parameters (i.e., volume, concentration, count, motility, and total progressively motile sperm) in older men.1 A meta-analysis examined up to 21 studies (varied based on semen endpoint) and demonstrated declines in volume of 3%–22%, motility of 3%–37%, and normal morphology of 4%–18% in men over 50.2 However, after reviewing 21 studies, no consistent pattern of change in sperm concentration was noted. Five studies showed a decline with age, six showed no change, eight showed an increase (0.3%–3.3% per year), and two showed a nonlinear relationship.
However, a more recent meta-analysis pooled data from 90 studies involving 93 839 patients.3 The authors reported that male age is associated with a decrease in semen volume, total sperm count, percent motility, percent progressive motility, and percent normal sperm. Moreover, the authors reported an increase in DNA fragmentation with age. Again, no consistent relationship was identified between male age and semen concentration. Thus, changes in sperm count reflect volume changes rather than concentration declines. They reported the greatest declines in sperm motility and DNA fragmentation.
However, a semen analysis provides only a crude measure of male fertility as demonstrated by data gathered as part of randomized clinical trial of intrauterine insemination and superovulation in the treatment of infertility from the National Cooperative Reproductive Medicine Network.4 Thus, other measures of male fertility have been studied. In a study of 2112 pregnant women attending antenatal clinics in England, Hassan and Killick demonstrated a longer time to pregnancy in older fathers. Indeed, the authors reported a 5-fold increase in time to pregnancy in men over 45.5
In addition, authors have explored assisted reproductive techniques to determine if and how paternal age may impact reproductive success. As with semen data, the literature is heterogeneous. Paulson and colleagues examined 441 donor egg cycles and found no effect of paternal age on fertilization rate or birth rate.6 In contrast, Frattarelli et al. examined 1023 donor oocyte cycles and identified lower live birth rates and higher miscarriage rates. In addition, there was an inverse association between paternal age and blastocyst formation.7 Robertshaw et al. also examined donor cycles and reported a decline in live birth rate with a corresponding increase in the spontaneous abortion and not pregnant rate.8
In addition to the donor oocyte model as a method to isolate the male contribution to IVF success, other authors have looked at other subgroups of IVF patients. de La Rochebrochard et al. looked at 1938 couples undergoing IVF for tubal disease and found a decline in pregnancy rates with older men.9 For example, the failure to conceive rose 70% in men older than 39 compared to those under 30.
A GENETIC BASIS FOR THE PATERNAL AGE EFFECT
Achondroplasia was the first genetic disease associated with advanced paternal age. As a common cause of dwarfism, achondroplasia is commonly caused by a sporadic mutation in the fibroblast growth factor receptor 3 (FGFR3) gene that leads to abnormal cartilage formation. In 1912, Weinberg published his observations on the inheritance of sporadic achondroplasia, noting that it had an increased incidence among last-born children of a siblingship. Then, in 1955, Penrose refined this observation to show that paternal age was the main variable influencing Weinberg's observation.10,11 Since that time, the epidemiologic, molecular, and epigenetic basis for the paternal age effect have been studied at length and a variety of diseases have been associated with paternal age.
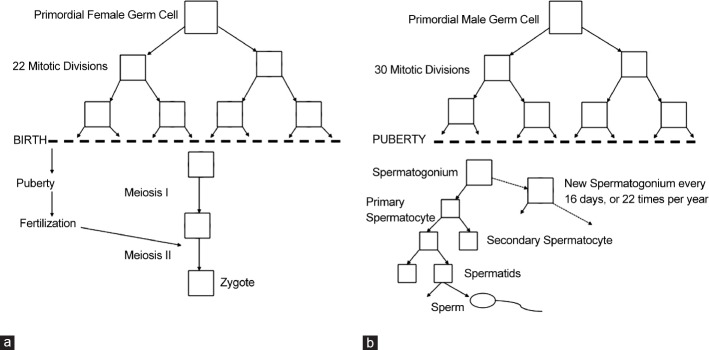
Based on the paternal bias in the origin of autosomal dominant disease, Penrose's “copy error” hypothesis was developed.11 Single base pair substitutions occurring more frequently in the paternal germline are responsible for sporadic cases of several diseases such as achondroplasia and Alpert syndrome. To explain the greater influence of paternal age compared to maternal age on the incidence of sporadic autosomal dominant congenital diseases, investigators have pointed to differences in gametogenesis between the sexes. Each oocyte produced by a female, regardless of her age, has undergone 22 germ-cell divisions and two meiotic divisions for a total of 23 chromosome replications (the final meiotic division does not require DNA replication). As a female grows older, her oocytes do not undergo any further DNA replication events. In contrast, spermatogenesis is continuous so that aging increases the number of chromosome replication events, which depends on the number of cell divisions (Figure 1). This leads to spermatic chromosomes that have undergone an estimated 35 replications by age 15, 150 by age 20, 380 by age 30, 610 by age 40, and 840 by age 50.12,13 With more cycles of DNA replication come more opportunities for copy error mutations, which may explain the higher rate of causative paternal mutation. In addition, it is important to note that elongated spermatids, immature, and mature spermatozoa do not have a DNA repair system leading to the persistence of DNA point mutations in the germline.14
Figure 1.
