Abstract
For men struggling to conceive with their partners, diagnostic tools are limited and often consist of only a standard semen analysis. This baseline test serves as a crude estimation of male fertility, leaving patients and clinicians in need of additional diagnostic biomarkers. Seminal fluid contains the highest concentration of molecules from the male reproductive glands, therefore, this review focuses on current and novel seminal biomarkers in certain male infertility scenarios, including natural fertility, differentiating azoospermia etiologies, and predicting assisted reproductive technique success. Currently available tests include antisperm antibody assays, DNA fragmentation index, sperm fluorescence in situ hybridization, and other historical sperm functional tests. The poor diagnostic ability of current assays has led to continued efforts to find more predictive biomarkers. Emerging research in the fields of genomics, epigenetics, proteomics, transcriptomics, and metabolomics holds promise for the development of novel male infertility biomarkers. Seminal protein-based assays of TEX101, ECM1, and ACRV1 are already available or under final development for clinical use. Additional panels of DNA, RNA, proteins, or metabolites are being explored as we attempt to understand the pathophysiologic processes of male infertility. Future ventures will need to continue data integration and validation for the development of clinically useful infertility biomarkers to aid in male infertility diagnosis, treatment, and counseling.
Keywords: assisted reproductive techniques, diagnosis, male infertility, semen
INTRODUCTION
A couple's inability to conceive after 1 year of regular unprotected intercourse clinically defines infertility, a condition which affects approximately 15% of the reproductive-aged population.1,2 A contributing male factor may be found in over half of cases with up to 40% of those being secondary to male factors alone.2,3 Male factor infertility is often characterized by abnormalities on semen analysis such as low or absent sperm counts and low motility. The diagnostic ability of available male investigative tools is limited, however, and likely underestimates the true prevalence of male factors in infertile couples.
Due to the high frequency of contributing male fertility problems, a thorough male evaluation should be performed early in a couple's investigation. The male fertility evaluation should include a thorough medical history and physical examination and at least two semen analyses (SAs), as recommended by the American Urological Association (AUA) best practice statement.4 While the history and physical examination may provide some clues, semen analyses serve as a baseline marker of male fecundity with data regarding sperm quantity and quality. Multiple SAs are necessary to establish a trend, as substantial variability may exist between samples secondary to biological and laboratory factors. Men should also be questioned carefully about possible confounding factors such as abstinence length, recent illnesses, or testicular heat exposure when interpreting semen analysis results.
Unfortunately, the “gold-standard” SA provides limited information and cannot discriminate fertile from infertile men on an individual basis. Widely overlapping ranges of semen parameters among these groups have left clinicians in search of better seminal biomarkers.5 The continued broadening of “normal” SA ranges by the World Health Organization (WHO) in its guidelines, now in their fifth iteration, will additionally classify more men who may be struggling to conceive with normal SA results and in search of alternative tests.6,7
Advancing research technology and techniques have allowed us to explore new potential biomarkers in the rapidly evolving fields of genomics, proteomics, transcriptomics, and metabolomics, together comprising the current “omics” era. Researchers are employing these novel methods to gather vast amounts of data on possible novel biomarkers of male fertility from blood plasma, urine, and semen. As a result of stringent cellular barriers between blood and the male reproductive organs, cell-free DNA, RNA and proteins typically found in the testicles and epididymis are absent or barely detectable in blood serum, however, and only appear in concentrated amounts in the semen.8
Several clinical areas in the field of male fertility are primed for development of seminal biomarkers due to the lack of alternative testing or need for invasive diagnostic procedures. This review will focus specifically on available seminal testing and advancements in biomarker research in the areas of natural male fertility, differentiating azoospermia etiologies, and predicting assisted reproductive technique (ART) success. Previous reviews have reported on general male health and fertility-related biomarkers;8,9 this review will serve as an update to prior reviews with specific focus on seminal markers in the clinical areas discussed.
NATURAL MALE FERTILITY
The fertility evaluation is typically initiated for couples who have been trying to conceive naturally for some period of time without success. They should first undergo an evaluation to determine any barriers or available treatments for their best opportunity to conceive naturally. As mentioned, the initial laboratory investigation of male infertility includes at least two semen analyses to establish a trend in reproductive potential. Prior studies revealed substantial variability in seminal parameters between and within male patients with >70% variability between total motile sperm counts in some men.10,11,12 Most guidelines suggest an initial two SAs while others have recommended three tests to provide a better overview of fecundity due to the intrinsic variability.4,13 Men should be counseled regarding ideal collection guidelines including abstinence period, quick delivery to the laboratory, and proper transportation temperature to produce consistent and accurate results. Ideally, the same laboratories should be used for multiple tests as significant inter-laboratory variability may exist.
Following appropriate collection, SA testing and interpretation should be completed in accordance with WHO 5th edition guidelines.6 Following incubation, qualitative observations of color and viscosity, and quantitative measurements of total ejaculate volume and pH are made. An unstained preparation of semen is used for manual quantification of sperm count and motility with further calculation of the total motile count (TMC). Sperm morphology may be assessed based on criteria of variable strictness with an additional stained preparation.6,14 Some laboratories will perform sperm viability testing with dye exclusion or hypotonic swelling tests to better characterize immotile sperm. Chemical testing of semen for micronutrients such as zinc, selenium, and carnitine may additionally be performed. Although there is some evidence demonstrating defects in sperm count or function with deficiencies in these micronutrients,15,16 testing may be unnecessary given that we routinely recommend multivitamin supplementation including these compounds to men undergoing a fertility evaluation.
SA reports should include, at the minimum, specimen measurements of ejaculate volume, sperm concentration, motility, TMC, and morphology with a comparison to 2010 WHO 5th edition reference values. A SA, though easily performed, is not as easily correlated to a man's fertility potential. The results do not directly measure fertility but rather serve as a surrogate, predicting the likelihood of achieving a pregnancy.17 When creating SA reference values, the WHO interpreted SA results from nearly 2000 fertile men and defined “normal” values at the 5th percentile, which indicates that a percentage of normally fertile men will have “abnormal” SA results.6 Among the array of semen parameters, sperm concentration and motility appear to most consistently correlate with fertility potential when comparing proven fertile and infertile male cohorts.5,18 Other semen parameters must be cautiously interpreted in the evaluation and treatment of individual patients.
Sperm quality tests
Given the limitations of the parameters reported on a standard semen analysis, other sperm quality assays were historically developed to aid further in the male infertility evaluation. One such historical test is the antisperm antibody (ASA) assay, which evaluates for the presence of immunoglobulins bound to a patient's sperm that may cause clumping with reduced sperm motility and function.19 Spermatozoa are normally located in an immunologically privileged site, protected from the systemic immune system via supporting cell tight junctions and physiologic processes which form the blood-testis barrier.20 Breaches in the form of trauma, surgery, or chronic obstruction may expose the germinal epithelium, evoking an immune response.21,22 Testing for surface ASAs can be completed with immunobead-binding or mixed antiglobulin reaction assays.23 The clinical ramifications of ASAs, however, remain controversial as they may not cause sperm agglutination and agglutination may be caused by factors other than ASAs.24 Results of extensive studies and reviews on the presence of ASAs have concluded that they ultimately have little to no correlation with semen quality or natural pregnancy rates.25,26 Additionally, attempted treatment of immunologic infertility with corticosteroids has shown no benefit in pregnancy rates in blinded trials.27,28
Another commonly reported assay, the DNA fragmentation index (DFI), measures sperm DNA integrity. Exposure to various cellular stress conditions may lead to broken or fragmented DNA, affecting fertilization and normal embryo development.29 The most frequently employed tests of sperm DNA damage are the Comet, terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL), sperm chromatin dispersion, and sperm chromatin structure assays (SCSAs).30 DNA fragmentation rates often correlate to SA parameters though a high abnormal DFI (>30%) may be found in up to 8% of infertile men with a normal SA, suggesting an adjunct role to the standard SA.31 In studies of natural pregnancy rates stratified by DFI, the rates of conception were statistically lower among couples with an elevated DFI.32,33,34,35 Additionally, an elevated DFI correlates to higher spontaneous miscarriage rates.36 While DNA integrity testing may, therefore, aid in natural pregnancy counseling, reported treatment options such as antioxidants have shown little clinical benefit in reducing DFI and improving pregnancy rates.4,37 The unclear prognostic ability of individual testing and possible treatment options has led the American Society of Reproductive Medicine (ASRM) to not routinely recommend DFI testing in infertile couples.38 We still recommend DFI testing in certain individual couples who are undecided regarding specific male infertility treatments such as varicocelectomy.
Reactive oxygen species (ROS) can produce an oxidative environment and have been implicated in DNA fragmentation. High seminal ROS levels may lead to spermatozoal damage or death with significantly higher levels found in infertile men.39 ROS cannot be eliminated, however, as a low concentration is required for critical steps in fertilization including capacitation, the acrosome reaction, and sperm-oocyte fusion.40 A lack of consensus on the physiologic versus pathologic ranges of seminal ROS has hampered its clinical applicability. In addition, studies demonstrating mild to no benefits with antioxidant therapy despite elevated ROS levels have prevented the ROS assay from becoming a routine clinical test for subfertile men.41,42
It has long been recognized that many cases of fertilization or implantation failure occur as a result of genetic imbalances, prompting the development of fluorescence in situ hybridization (FISH) testing for sperm aneuploidy, or an abnormal number of chromosomes. FISH utilizes fluorescent tags to specific DNA elements to identify aneuploidies which typically result from spermatogenic meiotic errors.43 The most commonly used tags report the frequency of numerical abnormalities involving chromosomes 13, 18, 21, X, and Y.44 Tags to other chromosomes and genetic loci are commercially available but not routinely used. Early studies of chromosomal numerical abnormalities established that most fertile men generally produce <2% aneuploid sperm.45,46 The clinical application of FISH has been studied in an array of infertile male populations including oligozoospermic, teratozoospermic, asthenozoospermic, and recurrent pregnancy loss.44,47 Although reduced SA parameters correlate with increased sperm aneuploidy rates, the cost of testing is somewhat prohibitive, and thus FISH tends to be used only in the most relevant clinical scenarios such as couples with recurrent miscarriages. Estimation of sperm aneuploidy for couples in this population may aid in patient counseling and treatment decisions, including in vitro fertilization (IVF) with preimplantation genetic determination or reproductive alternatives such as adoption or use of a sperm donor.
Sperm functional aspects have been previously studied as well including the sperm-mucus interaction, acrosome reaction (AR), and zona pellucida binding/penetration. The sperm-mucus interaction can be assessed with postcoital or in vitro tests although the ASRM no longer recommends postcoital testing due to poor reproducibility and patient inconvenience.48 While in vitro sperm-mucus assays may demonstrate cervical infertility, the most common treatment, barring any severe male factors, would be to proceed with intrauterine insemination (IUI) regardless of test results. Many clinicians will now forgo testing and proceed directly to IUI in appropriately-selected couples. Testing of the AR and zona binding/penetration will be further discussed in the “Predicting ART Success” section.
Molecular and epigenetic markers
More than being a simple carrier of the male genetic complement, spermatozoa supply an epigenetically-modified genome with RNA and protein components critical for fertilization and embryonic development. The sperm epigenome is characterized by DNA methylation, which modifies the genetic material, and extensive protamination, or DNA repackaging. Protamines 1 and 2 (P1 and P2) replace histones during spermatogenesis, leading to a more compact chromatin packaging structure necessary for sperm function.49 The relative concentrations of P1 and P2, normally equally expressed, may be abnormal in some groups of infertile men. Carrell and Liu found an undetectable P2 level in 17% of men requiring IVF with an associated reduced penetration capacity, possibly explaining their inability to conceive naturally.50 Among a comparison group of 50 fertile men, all had measurable P2 concentrations. Similarly, aberrant DNA methylation, often in the form of hypermethylation, in several genes has been implicated as a contributing factor in male infertility cases.51 The cAMP response element modulator (CREM) gene is one example where hypermethylation was found to correlate negatively with sperm concentration, motility, and normal morphology.52 Other groups evaluating abnormally increased methylation of imprinted loci, such as mesoderm specific transcript (MEST), have likewise noted associations with abnormal semen parameters and male factor infertility.53,54,55
Novel biomarker research has associated spermatozoal RNA elements with natural fertility success.56 In a pilot study comparing fertile to infertile men, Garrido et al. identified unique transcriptomes between cohorts, reporting 26 specific differentially expressed messenger RNAs.57 Next generation sequencing (NGS) has more recently been applied to identify a larger population of coding and noncoding RNAs including thousands of microRNAs.58,59 MicroRNAs are delivered to the oocyte at the time of fertilization and believed to be involved in regulation of early embryogenesis. Utilizing NGS allowed Jodar and colleagues to characterize a larger group of 648 sperm required elements (SREs) for male fecundity.60 Natural pregnancies (timed intercourse or intrauterine insemination) were achieved in 73% versus 27% of those couples with and without the full complement of SREs, respectively. A recent study by Salas-Huetos et al. similarly demonstrated differences between microRNA profiles between fertile and infertile men and drilled down further, finding defect-specific altered microRNA profiles in groups of infertile men with isolated oligozoospermia, asthenozoospermia, and teratozoospermia.61 With increasingly complex descriptions of the complete spermatozoal transcriptome including microRNAs and Piwi-interacting piRNAs, efforts to integrate this data are needed to understand better the information quickly becoming available. Characterization and validation of the data with collaborative efforts across groups will be needed before these biomarkers may be available in the clinic.
Semen proteomics
Seminal fluid is made up of a small volume of sperm with additional secretions from other male reproductive organs including the epididymides, seminal vesicles, prostate gland, and bulbourethral glands (Figure 1). The total sperm count constitutes only approximately 10% of the total ejaculate volume while the remaining 90%, referred to as the seminal plasma, contains a diverse molecular composition. The high concentration of tissue-specific proteins within the seminal plasma provides a rich source of potential biomarkers in male fertility evaluation.8 Proteomic and biomarker discovery technologies have advanced greatly, progressing from basic electrophoresis techniques to liquid chromatography and mass spectrometry platforms, enabling high-throughput identification of thousands of high-, medium- and low-abundance proteins. Original investigations of seminal protein biomarkers in the 1940s identified only four unique protein separations.62 In 2011, Batruch et al. identified over 2300 seminal proteins in groups of infertile and fertile men using modern mass spectrometry techniques.63 Researchers hope to develop natural fertility biomarker panels for male infertility diagnosis and treatment by studying these cohorts.
Figure 1.