Female (a) and male (b) meiosis and DNA replication cycles.
In contrast to germline point mutations, aneuploidy is predominantly maternal in origin.15 Aneuploidy is defined as an abnormal number of chromosomes and may occur as a result of a mitotic or meiotic error. Indeed, as women age, higher rates of oocyte aneuploidy are found.16 The mechanisms of aneuploidy are likely multifactorial and are partially attributed to the missegregation of sister chromatids in Meiosis I of the oocyte.15 Trisomy 21 has been attributed to maternal gamete aneuploidy in 93% of cases.17 Other trisomies have been shown to have similar rates of maternal origin. The significant role that maternal aging plays in this process has been well described with the incidence of Trisomy 21 rising from 1/1250 in a 20-year-old female to 1/100 in a 40-year-old female. Determining the effect of advanced paternal age on aneuploidy has proven more difficult. Nevertheless, there appears to be an association. In a study by Zhu et al. on a Danish population, investigators found that men who were over 45 had a 4.50-fold (1.00–20.30) higher risk of siring a child with Trisomy 21 compared to men younger than 30.18 It is important to note that while maternal age was restricted to mothers who were 20–29 years of age, maternal age was not included in the study's final model.
Given the relatively low rates of trisomy and the confounding effect of maternal age, investigators have examined sperm DNA content directly to assess the effects of paternal aging. Indeed, Wyrobek et al. described an increase in sperm chromatin defects and DNA damage in aging men, postulating this as an explanation for impaired fertility in older men.19 Moreover, the same group noted higher rates of mutations in the FGFR3 gene in older men suggesting a 3% increase in the frequency of the mutation with each additional year of age. In contrast, other measures of DNA quality, such as high DNA instability and sperm aneuploidy or diploidy, did not appear to vary based on age. However, other studies show an increased incidence of sperm aneuploidly in men aged over 59 when compared to men under 40.20 The mechanisms underlying these observations are not completely understood.
Autosomal dominant
To date, the most well characterized paternal age effect disease is seen with sporadic autosomal dominant diseases. Perhaps, the best example is the family of disease affecting the fibroblast growth factor receptor 2 (FGFR2) and the fibroblast growth factor receptor 3 (FGFR3) namely Apert's syndrome and Achondroplasia, respectively. Apert's syndrome is characterized by acrocephalosyndactyly or malformations of the skull, face, hands, and feet. In a study by Wilkin et al., it was noted that all 40 patients (100%) affected with Achondroplasia had mutations in these receptor genes that were of paternal origin.21 The mutations are single base pair substitutions, and most cases are due to a glycine to arginine substitution at codon 1138 which leads to an alteration in the transmembrane domain of FGFR3 and the achondroplasia phenotype.
Interestingly, while men who are ages 50–54 have a 12-fold increased chance of having a child with achondroplasia, the relative risk of having sperm that carry the mutation is only 2-fold.22 This discrepancy is troubling for the hypothesis that the prevalence of autosomal dominant disease in the setting of APA is solely due to the increasing number of DNA replication cycles (Penrose's copy error hypothesis) in older males and suggests that there is more to understand about this observation.