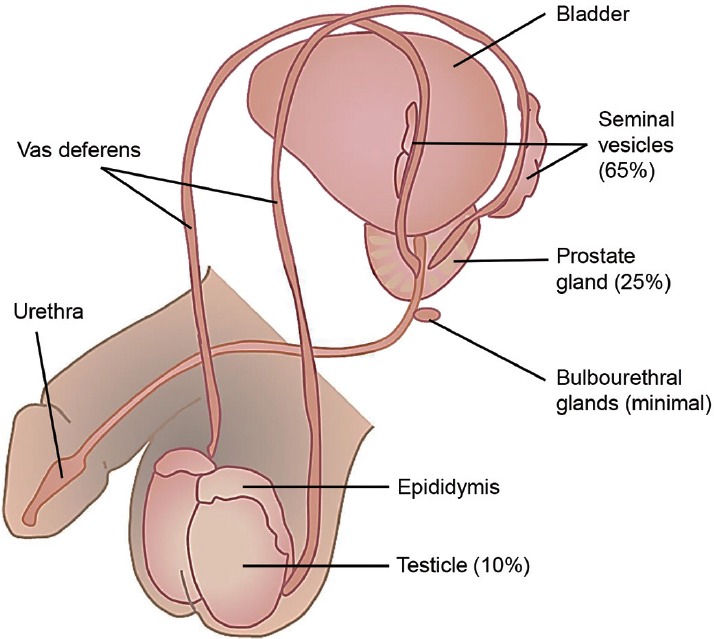
Male reproductive anatomy and relative contributions of individual organs to the total ejaculate volume (adapted from Drabovich et al.8 with permission).
Comparing seminal plasma protein levels to routine semen analysis findings has been performed by a number of groups. In a comparison of normal and asthenozoospermic (AS) men, Wang et al. reported an increased expression of 45 proteins and downregulation of 56 in the AS group.64 They identified significantly decreased expression of DJ-1-a, a protein implicated in oxidative stress regulation, in the AS men, which may indicate a loss of oxidative stress regulation in men with low sperm motility. Diamandis et al. chose a panel of candidate proteins to analyze in 202 ejaculates noting a positive correlation between seminal prostaglandin D synthase (PTGDS) concentration and SA concentration, motility, and normal morphology.65 Additionally, a progressive decline in PTGDS was seen from the normal to oligozoospermic to azoospermic to postvasectomy men, suggesting a relation to testicular obstruction. A similar study by Rolland et al. characterized the seminal proteomes among a group of men with normozoospermia, azoospermia, and postvasectomy, identifying a total of 699 proteins.66 They then compared testis-specific proteins identified on proteomic analysis, finding that transketolase-like protein 1 (TKTL1), L-lactate dehydrogenase C chain (LDHC), and phosphoglycerate kinase 2 (PGK2), which were easily detected in the normal SA men, were undetectable or barely detected in the azoospermia and postvasectomy groups. Azoospermic men separately demonstrated upregulation of eight proteins (fibronectin, prostatic acid phosphatase, prolactin-inducible protein, beta-2-microglobulin, proteasome subunit alpha type-3, galectin-3-binding protein, and cytosolic nonspecific dipeptidase) in another cohort comparison.67 Results of these studies augment the proteomic understanding of male subfertility but have yet to add diagnostic abilities above and beyond the currently-available semen analysis.
Milardi and colleagues took this approach one step further, evaluating the seminal protein profiles among a pool of five men with proven fertility and hoping to identify a set of commonly expressed proteins required for fecundity.68 Over 900 proteins were identified in each patient with 83 proteins present in samples from all men. The authors concluded that some of the proteins identified, such as olfactory receptor 5R1, lactoferrin, hCAP18, spindlin 1, and clusterin, may be required in male fertility. No subfertile population was available for comparison, however. Another small cohort study by Cadavid et al. observed increased concentrations of 10 seminal proteins in nine infertile men versus seven fertile individuals.69 Though they did not finalize the identification of the proteins, the group suggested that several of them are involved in the ubiquitination pathway. It is important to note that significant intra-group variation may exist in seminal protein concentrations, making the development and interpretation of future biomarker assays more difficult.70 Moving forward, big data studies involving multi-institutional cohorts will likely be needed to determine diagnostic panels of fertility required seminal proteins and adequately power their validation.
Proteins detected in the previously mentioned research studies represent the soluble protein composition of seminal plasma and do not account for the approximately 3% contained within secreted microvesicles, such as epididymosomes and prostasomes.71 This burgeoning area of male fertility research is just beginning to characterize the diversity of proteins contained within these membranous vesicles.72,73 In a mass spectrometry analysis of epididymosomes collected during vasovasostomy, Thimon and colleagues reported the presence of 146 individual intravesicular proteins.74 Further studies are being designed to determine the applicability of seminal secreted microvesicles in male disorders such as infertility, benign prostatic hyperplasia, and prostate cancer.75
DIFFERENTIATING AZOOSPERMIA ETIOLOGIES
Emerging proteomic technologies promised to discover biomarkers for the noninvasive diagnosis of multiple urological disorders and male infertility.76,77 This included differentiation of azoospermia forms, obstructive (OA) versus nonobstructive (NOA), and histopathological subtypes of the nonobstructive azoospermia (hypospermatogenesis, maturation arrest, and Sertoli cell-only syndrome), as well as prediction of sperm retrieval outcomes by surgical techniques.
In an attempt to diagnose azoospermia subtypes and predict sperm retrieval outcomes, multiple studies thoroughly examined a variety of blood serum proteins. Proteins studied included follicle-stimulating hormone,78 anti-Mόllerian hormone,79 and inhibin B.80 The most promising biomarker, follicle-stimulating hormone, had a sensitivity of only around 77% and specificity of 85% to predict spermatogenesis.81 Due to their relatively low diagnostic specificities and sensitivities, blood-based markers may require more invasive confirmatory testing methods.
In a search for biomarkers of azoospermia, recent proteomic studies also profiled thousands of semen and seminal plasma proteins, a number of which have already been mentioned above.63,82,83,84 Some protein biomarkers, such as PTGDS,85,86 ACRV1,87,88 LGALS3BP,89 ECM1,86 and TEX10186,90 were further verified and validated (Table 1). Lateral flow immunochromatographic assay of ACRV1 protein was recently implemented into commercially available home tests (SpermCheck Fertility® and SpermCheck Vasectomy®)87,88 while commercial TEX101- and ECM1-based immunodiagnostic assays are currently under development.91
Table 1.
Differentiating azoospermia etiologies with seminal plasma-based protein biomarkers
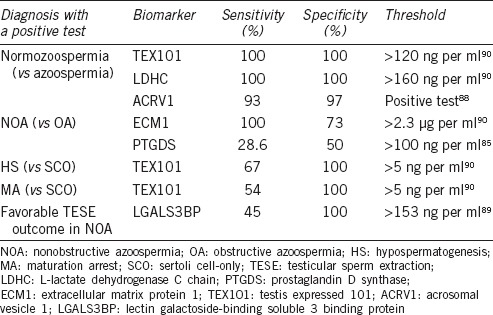
Even though some biomarkers already deliver near-absolute diagnostic sensitivities and specificities (Table 1), there is still a need for novel biomarkers with better diagnostic sensitivity to predict testicular sperm extraction (TESE) outcome in NOA patients and to differentiate between hypospermatogenesis, maturation arrest, and Sertoli cell-only syndrome. Accounting for etiologies of OA (absence of vas deference or physical obstruction of the vas deferens) and considering all the glands which secrete proteins into semen, we may suggest that epididymis-specific proteins would emerge as biomarkers for differentiation between NOA and OA. ECM1, a protein secreted into semen predominantly by epididymis supports this hypothesis.90 Likewise, proteins with a specific expression in testis, such as TEX101, would emerge as biomarkers for the prediction of TESE outcome and differentiation between NOA subtypes of hypospermatogenesis, maturation arrest, and Sertoli cell-only syndrome.90 Identification of both testis-specific and germ cell type-specific proteins secreted into semen exclusively by spermatocytes, spermatids, or spermatozoa should provide markers to accurately pinpoint the stage of spermatogenesis failure and thus predict TESE outcome with a better diagnostic performance.