A similar discrepancy is found in studies of Apert syndrome, in that the incidence of the phenotype increases exponentially with age starting at age 37.23 However, the incidence of the mutation in sperm only rises with approximately a 2.5-fold increase in incidence among men older than 65-year-old compared to men younger than 30.24 As with achondroplasia, over 60% of the cases are due to a single mutation leading to a Ser252Trp mutation, which affects the ligand binding to the receptor.25 Other diseases with phenotypes that have some similarities to Apert such as Crouzon and Pfeiffer's syndromes are also associated with increased paternal age and cause craniosynostoses and are associated with a mutation on the FGFR2 gene. Analyses of the genetic causes of Crouzon and Pfeiffer's syndromes show the mutations to be of paternal origin with the average age of men siring affected offspring almost 5 years older than the controls.26
As a group, mutations in the FGR have been the focus of more recent efforts to explain the biologic basis for the paternal age effect. While the copy number theory clearly overestimates the rate at which these mutations should lead to affected offspring, it has also been suggested that these mutations offer a selective advantage for the affected germ cells. Quantification of mutations associated with the paternal age effect in sperm and testes determined that these mutations may lead to clonal expansion of the affected cells. It has been further suggested that this occurs through the growth factor receptor-RAS signal transduction pathway.27 Further mapping of the testis affected by this mutation has shown that these mutations seem to be clustered rather than randomly distributed, further supporting the idea that the germline is affected only in certain spermatogonium and that there is a clonal expansion in these regions.28
Aneuploidy
Advanced paternal age has also been noted to affect aneuploidy in autosomes and sex chromosomes. The autosomal trisomies are difficult to study with respect to paternal age because of the strong influence of maternal age, especially given the fact that partner ages are often similar. As stated earlier, Zhu et al. examined a Danish cohort and reported an increasing risk of trisomy 21 with advancing paternal age.18
As described above, maternal origin of the aneuploidy is the predominant pattern in most trisomy, with the exception of sex chromosome aneuploidy, where the origin is of equal maternal and paternal frequency. In fact, there is a 160% increased incidence of having XY bearing sperm in 50-year-old fathers compared to fathers younger than 30.29
Cancer
There is evidence suggesting that the lifetime risk of cancers may be associated with both advanced paternal and maternal age. Not only is the overall incidence of cancer higher but also the onset of malignancies is earlier. Potential causes include increased damage to paternal DNA from prolonged environmental exposures, de novo germline mutations as hypothesized above, and aberrant epigenetic regulation that activates oncogenes or inactivates tumor suppressor genes.30,31,32 For instance, DNA methylation has been shown to be increased in newborns with advanced parental age suggesting that epigenetic mechanisms are corrupted by somatic aging.33 While some data support an increased rate of cancer among offspring conceived by older fathers, the data are conflicting.
Investigators from Sweden have explored birth and cancer registries to demonstrate a 25% increased risk of brain tumors in children born to fathers over 30 compared to those younger than 25.34 Importantly, many other solid and hematologic malignancies were also explored in this same study with no consistent relationship.
Childhood leukemia has a risk of 1 in 25 000 in the general population. Epidemiologic studies have shown that when paternal age is >35 years, this rate increases to 1 in 17 000. The same study calculates the relative risk of developing childhood leukemia of 1.5 for children of men aged 35 or older.35 Adult onset hematologic malignancies such as non-Hodgkin's lymphoma also have an association with advanced paternal age with a California database reporting a 50% increased risk for paternal age >40 compared to paternal age of 25.36 Similar observations have been made in the setting of central nervous system tumors of childhood.37
Breast and prostate cancers have been shown to have similar epidemiologic trends. Using data from the Framingham study, Zhang et al. noted that the incidence rate of prostate cancer increased from 1.70 per 1000 person-years among sons in the lowest quartile of paternal age (paternal age <27) to 2.00 (27≤ paternal age <32), 2.32 (32≤ paternal age <38), and 2.74 (paternal age ≥38) among those of each increased paternal age category, respectively. Overall, the risk of prostate cancer in offspring increased 70% for sons sired by fathers 38 or over compared to those whose fathers were <27 at birth. Importantly, no relationship existed for maternal age.38 A Korean case-control study found that paternal age >40 increases a daughter's lifetime incidence of breast cancer with 1 in 5.3 compared to 1 in 8.5 if the father's age was <30. In addition to an increase in overall breast cancer incidence, investigators noted a 90% increased odds of earlier onset breast cancer for daughters of older fathers.39
Nevertheless, most of the data examining the relationship between paternal age and malignancies are conflicting without consistency among populations. The proposed hypotheses suggest that increased germline mutations and epigenetic dysregulation lead to higher cancer incidences. However, given the heterogeneity of the current literature, it is likely that the etiology is multifactorial and heavily influenced by external factors (i.e., environmental).
NEUROPSYCHICATRIC CONDITIONS
Perhaps the largest body of recent literature on advanced paternal age exists on its effect on behavioral and psychiatric conditions. While the evidence linking advanced paternal age and neuropsychiatric diseases is strong, especially in the realm of autism, it is not clear which is cause and which is effect. Does a germline mutation cause autism or are men who have children at an advanced age more likely to carry phenotypes on the autistic spectrum? Some have suggested that traits that lead to siring children at an advanced age, such as aloofness, shyness, and trouble communicating with women, may be in part responsible for the association of autism with advanced paternal age.40
Autism
Autism spectrum disorders include a broad range of entities. The three prominent characteristics of autism spectrum disorders (ASDs) are impairments in social interaction, communication and restricted, repetitive and stereotyped patterns of behavior, interests, and activities.
Reichenberg and colleagues examined a cohort of nearly 400 000 men and women born in Israel in the 1980s and found that paternal age above 40 increased the odds of having a child diagnosed with autism (Odds Ratio [OR]: 5.75, 95% CI: 2.65–12.46). Similar findings resulted from examining age as a continuous variable so that for each 10-year increase in age, the odds of siring an autistic child more than doubled after adjusting for sociodemographic and maternal factors (OR: 2.14, 95% CI: 1.44–3.16). Importantly, the same data were used in a model for advancing maternal age, which showed no significant effect.41
Another study compared autism to the previously noted sporadic congenital autosomal dominant disorders. Investigators stratified 677 patients with autism from 340 distinct families into either multiplex (having a family history of autism) or simplex (being the only known affected individual in a family). They then found that after controlling for sex, birth order, and family size, there was a linear association with advancing paternal age and probability of being a simplex family. This suggests a correlation with the autosomal dominant diseases that show associations with paternal age in their sporadic forms because of the increased rates of germline mutations.42 The understanding that germline mutations can affect the autistic phenotype was championed by Sebat and colleagues, who described a stronger than expected association of de novo copy number variations (CNVs) with autism in cases of sporadic autism.43 CNVs are alterations in the number of specific regions of DNA so that cells may contain deletions or duplications of given regions. The authors demonstrated that de novo mutations resulting in both gain and loss of DNA region copy number might explain some portion of ASD.
Using a population-based cohort of all individuals born in Sweden between 1973 and 2001, D’Onofrio and colleagues reported that advanced paternal age was associated with a higher risk of autism in the study population. Moreover, when performing a sibling comparison analysis, the authors reported a higher risk for autism (HR: 3.45, 95% CI: 1.62–7.33) for offspring of fathers 45 years and older compared to offspring born to men between 20- and 24-year-old.44
Certainly, the etiology of autism is more complicated than a base-pair substitution in a single gene. The genetics of autism spectrum disorders are likely quite complex involving genetic, epigenetic, and environmental factors. Nevertheless, its heritability is established as siblings of autistic children have a 25-fold increased risk of being diagnosed with ASD compared to the general population.43 Further evidence of the importance of genetics on ASD is revealed by examining the concordance rates of autism in monozygotic (60%–90%) and dizogotic (0%–10%) twins.45
Schizophrenia