It is also intriguing to speculate if any of the aforementioned protein biomarkers in seminal plasma (Table 1) would also be informative in the blood serum and thus facilitate blood-based diagnostics of male infertility and azoospermia. Careful consideration of the male urogenital anatomy reveals that all reproductive glands are sequestered from the systemic circulation by the stringent tissue-blood barriers and are thus immune privileged.20 As a result, reproductive gland-specific proteins are typically not found in the blood serum, and it is unlikely that seminal plasma-based biomarkers will be informative in blood. However, if male infertility is associated with the destruction of the blood-testis barrier due to inflammation, presence of testis-specific proteins in the blood can be assumed. In addition, such proteins leaked even in negligible amounts can lead to an autoimmune reaction, and the presence of the corresponding autoantibodies may facilitate the development of blood-based diagnostics of male infertility.92
PREDICTING ART SUCCESS
Assisted reproductive techniques employ artificial methods in an attempt to augment a couple's chances at conception and include intrauterine insemination and in vitro fertilization with or without intracytoplasmic sperm injection (ICSI). IUI may slightly improve pregnancy rates over natural attempts, especially if ovarian stimulation is added during a cycle.93 IVF/ICSI is often recommended for couples in certain clinical situations (e.g., severe oligozoospermia or Fallopian tube obstruction) or when IUI has failed. Despite the weighty costs and advanced technology of IVF/ICSI, success rates may remain lower than patients’ expectation. According to the Centers for Disease Control 2012 IVF data, only 36% and 29.4% of the IVF cycles resulted in pregnancies and live births, respectively.94 Age significantly impacts the success rates with live births resulting from 40% of IVF cycles in women <35-year-old compared to <10% of cycles in women 42 and older. Despite these limitations, over 150 000 IVF procedures were performed in the United States that year.95 In total, IVF procedures resulted in the birth of over 65 000 infants or 1.5% of all US childbirths. Some European countries have reported up to 5% of the live births conceived using assisted reproduction.96
Before ICSI, additional sperm tests were frequently used in an effort to assess the functional capacity of sperm to complete the final steps of fertilization necessary for IVF, namely capacitation, the acrosome reaction, and oocyte zona pellucida binding/penetration.97 Sperm capacitation normally occurs during transport through the female genital tract with the acrosome reaction taking place as the sperm approaches the oocyte.98 In vitro testing of this process may employ a calcium ionophore or natural agonists such as progesterone or zona pellucida proteins to induce the acrosome reaction and may aid in predicting fertilization success with IVF.99,100 The spermatozoa penetration assay (SPA) measures the ability of human spermatozoa to bind and penetrate a zona-free hamster oocyte, thereby collectively assessing capacitation, acrosome reaction, sperm-oocyte binding, penetration, and sperm chromatin decondensation.101 SPA was frequently used in the pre-ICSI era though interpretation of the test remained challenging. While a few may advocate for ICSI-SPA to directly assess chromatin decondensation,102 the clinical application of ICSI has largely replaced the need for SPA testing.
As previously discussed, ASA testing provided little benefit in predicting natural pregnancy rates. Additional reviews of ASA presence among couples undergoing IVF with or without ICSI revealed no relationship to ART success rates.103 DFI, on the other hand, was found to have a minimal but significant negative correlation with IVF results among meta-analyses, although individual studies demonstrate a great deal of heterogeneity.104,105,106 The small predictive value of DFI with ART may not be clinically significant enough to warrant DFI testing in all couples undergoing IVF. ICSI pregnancy rates, on the other hand, do not appear to be affected by DNA fragmentation levels.107 There is also evidence that couples with an elevated sperm DFI may be at a greater risk of pregnancy loss with IVF and ICSI.108 These studies collectively suggest that bypassing natural barriers to conception with IVF/ICSI may improve pregnancy rates among couples with an elevated DFI, but concerns are now being expressed about possible genomic effects on offspring.109 We need to adequately capture the results of DFI testing, IVF outcomes, and birth defects/developmental outcomes in properly-collected databases to determine the true implications of using sperm with compromised DNA for assisted reproductive techniques.
Seminal reactive oxygen species are often a causal factor in DNA fragmentation and, therefore, may additionally affect ART success rates. In a meta-analysis by Agarwal and colleagues, the seminal ROS concentration was found to inversely correlate with IVF fertilization rates.110 They concluded that assessing ROS level may aid in predicting ART success and counseling patients. A review of patients undergoing IVF or ICSI by Hammadeh et al. conversely found that, despite significantly higher ROS concentrations among the IVF cohort, fertilization rates were similar between groups.111 A better understanding of the “normal” and “abnormal” reference values is needed before additional studies will be able to determine the relationship between ROS as well as antioxidant therapies on assisted reproductive outcomes.
For couples failing multiple attempts at IVF/ICSI, additional testing with sperm fluorescence in situ hybridization may be warranted. Petit and colleagues noted elevated sperm aneuploidy rates among couples with repeated ICSI failures.112 Discovery of increased sperm chromosomal numerical abnormalities with FISH analysis may aid in counseling these couples regarding preimplantation genetic diagnosis or alternative reproductive options. Other genomic factors including the epigenome have been shown to impact assisted reproduction outcomes in small series. As previously discussed, protamines 1 and 2 are necessary for compact chromatin packaging and the two proteins are typically expressed in similar concentrations. In a review of men undergoing standard IVF, those with abnormal protamine 1/protamine 2 ratios were found to have reduced fertilization rates compared to men with a normal ratio.113 Significantly lower pregnancy rates were seen among men who had an abnormally reduced protamine ratio.
In addition to the genetic material it delivers to the oocyte at fertilization, the spermatozoon also provides a complement of proteins necessary for early embryo development. IVF pregnancy rates may thus be affected by the seminal plasma proteome, as reported by Zhu and colleagues.114 They analyzed the seminal proteome from 12 men, six who conceived with ART and six who did not. Out of a total of 2045 proteins identified, 21 were differentially expressed between the groups. With confirmatory analyses, three proteins (A2LD1, ATP1B3, and FBXO2) were shown to be significantly differently expressed. Azpiazu et al. similarly reviewed the seminal proteome in 31 men subdivided based on IVF success.115 They observed differential expression of 66 proteins with confirmatory testing of one protein, SRSF protein kinase 1. Based on functional clustering, many of the proteins were involved in lipoprotein metabolism and chromatin assembly. Again, additional validation of sperm and seminal plasma proteomes with respect to ART outcomes in big data multi-institutional cohorts may provide better diagnostic tools for counseling couples considering assisted reproduction.
FUTURE OF MALE INFERTILITY BIOMARKERS IN THE CLINIC
Recent research holds promising results for the development of novel, clinically useful male fertility biomarkers to better inform our patients and possibly avoid the need for more invasive testing. Several clinically relevant areas still need to be explored including markers that predict success of sperm retrieval and provide insight to the health of offspring conceived via ART with male factor infertility. Challenges will be faced in more advanced proteomic applications given the variety of chemically modified protein forms that can be present and the evolving proteome as ejaculated semen progresses from coagulated to liquefied states.116
As we have discovered, a number of promising molecules and panels are currently being explored to aid in the assessment of men's fertility potential, azoospermia etiologies, and predicting ART success. This research in the field of male fertility biomarkers has led to an accumulation of diverse genomics, proteomics, transcriptomics, and metabolomics data, as discussed in this review. Individual studies may over-simplify the human body by analyzing one “omics” field with resulting collections of disjointed individual data sets. A few groups are beginning to integrate data across multiple fields and will need to be a focus of collaborations moving forward to better define biomarkers of male fertility.117 With a better understanding of the pathophysiologic processes of male infertility, we can further translate the results of biomedical research into future clinical diagnostic tools. Additionally, though ELISA assays for many protein biomarkers are quite affordable ($2–5 per sample), further studies will need to address costs across the array of novel testing for cost-effectiveness comparisons to our standard semen analysis.118
AUTHOR CONTRIBUTIONS
The manuscript design, literature review, drafting, and final approval were performed by all authors.
COMPETING INTERESTS
All authors declare no competing financial interests.
REFERENCES
- 1.Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, et al. International committee for monitoring assisted reproductive technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92:1520–4. doi: 10.1016/j.fertnstert.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Mosher WD, Pratt WF. Fecundity and infertility in the United States: incidence and trends. Fertil Steril. 1991;56:192–3. [PubMed] [Google Scholar]
- 3.Thonneau P, Marchand S, Tallec A, Ferial ML, Ducot B, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989) Hum Reprod. 1991;6:811–6. doi: 10.1093/oxfordjournals.humrep.a137433. [DOI] [PubMed] [Google Scholar]
- 4.Jarow J, Sigman M, Kolettis PN, Lipshultz LR, McClure D, et al. The Optimal Evaluation of the Infertile Male: AUA Best Practice Statement. 2010. [Last accessed on 2015 Aug 03]. Availble from: https://www.auanet.org/common/pdf/education/clinical-guidance/Male-Infertility-d.pdf .
- 5.Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388–93. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- 6.5th ed. Geneva: World Health Organization; 2010. World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. [Google Scholar]
- 7.Murray KS, James A, McGeady JB, Reed ML, Kuang WW, et al. The effect of the new 2010 World Health Organization criteria for semen analyses on male infertility. Fertil Steril. 2012;98:1428–31. doi: 10.1016/j.fertnstert.2012.07.1130. [DOI] [PubMed] [Google Scholar]
- 8.Drabovich AP, Saraon P, Jarvi K, Diamandis EP. Seminal plasma as a diagnostic fluid for male reproductive system disorders. Nat Rev Urol. 2014;11:278–88. doi: 10.1038/nrurol.2014.74. [DOI] [PubMed] [Google Scholar]
- 9.Kovac JR, Pastuszak AW, Lamb DJ. The use of genomics, proteomics, and metabolomics in identifying biomarkers of male infertility. Fertil Steril. 2013;99:998–1007. doi: 10.1016/j.fertnstert.2013.01.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keel BA. Within- and between-subject variation in semen parameters in infertile men and normal semen donors. Fertil Steril. 2006;85:128–34. doi: 10.1016/j.fertnstert.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 11.Francavilla F, Barbonetti A, Necozione S, Santucci R, Cordeschi G, et al. Within-subject variation of seminal parameters in men with infertile marriages. Int J Androl. 2007;30:174–81. doi: 10.1111/j.1365-2605.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 12.Leushuis E, van der Steeg JW, Steures P, Repping S, Bossuyt PM, et al. Reproducibility and reliability of repeated semen analyses in male partners of subfertile couples. Fertil Steril. 2010;94:2631–5. doi: 10.1016/j.fertnstert.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Castilla JA, Alvarez C, Aguilar J, Gonzalez-Varea C, Gonzalvo MC, et al. Influence of analytical and biological variation on the clinical interpretation of seminal parameters. Hum Reprod. 2006;21:847–51. doi: 10.1093/humrep/dei423. [DOI] [PubMed] [Google Scholar]
- 14.Menkveld R, Stander FS, Kotze TJ, Kruger TF, van Zyl JA. The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod. 1990;5:586–92. doi: 10.1093/oxfordjournals.humrep.a137150. [DOI] [PubMed] [Google Scholar]
- 15.Taravati A, Tohidi F. Association between seminal plasma zinc level and asthenozoospermia: a meta-analysis study. Andrologia. 2015 doi: 10.1111/and.12494. doi: 10.1111/and.12494, Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Marzec-Wroblewska U, Kaminski P, Lakota P, Szymanski M, Wasilow K, et al. The employment of IVF techniques for establishment of sodium, copper and selenium impact upon human sperm quality. Reprod Fertil Dev. 2015 doi: 10.1071/RD15041. doi: 10.1071/RD15041, Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Jequier AM. Semen analysis: a new manual and its application to the understanding of semen and its pathology. Asian J Androl. 2010;12:11–3. doi: 10.1038/aja.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nallella KP, Sharma RK, Aziz N, Agarwal A. Significance of sperm characteristics in the evaluation of male infertility. Fertil Steril. 2006;85:629–34. doi: 10.1016/j.fertnstert.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 19.Wilson L. Sperm agglutinins in human semen and blood. Proc Soc Exp Biol Med. 1954;85:652–5. doi: 10.3181/00379727-85-20982. [DOI] [PubMed] [Google Scholar]
- 20.Mital P, Hinton BT, Dufour JM. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol Reprod. 2011;84:851–8. doi: 10.1095/biolreprod.110.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarow JP, Sanzone JJ. Risk factors for male partner antisperm antibodies. J Urol. 1992;148:1805–7. doi: 10.1016/s0022-5347(17)37034-9. [DOI] [PubMed] [Google Scholar]
- 22.Lee R, Goldstein M, Ullery BW, Ehrlich J, Soares M, et al. Value of serum antisperm antibodies in diagnosing obstructive azoospermia. J Urol. 2009;181:264–9. doi: 10.1016/j.juro.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Hellstrom WJ, Samuels SJ, Waits AB, Overstreet JW. A comparison of the usefulness of SpermMar and immunobead tests for the detection of antisperm antibodies. Fertil Steril. 1989;52:1027–31. doi: 10.1016/s0015-0282(16)53170-7. [DOI] [PubMed] [Google Scholar]
- 24.Chiu WW, Chamley LW. Clinical associations and mechanisms of action of antisperm antibodies. Fertil Steril. 2004;82:529–35. doi: 10.1016/j.fertnstert.2003.09.084. [DOI] [PubMed] [Google Scholar]
- 25.Munuce MJ, Berta CL, Pauluzzi F, Caille AM. Relationship between antisperm antibodies, sperm movement, and semen quality. Urol Int. 2000;65:200–3. doi: 10.1159/000064876. [DOI] [PubMed] [Google Scholar]
- 26.Leushuis E, van der Steeg JW, Steures P, Repping S, Schols W, et al. Immunoglobulin G antisperm antibodies and prediction of spontaneous pregnancy. Fertil Steril. 2009;92:1659–65. doi: 10.1016/j.fertnstert.2008.08.082. [DOI] [PubMed] [Google Scholar]
- 27.Haas GG, Jr, Manganiello P.A. double-blind, placebo-controlled study of the use of methylprednisolone in infertile men with sperm-associated immunoglobulins. Fertil Steril. 1987;47:295–301. [PubMed] [Google Scholar]
- 28.Bals-Pratsch M, Doren M, Karbowski B, Schneider HP, Nieschlag E. Cyclic corticosteroid immunosuppression is unsuccessful in the treatment of sperm antibody-related male infertility: a controlled study. Hum Reprod. 1992;7:99–104. doi: 10.1093/oxfordjournals.humrep.a137568. [DOI] [PubMed] [Google Scholar]
- 29.Aitken RJ, De Iuliis GN, Finnie JM, Hedges A, McLachlan RI. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: development of diagnostic criteria. Hum Reprod. 2010;25:2415–26. doi: 10.1093/humrep/deq214. [DOI] [PubMed] [Google Scholar]