Schizophrenia and the spectrum of schizophrenia disorders (i.e., schizoaffective personality disorder, schizotypal personality disorder, delusional disorders, or other psychoses) are also associated with older paternal age. In 2002, using over 12 000 participants from the Child Health and Development Study Cohort in the United States, investigators sought to determine the effect of paternal age on the diagnosis of a schizophrenia spectrum disorder. In an analysis of paternal age as a continuous variable in schizophrenia spectrum disorders, each 10 years increase in paternal age had an adjusted rate ratio of 1.86 (1.20–2.87).46 The results of this study were similar to an earlier study of Israeli subjects.47 Investigators examined over 750 000 Swedes and found that for each 10 years increase in paternal age, the risk of schizophrenia in the offspring increased by nearly 50% (Hazard Ratio: 1.47, 95% CI: 1.23–1.76). Interestingly, the association only existed in those with no family history of schizophrenia suggesting that the accumulation of de novo mutations in the sperm contributes to the risk of schizophrenia.48
Bipolar disorder
Bipolar disorder, a common severe mood disorder, is associated with significant comorbidities including suicidality and substance abuse. In a nested case–control study from Sweden that controlled for maternal age, 13 000 patients with bipolar disorder were identified and matched with five healthy control subjects by sex and year of birth. This study found that the offspring of men 55 years or older were 1.37 (1.02–1.84) times more likely to be diagnosed with bipolar disorder than if the age of the father was <25. Moreover, earlier age of disease onset was also associated with advanced paternal age. Interestingly, when analyzed in a similar fashion, maternal age had no significant effect.49
BEHAVIOR AND COGNITIVE ABILITY
In their analyses of a cohort from the US Collaborative Perinatal Project, Saha and colleagues have published two studies that elucidate the effect of advanced paternal age on neurocognitive and behavioral outcomes. One examined advanced paternal age and its effect on neurocognitive measures during childhood while the other showed that advanced paternal age was associated with more “externalizing” adverse behaviors (e.g., aggression, vandalism, and disruptive behavior). These researchers found that using five neurocognitive measures (Bayley scales, Stanford–Binet Intelligence Scale, Graham-Ernhart Block Sort Test, Wechsler Intelligence Scale for Children, and Wide Range Achievement Test) at various developmental ages revealed that children of men with advancing paternal age scored worse. This trend followed a linear pattern when children were grouped into paternal age groups in 5 years intervals. Interestingly, maternal age had the opposite effect.50 In their study of behavioral effects, it was found that for every 5 years increase in paternal age, the odds of higher “externalizing” behaviors was increased by 12% (OR = 1.12, 95% CI: 1.03–1.21; P < 0.0001).51
Data from Sweden also support the association between paternal age and academic struggles. Compared with offspring born to fathers 20–24 years old, offspring of fathers 45 years and older was at heightened risk of failing a grade (OR = 1.59, 95% CI: 1.37–1.85) and low educational attainment (OR = 1.70, 95% CI: 1.50–1.93) in within-sibling comparisons.44 The notion that cognitive decline exists in the setting of older fathers is not novel, as an animal study of rats from 1983 showed that rats with older fathers exhibited impaired learning and conditioning.52
CONCLUSIONS
Careful examination of the association between paternal age and diseases in the offspring reveals the variety of mechanisms which lead to phenotypic pathology from syndromes caused by single base-pair substitutions, genomic imprinting, and increasing numbers of trinucleotide repeats, to complex and poorly understood transmission of neuropsychiatric conditions. The implications for a society with aging fathers could be quite significant. As many of these diseases do not manifest until after reproductive age, these genomic and epigenetic mutations will be passed on to future generations. As specific genetic targets are identified, it is conceivable that some risks could be mitigated through preimplanation genetic diagnosis. However, while the relative risks of several of these disorders do increase with advanced paternal age, the absolute risk remains low and may render such strategies cost prohibitive. Currently, no specific treatment can be offered to older patients interested in reproduction, other than education, and encouraging couples to begin reproductive efforts earlier. However, some have considered “social freezing” of sperm to mitigate the risks of paternal aging.53
COMPETING INTERESTS
All authors declare no competing interests.