- 30.Lewis SE, John Aitken R, Conner SJ, Iuliis GD, Evenson DP, et al. The impact of sperm DNA damage in assisted conception and beyond: recent advances in diagnosis and treatment. Reprod Biomed Online. 2013;27:325–37. doi: 10.1016/j.rbmo.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Zini A, Bielecki R, Phang D, Zenzes MT. Correlations between two markers of sperm DNA integrity, DNA denaturation and DNA fragmentation, in fertile and infertile men. Fertil Steril. 2001;75:674–7. doi: 10.1016/s0015-0282(00)01796-9. [DOI] [PubMed] [Google Scholar]
- 32.Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod. 1999;14:1039–49. doi: 10.1093/humrep/14.4.1039. [DOI] [PubMed] [Google Scholar]
- 33.Spano M, Bonde JP, Hjollund HI, Kolstad HA, Cordelli E, et al. Sperm chromatin damage impairs human fertility. The Danish First Pregnancy Planner Study Team. Fertil Steril. 2000;73:43–50. doi: 10.1016/s0015-0282(99)00462-8. [DOI] [PubMed] [Google Scholar]
- 34.Evenson DP, Wixon R. Data analysis of two in vivo fertility studies using sperm chromatin structure assay-derived DNA fragmentation index vs.pregnancy outcome. Fertil Steril. 2008;90:1229–31. doi: 10.1016/j.fertnstert.2007.10.066. [DOI] [PubMed] [Google Scholar]
- 35.Evenson D, Wixon R. Meta-analysis of sperm DNA fragmentation using the sperm chromatin structure assay. Reprod Biomed Online. 2006;12:466–72. doi: 10.1016/s1472-6483(10)62000-7. [DOI] [PubMed] [Google Scholar]
- 36.Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012;27:2908–17. doi: 10.1093/humrep/des261. [DOI] [PubMed] [Google Scholar]
- 37.Gharagozloo P, Aitken RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod. 2011;26:1628–40. doi: 10.1093/humrep/der132. [DOI] [PubMed] [Google Scholar]
- 38.Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril. 2015;103:e18–25. doi: 10.1016/j.fertnstert.2014.12.103. [DOI] [PubMed] [Google Scholar]
- 39.Agarwal A, Sharma RK, Nallella KP, Thomas AJ, Jr, Alvarez JG, et al. Reactive oxygen species as an independent marker of male factor infertility. Fertil Steril. 2006;86:878–85. doi: 10.1016/j.fertnstert.2006.02.111. [DOI] [PubMed] [Google Scholar]
- 40.Ko EY, Sabanegh ES, Jr, Agarwal A. Male infertility testing: reactive oxygen species and antioxidant capacity. Fertil Steril. 2014;102:1518–27. doi: 10.1016/j.fertnstert.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 41.Agarwal A, Nallella KP, Allamaneni SS, Said TM. Role of antioxidants in treatment of male infertility: an overview of the literature. Reprod Biomed Online. 2004;8:616–27. doi: 10.1016/s1472-6483(10)61641-0. [DOI] [PubMed] [Google Scholar]
- 42.Lombardo F, Sansone A, Romanelli F, Paoli D, Gandini L, et al. The role of antioxidant therapy in the treatment of male infertility: an overview. Asian J Androl. 2011;13:690–7. doi: 10.1038/aja.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarrate Z, Blanco J, Anton E, Egozcue S, Egozcue J, et al. FISH studies of chromosome abnormalities in germ cells and its relevance in reproductive counseling. Asian J Androl. 2005;7:227–36. doi: 10.1111/j.1745-7262.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 44.Hwang K, Weedin JW, Lamb DJ. The use of fluorescent in situ hybridization in male infertility. Ther Adv Urol. 2010;2:157–69. doi: 10.1177/1756287210373758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin RH, Balkan W, Burns K, Rademaker AW, Lin CC, et al. The chromosome constitution of 1000 human spermatozoa. Hum Genet. 1983;63:305–9. doi: 10.1007/BF00274750. [DOI] [PubMed] [Google Scholar]
- 46.Brandriff B, Gordon L, Ashworth L, Watchmaker G, Moore D, 2nd, et al. Chromosomes of human sperm: variability among normal individuals. Hum Genet. 1985;70:18–24. doi: 10.1007/BF00389451. [DOI] [PubMed] [Google Scholar]
- 47.Bernardini LM, Costa M, Bottazzi C, Gianaroli L, Magli MC, et al. Sperm aneuploidy and recurrent pregnancy loss. Reprod Biomed Online. 2004;9:312–20. doi: 10.1016/s1472-6483(10)62147-5. [DOI] [PubMed] [Google Scholar]
- 48.Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril. 2015;103:e44–50. doi: 10.1016/j.fertnstert.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 49.Carrell DT, Emery BR, Hammoud S. Altered protamine expression and diminished spermatogenesis: what is the link? Hum Reprod Update. 2007;13:313–27. doi: 10.1093/humupd/dml057. [DOI] [PubMed] [Google Scholar]
- 50.Carrell DT, Liu L. Altered protamine 2 expression is uncommon in donors of known fertility, but common among men with poor fertilizing capacity, and may reflect other abnormalities of spermiogenesis. J Androl. 2001;22:604–10. [PubMed] [Google Scholar]
- 51.Rajender S, Avery K, Agarwal A. Epigenetics, spermatogenesis and male infertility. Mutat Res. 2011;727:62–71. doi: 10.1016/j.mrrev.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Nanassy L, Carrell DT. Abnormal methylation of the promoter of CREM is broadly associated with male factor infertility and poor sperm quality but is improved in sperm selected by density gradient centrifugation. Fertil Steril. 2011;95:2310–4. doi: 10.1016/j.fertnstert.2011.03.096. [DOI] [PubMed] [Google Scholar]
- 53.Poplinski A, Tuttelmann F, Kanber D, Horsthemke B, Gromoll J. Idiopathic male infertility is strongly associated with aberrant methylation of MEST and IGF2/H19 ICR1. Int J Androl. 2010;33:642–9. doi: 10.1111/j.1365-2605.2009.01000.x. [DOI] [PubMed] [Google Scholar]
- 54.Hammoud SS, Purwar J, Pflueger C, Cairns BR, Carrell DT. Alterations in sperm DNA methylation patterns at imprinted loci in two classes of infertility. Fertil Steril. 2010;94:1728–33. doi: 10.1016/j.fertnstert.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 55.Kobayashi H, Sato A, Otsu E, Hiura H, Tomatsu C, et al. Aberrant DNA methylation of imprinted loci in sperm from oligospermic patients. Hum Mol Genet. 2007;16:2542–51. doi: 10.1093/hmg/ddm187. [DOI] [PubMed] [Google Scholar]
- 56.Platts AE, Dix DJ, Chemes HE, Thompson KE, Goodrich R, et al. Success and failure in human spermatogenesis as revealed by teratozoospermic RNAs. Hum Mol Genet. 2007;16:763–73. doi: 10.1093/hmg/ddm012. [DOI] [PubMed] [Google Scholar]
- 57.Garrido N, Martinez-Conejero JA, Jauregui J, Horcajadas JA, Simon C, et al. Microarray analysis in sperm from fertile and infertile men without basic sperm analysis abnormalities reveals a significantly different transcriptome. Fertil Steril. 2009;91:1307–10. doi: 10.1016/j.fertnstert.2008.01.078. [DOI] [PubMed] [Google Scholar]