REFERENCES
- 1.Eskenazi B, Wyrobek AJ, Sloter E, Kidd SA, Moore L, et al. The association of age and semen quality in healthy men. Hum Reprod. 2003;18:447–54. doi: 10.1093/humrep/deg107. [DOI] [PubMed] [Google Scholar]
- 2.Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril. 2001;75:237–48. doi: 10.1016/s0015-0282(00)01679-4. [DOI] [PubMed] [Google Scholar]
- 3.Johnson SL, Dunleavy J, Gemmell NJ, Nakagawa S. Consistent age-dependent declines in human semen quality: a systematic review and meta-analysis. Ageing Res Rev. 2015;19:22–33. doi: 10.1016/j.arr.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388–93. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- 5.Hassan MA, Killick SR. Effect of male age on fertility: evidence for the decline in male fertility with increasing age. Fertil Steril. 2003;79(Suppl 3):1520–7. doi: 10.1016/s0015-0282(03)00366-2. [DOI] [PubMed] [Google Scholar]
- 6.Paulson RJ, Milligan RC, Sokol RZ. The lack of influence of age on male fertility. Am J Obstet Gynecol. 2001;184:818–22. doi: 10.1067/mob.2001.113852. [DOI] [PubMed] [Google Scholar]
- 7.Frattarelli JL, Miller KA, Miller BT, Elkind-Hirsch K, Scott RT., Jr Male age negatively impacts embryo development and reproductive outcome in donor oocyte assisted reproductive technology cycles. Fertil Steril. 2008;90:97–103. doi: 10.1016/j.fertnstert.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Robertshaw I, Khoury J, Abdallah ME, Warikoo P, Hofmann GE. The effect of paternal age on outcome in assisted reproductive technology using the ovum donation model. Reprod Sci. 2014;21:590–3. doi: 10.1177/1933719113506497. [DOI] [PubMed] [Google Scholar]
- 9.de La Rochebrochard E, de Mouzon J, Thepot F, Thonneau P. Fathers over 40 and increased failure to conceive: the lessons of in vitro fertilization in France. Fertil Steril. 2006;85:1420–4. doi: 10.1016/j.fertnstert.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 10.Weinberg W. The inheritance of hypopituitary dwarfism. Arch Rassenu Gesel Biol. 1912;9:710–18. [Google Scholar]
- 11.Penrose L. Paternal age and mutation. Lancet. 1955;269:312–13. doi: 10.1016/s0140-6736(55)92305-9. [DOI] [PubMed] [Google Scholar]
- 12.Vogel F, Motulsky AG. Human Genetics: Problems and Approaches. 3rd edition. New York: Springer; 1997. [Google Scholar]
- 13.Kong A, Frigge ML, Masson G, Besenbacher S, Sulem P, et al. Rate of de novo mutations and the importance of father's age to disease risk. Nature. 2012;488:471–5. doi: 10.1038/nature11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarín JJ, Brines J, Cano A. Long-term effects of delayed parenthood. Hum Reprod. 1998;13:2371–6. doi: 10.1093/humrep/13.9.2371. [DOI] [PubMed] [Google Scholar]
- 15.Yoon PW, Freeman SB, Sherman SL, Taft LF, Gu Y, et al. Advanced maternal age and the risk of Down syndrome characterized by the meiotic stage of chromosomal error: a population-based study. Am J Hum Genet. 1996;58:628–33. [PMC free article] [PubMed] [Google Scholar]
- 16.Kurahashi H, Tsutsumi M, Nishiyama S, Kogo H, Inagaki H, et al. Molecular basis of maternal age-related increase in oocyte aneuploidy. Congenit Anom (Kyoto) 2012;52:8–15. doi: 10.1111/j.1741-4520.2011.00350.x. [DOI] [PubMed] [Google Scholar]
- 17.Sartorelli EM, Mazzucatto LF, de Pina-Neto JM. Effect of paternal age on human sperm chromosomes. Fertil Steril. 2001;76:1119–23. doi: 10.1016/s0015-0282(01)02894-1. [DOI] [PubMed] [Google Scholar]
- 18.Zhu JL, Madsen KM, Vestergaard M, Olesen AV, Basso O, et al. Paternal age and congenital malformations. Hum Reprod. 2005;20:3173–7. doi: 10.1093/humrep/dei186. [DOI] [PubMed] [Google Scholar]