- 58.Krawetz SA, Kruger A, Lalancette C, Tagett R, Anton E, et al. A survey of small RNAs in human sperm. Hum Reprod. 2011;26:3401–12. doi: 10.1093/humrep/der329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jodar M, Selvaraju S, Sendler E, Diamond MP, Krawetz SA, et al. The presence, role and clinical use of spermatozoal RNAs. Hum Reprod Update. 2013;19:604–24. doi: 10.1093/humupd/dmt031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jodar M, Sendler E, Moskovtsev SI, Librach CL, Goodrich R, et al. Absence of sperm RNA elements correlates with idiopathic male infertility. Sci Transl Med. 2015;7:295–6. doi: 10.1126/scitranslmed.aab1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salas-Huetos A, Blanco J, Vidal F, Godo A, Grossmann M, et al. Spermatozoa from patients with seminal alterations exhibit a differential micro-ribonucleic acid profile. Fertil Steril. 2015;104:591–601. doi: 10.1016/j.fertnstert.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 62.Gray S, Huggins C. Electrophoretic analysis of human semen. Exp Biol Med. 1942;50:351–3. [Google Scholar]
- 63.Batruch I, Lecker I, Kagedan D, Smith CR, Mullen BJ, et al. Proteomic analysis of seminal plasma from normal volunteers and post-vasectomy patients identifies over 2000 proteins and candidate biomarkers of the urogenital system. J Proteome Res. 2011;10:941–53. doi: 10.1021/pr100745u. [DOI] [PubMed] [Google Scholar]
- 64.Wang J, Wang J, Zhang HR, Shi HJ, Ma D, et al. Proteomic analysis of seminal plasma from asthenozoospermia patients reveals proteins that affect oxidative stress responses and semen quality. Asian J Androl. 2009;11:484–91. doi: 10.1038/aja.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diamandis EP, Arnett WP, Foussias G, Pappas H, Ghandi S, et al. Seminal plasma biochemical markers and their association with semen analysis findings. Urology. 1999;53:596–603. doi: 10.1016/s0090-4295(98)00550-0. [DOI] [PubMed] [Google Scholar]
- 66.Rolland AD, Lavigne R, Dauly C, Calvel P, Kervarrec C, et al. Identification of genital tract markers in the human seminal plasma using an integrative genomics approach. Hum Reprod. 2013;28:199–209. doi: 10.1093/humrep/des360. [DOI] [PubMed] [Google Scholar]
- 67.Davalieva K, Kiprijanovska S, Noveski P, Plaseski T, Kocevska B, et al. Proteomic analysis of seminal plasma in men with different spermatogenic impairment. Andrologia. 2012;44:256–64. doi: 10.1111/j.1439-0272.2012.01275.x. [DOI] [PubMed] [Google Scholar]
- 68.Milardi D, Grande G, Vincenzoni F, Messana I, Pontecorvi A, et al. Proteomic approach in the identification of fertility pattern in seminal plasma of fertile men. Fertil Steril. 2012;97:67–73. doi: 10.1016/j.fertnstert.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 69.Cadavid JA, Alvarez A, Markert UR, Cardona Maya W. Differential protein expression in seminal plasma from fertile and infertile males. J Hum Reprod Sci. 2014;7:206–11. doi: 10.4103/0974-1208.142485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamakawa K, Yoshida K, Nishikawa H, Kato T, Iwamoto T. Comparative analysis of interindividual variations in the seminal plasma proteome of fertile men with identification of potential markers for azoospermia in infertile patients. J Androl. 2007;28:858–65. doi: 10.2164/jandrol.107.002824. [DOI] [PubMed] [Google Scholar]
- 71.Ronquist G, Brody I. The prostasome: its secretion and function in man. Biochim Biophys Acta. 1985;822:203–18. doi: 10.1016/0304-4157(85)90008-5. [DOI] [PubMed] [Google Scholar]
- 72.Utleg AG, Yi EC, Xie T, Shannon P, White JT, et al. Proteomic analysis of human prostasomes. Prostate. 2003;56:150–61. doi: 10.1002/pros.10255. [DOI] [PubMed] [Google Scholar]
- 73.Poliakov A, Spilman M, Dokland T, Amling CL, Mobley JA. Structural heterogeneity and protein composition of exosome-like vesicles (prostasomes) in human semen. Prostate. 2009;69:159–67. doi: 10.1002/pros.20860. [DOI] [PubMed] [Google Scholar]
- 74.Thimon V, Frenette G, Saez F, Thabet M, Sullivan R. Protein composition of human epididymosomes collected during surgical vasectomy reversal: a proteomic and genomic approach. Hum Reprod. 2008;23:1698–707. doi: 10.1093/humrep/den181. [DOI] [PubMed] [Google Scholar]
- 75.Sandvig K, Llorente A. Proteomic analysis of microvesicles released by the human prostate cancer cell line PC-3. Mol Cell Proteomics. 2012;11:M111.012914. doi: 10.1074/mcp.M111.012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Drabovich AP, Martinez-Morillo E, Diamandis EP. Toward an integrated pipeline for protein biomarker development. Biochim Biophys Acta. 2015;1854:677–86. doi: 10.1016/j.bbapap.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 77.Drabovich AP, Pavlou MP, Batruch I, Diamandis EP. Proteomic and mass spectrometry technologies for protein biomarker discovery. In: Isaaq HJ, Veenstra TD, editors. Proteomic and Metabolomic Approaches to Biomarker Discovery. Amsterdam: Elsevier; 2013. p. 472. [Google Scholar]
- 78.Carpi A, Sabanegh E, Mechanick J. Controversies in the management of nonobstructive azoospermia. Fertil Steril. 2009;91:963–70. doi: 10.1016/j.fertnstert.2009.01.083. [DOI] [PubMed] [Google Scholar]
- 79.Tsametis C, Mintziori G, Iliadou PK, Tarlatzis BC, Papadimas I, et al. Dynamic endocrine test of inhibin B and anti-Mullerian hormone in men with non-obstructive azoospermia. Gynecol Endocrinol. 2011;27:661–5. doi: 10.3109/09513590.2010.521267. [DOI] [PubMed] [Google Scholar]
- 80.Muttukrishna S, Yussoff H, Naidu M, Barua J, Arambage K, et al. Serum anti-Mullerian hormone and inhibin B in disorders of spermatogenesis. Fertil Steril. 2007;88:516–8. doi: 10.1016/j.fertnstert.2006.11.110. [DOI] [PubMed] [Google Scholar]
- 81.Madani AH, Falahatkar S, Heidarzadeh A, Roshan ZA, Sazgari E, et al. Sensitivity and specificity of serum FSH and testis size in predicting the existence of spermatogenesis in azoospermic infertile men. Andrologia. 2012;44:205–9. doi: 10.1111/j.1439-0272.2010.01130.x. [DOI] [PubMed] [Google Scholar]
- 82.Batruch I, Smith CR, Mullen BJ, Grober E, Lo KC, et al. Analysis of seminal plasma from patients with non-obstructive azoospermia and identification of candidate biomarkers of male infertility. J Proteome Res. 2012;11:1503–11. doi: 10.1021/pr200812p. [DOI] [PubMed] [Google Scholar]
- 83.Pilch B, Mann M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol. 2006;7:R40. doi: 10.1186/gb-2006-7-5-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rolland AD, Lavigne R, Dauly C, Calvel P, Kervarrec C, et al. Identification of genital tract markers in the human seminal plasma using an integrative genomics approach. Hum Reprod. 2012;28:199–209. doi: 10.1093/humrep/des360. [DOI] [PubMed] [Google Scholar]
- 85.Heshmat SM, Mullen JB, Jarvi KA, Soosaipillai A, Diamandis EP, et al. Seminal plasma lipocalin-type prostaglandin D synthase: a potential new marker for the diagnosis of obstructive azoospermia. J Urol. 2008;179:1077–80. doi: 10.1016/j.juro.2007.10.070. [DOI] [PubMed] [Google Scholar]