- 19.Wyrobek AJ, Eskenazi B, Young S, Arnheim N, Tiemann-Boege I, et al. Advancing age has differential effects on DNA damage, chromatin, integrity, gene mutations, and aneuploidies in sperm. Proc Natl Acad Sci U S A. 2006;103:9601–6. doi: 10.1073/pnas.0506468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassold T, Abruzzo M, Adkins K, Griffin D, Merrill M, et al. Human aneuploidy: incidence, origin, and etiology. Environ Mol Mutagen. 1996;28:167–75. doi: 10.1002/(SICI)1098-2280(1996)28:3<167::AID-EM2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 21.Wilkin DJ, Szabo JK, Cameron R, Henderson S, Bellus GA, et al. Mutations in fibroblast growth-factor receptor 3 in sporadic cases of achondroplasia occur exclusively on the paternally derived chromosome. Am J Hum Genet. 1998;63:711–6. doi: 10.1086/302000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiemann-Boege I, Navidi W, Grewal R, Cohn D, Eskenazi B, et al. The observed human sperm mutation frequency cannot explain the achondroplasia paternal age effect. Proc Natl Acad Sci U S A. 2002;99:14952–7. doi: 10.1073/pnas.232568699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Risch N, Reich EW, Wishnick MM, McCarthy JG. Spontaneous mutation and parental age in humans. Am J Hum Genet. 1987;41:218–48. [PMC free article] [PubMed] [Google Scholar]
- 24.Glaser RL, Broman KW, Schulman RL, Eskenazi B, Wyrobek AJ, et al. The paternal-age effect in Apert syndrome is due, in part, to the increased frequency of mutations in sperm. Am J Hum Genet. 2003;73:939–47. doi: 10.1086/378419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goriely A, McVean GA, Röjmyr M, Ingemarsson B, Wilkie AO. Evidence for selective advantage of pathogenic FGFR2 mutations in the male germ line. Science. 2003;301:643–6. doi: 10.1126/science.1085710. [DOI] [PubMed] [Google Scholar]
- 26.Glaser RL, Jiang W, Boyadjiev SA, Tran AK, Zachary AA, et al. Paternal origin of FGFR2 mutations in sporadic cases of Crouzon syndrome and Pfeiffer syndrome. Am J Hum Genet. 2000;66:768–77. doi: 10.1086/302831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goriely A, Wilkie AO. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet. 2012;90:175–200. doi: 10.1016/j.ajhg.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shinde DN, Elmer DP, Calabrese P, Boulanger J, Arnheim N, et al. New evidence for positive selection helps explain the paternal age effect observed in achondroplasia. Hum Mol Genet. 2013;22:4117–26. doi: 10.1093/hmg/ddt260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowe X, Eskenazi B, Nelson DO, Kidd S, Alme A, et al. Frequency of XY sperm increases with age in fathers of boys with Klinefelter syndrome. Am J Hum Genet. 2001;69:1046–54. doi: 10.1086/323763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh NP, Muller CH, Berger RE. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil Steril. 2003;80:1420–30. doi: 10.1016/j.fertnstert.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Crow JF. Development. There's something curious about paternal-age effects. Science. 2003;301:606–7. doi: 10.1126/science.1088552. [DOI] [PubMed] [Google Scholar]
- 32.Bennett-Baker PE, Wilkowski J, Burke DT. Age-associated activation of epigenetically repressed genes in the mouse. Genetics. 2003;165:2055–62. doi: 10.1093/genetics/165.4.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adkins RM, Thomas F, Tylavsky FA, Krushkal J. Parental ages and levels of DNA methylation in the newborn are correlated. BMC Med Genet. 2011;12:47. doi: 10.1186/1471-2350-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hemminki K, Kyyrönen P, Vaittinen P. Parental age as a risk factor of childhood leukemia and brain cancer in offspring. Epidemiology. 1999;10:271–5. [PubMed] [Google Scholar]
- 35.Murray L, McCarron P, Bailie K, Middleton R, Davey Smith G, et al. Association of early life factors and acute lymphoblastic leukaemia in childhood: historical cohort study. Br J Cancer. 2002;86:356–61. doi: 10.1038/sj.bjc.6600012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu Y, Ma H, Sullivan-Halley J, Henderson KD, Chang ET, et al. Parents’ ages at birth and risk of adult-onset hematologic malignancies among female teachers in California. Am J Epidemiol. 2010;171:1262–9. doi: 10.1093/aje/kwq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yip BH, Pawitan Y, Czene K. Parental age and risk of childhood cancers: a population-based cohort study from Sweden. Int J Epidemiol. 2006;35:1495–503. doi: 10.1093/ije/dyl177. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Kreger BE, Dorgan JF, Cupples LA, Myers RH, et al. Parental age at child's birth and son's risk of prostate cancer. The Framingham Study. Am J Epidemiol. 1999;150:1208–12. doi: 10.1093/oxfordjournals.aje.a009947. [DOI] [PubMed] [Google Scholar]
- 39.Choi JY, Lee KM, Park SK, Noh DY, Ahn SH, et al. Association of paternal age at birth and the risk of breast cancer in offspring: a case control study. BMC Cancer. 2005;5:143. doi: 10.1186/1471-2407-5-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puleo CM, Reichenberg A, Smith CJ, Kryzak LA, Silverman JM. Do autism-related personality traits explain higher paternal age in autism? Mol Psychiatry. 2008;13:243–4. doi: 10.1038/sj.mp.4002102. [DOI] [PubMed] [Google Scholar]
- 41.Reichenberg A, Gross R, Weiser M, Bresnahan M, Silverman J, et al. Advancing paternal age and autism. Arch Gen Psychiatry. 2006;63:1026–32. doi: 10.1001/archpsyc.63.9.1026. [DOI] [PubMed] [Google Scholar]
- 42.Puleo CM, Schmeidler J, Reichenberg A, Kolevzon A, Soorya LV, et al. Advancing paternal age and simplex autism. Autism. 2012;16:367–80. doi: 10.1177/1362361311427154. [DOI] [PubMed] [Google Scholar]
- 43.Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–9. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D’Onofrio BM, Rickert ME, Frans E, Kuja-Halkola R, Almqvist C, et al. Paternal age at childbearing and offspring psychiatric and academic morbidity. JAMA Psychiatry. 2014;71:432–8. doi: 10.1001/jamapsychiatry.2013.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geschwind DH. Advances in autism. Annu Rev Med. 2009;60:367–80. doi: 10.1146/annurev.med.60.053107.121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown AS, Schaefer CA, Wyatt RJ, Begg MD, Goetz R, et al. Paternal age and risk of schizophrenia in adult offspring. Am J Psychiatry. 2002;159:1528–33. doi: 10.1176/appi.ajp.159.9.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malaspina D, Harlap S, Fennig S, Heiman D, Nahon D, et al. Advancing paternal age and the risk of schizophrenia. Arch Gen Psychiatry. 2001;58:361–7. doi: 10.1001/archpsyc.58.4.361. [DOI] [PubMed] [Google Scholar]
- 48.Sipos A, Rasmussen F, Harrison G, Tynelius P, Lewis G, et al. Paternal age and schizophrenia: a population based cohort study. BMJ. 2004;329:1070. doi: 10.1136/bmj.38243.672396.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frans EM, Sandin S, Reichenberg A, Lichtenstein P, Långström N, et al. Advancing paternal age and bipolar disorder. Arch Gen Psychiatry. 2008;65:1034–40. doi: 10.1001/archpsyc.65.9.1034. [DOI] [PubMed] [Google Scholar]
- 50.Saha S, Barnett AG, Foldi C, Burne TH, Eyles DW, et al. Advanced paternal age is associated with impaired neurocognitive outcomes during infancy and childhood. PLoS Med. 2009;6:e40. doi: 10.1371/journal.pmed.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saha S, Barnett AG, Buka SL, McGrath JJ. Maternal age and paternal age are associated with distinct childhood behavioural outcomes in a general population birth cohort. Schizophr Res. 2009;115:130–5. doi: 10.1016/j.schres.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 52.Auroux M. Decrease of learning capacity in offspring with increasing paternal age in the rat. Teratology. 1983;27:141–8. doi: 10.1002/tera.1420270202. [DOI] [PubMed] [Google Scholar]
- 53.Campagne DM. Delayed childbearing: determining responsibilities for prime gamete quality. J Reprod Med. 2013;58:531–7. [PubMed] [Google Scholar]