- 86.Drabovich AP, Jarvi K, Diamandis EP. Verification of male infertility biomarkers in seminal plasma by multiplex selected reaction monitoring assay. Mol Cell Proteomics. 2011;10:M110.004127. doi: 10.1074/mcp.M110.004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coppola MA, Klotz KL, Kim KA, Cho HY, Kang J, et al. SpermCheck Fertility, an immunodiagnostic home test that detects normozoospermia and severe oligozoospermia. Hum Reprod. 2010;25:853–61. doi: 10.1093/humrep/dep413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Klotz KL, Coppola MA, Labrecque M, Brugh VM, 3rd, Ramsey K, et al. Clinical and consumer trial performance of a sensitive immunodiagnostic home test that qualitatively detects low concentrations of sperm following vasectomy. J Urol. 2008;180:2569–76. doi: 10.1016/j.juro.2008.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Freour T, Com E, Barriere P, Bouchot O, Jean M, et al. Comparative proteomic analysis coupled with conventional protein assay as a strategy to identify predictors of successful testicular sperm extraction in patients with non-obstructive azoospermia. Andrology. 2013;1:414–20. doi: 10.1111/j.2047-2927.2012.00059.x. [DOI] [PubMed] [Google Scholar]
- 90.Drabovich AP, Dimitromanolakis A, Saraon P, Soosaipillai A, Batruch I, et al. Differential diagnosis of azoospermia with proteomic biomarkers ECM1 and TEX101 quantified in seminal plasma. Sci Transl Med. 2013;5:212ra160. doi: 10.1126/scitranslmed.3006260. [DOI] [PubMed] [Google Scholar]
- 91.Korbakis D, Brinc D, Schiza C, Soosaipillai A, Jarvi K, et al. Immunocapture-selected reaction monitoring screening facilitates the development of ELISA for the measurement of native TEX101 in biological fluids. Mol Cell Proteomics. 2015;14:1517–26. doi: 10.1074/mcp.M114.047571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fijak M, Zeller T, Huys T, Klug J, Wahle E, et al. Autoantibodies against protein disulfide isomerase ER-60 are a diagnostic marker for low-grade testicular inflammation. Hum Reprod. 2014;29:2382–92. doi: 10.1093/humrep/deu226. [DOI] [PubMed] [Google Scholar]
- 93.Veltman-Verhulst SM, Cohlen BJ, Hughes E, Heineman MJ. Intra-uterine insemination for unexplained subfertility. Cochrane Database Syst Rev. 2012;9:CD001838. doi: 10.1002/14651858.CD001838.pub4. [DOI] [PubMed] [Google Scholar]
- 94.Atlanta, GA: US Dept of Health and Human Services; 2014. Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. 2012 Assisted Reproductive Technology National Summary Report. [Google Scholar]
- 95.Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, et al. Assisted reproductive technology surveillance – United States, 2012. MMWR Surveill Summ. 2015;64(Suppl 6):1–29. [PubMed] [Google Scholar]
- 96.de Mouzon J, Goossens V, Bhattacharya S, Castilla JA, Ferraretti AP, et al. Assisted reproductive technology in Europe, 2007: results generated from European registers by ESHRE. Hum Reprod. 2012;27:954–66. doi: 10.1093/humrep/des023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Muller CH. Rationale, interpretation, validation, and uses of sperm function tests. J Androl. 2000;21:10–30. [PubMed] [Google Scholar]
- 98.Zaneveld LJ, De Jonge CJ, Anderson RA, Mack SR. Human sperm capacitation and the acrosome reaction. Hum Reprod. 1991;6:1265–74. doi: 10.1093/oxfordjournals.humrep.a137524. [DOI] [PubMed] [Google Scholar]
- 99.Katsuki T, Hara T, Ueda K, Tanaka J, Ohama K. Prediction of outcomes of assisted reproduction treatment using the calcium ionophore-induced acrosome reaction. Hum Reprod. 2005;20:469–75. doi: 10.1093/humrep/deh636. [DOI] [PubMed] [Google Scholar]
- 100.Bronson RA, Peresleni T, Golightly M. Progesterone promotes the acrosome reaction in capacitated human spermatozoa as judged by flow cytometry and CD46 staining. Mol Hum Reprod. 1999;5:507–12. doi: 10.1093/molehr/5.6.507. [DOI] [PubMed] [Google Scholar]
- 101.Oehninger S, Franken DR, Ombelet W. Sperm functional tests. Fertil Steril. 2014;102:1528–33. doi: 10.1016/j.fertnstert.2014.09.044. [DOI] [PubMed] [Google Scholar]
- 102.Gvakharia MO, Lipshultz LI, Lamb DJ. Human sperm microinjection into hamster oocytes: a new tool for training and evaluation of the technical proficiency of intracytoplasmic sperm injection. Fertil Steril. 2000;73:395–401. doi: 10.1016/s0015-0282(99)00500-2. [DOI] [PubMed] [Google Scholar]
- 103.Zini A, Fahmy N, Belzile E, Ciampi A, Al-Hathal N, et al. Antisperm antibodies are not associated with pregnancy rates after IVF and ICSI: systematic review and meta-analysis. Hum Reprod. 2011;26:1288–95. doi: 10.1093/humrep/der074. [DOI] [PubMed] [Google Scholar]
- 104.Collins JA, Barnhart KT, Schlegel PN. Do sperm DNA integrity tests predict pregnancy with in vitro fertilization? Fertil Steril. 2008;89:823–31. doi: 10.1016/j.fertnstert.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 105.Zini A, Sigman M. Are tests of sperm DNA damage clinically useful? Pros and cons. J Androl. 2009;30:219–29. doi: 10.2164/jandrol.108.006908. [DOI] [PubMed] [Google Scholar]
- 106.Payne JF, Raburn DJ, Couchman GM, Price TM, Jamison MG, et al. Redefining the relationship between sperm deoxyribonucleic acid fragmentation as measured by the sperm chromatin structure assay and outcomes of assisted reproductive techniques. Fertil Steril. 2005;84:356–64. doi: 10.1016/j.fertnstert.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 107.Zini A. Are sperm chromatin and DNA defects relevant in the clinic? Syst Biol Reprod Med. 2011;57:78–85. doi: 10.3109/19396368.2010.515704. [DOI] [PubMed] [Google Scholar]
- 108.Zini A, Boman JM, Belzile E, Ciampi A. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: systematic review and meta-analysis. Hum Reprod. 2008;23:2663–8. doi: 10.1093/humrep/den321. [DOI] [PubMed] [Google Scholar]
- 109.Bungum M, Bungum L, Lynch KF, Wedlund L, Humaidan P, et al. Spermatozoa DNA damage measured by sperm chromatin structure assay (SCSA) and birth characteristics in children conceived by IVF and ICSI. Int J Androl. 2012;35:485–90. doi: 10.1111/j.1365-2605.2011.01222.x. [DOI] [PubMed] [Google Scholar]
- 110.Agarwal A, Allamaneni SS, Nallella KP, George AT, Mascha E. Correlation of reactive oxygen species levels with the fertilization rate after in vitro fertilization: a qualified meta-analysis. Fertil Steril. 2005;84:228–31. doi: 10.1016/j.fertnstert.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 111.Hammadeh ME, Al Hasani S, Rosenbaum P, Schmidt W, Fischer Hammadeh C. Reactive oxygen species, total antioxidant concentration of seminal plasma and their effect on sperm parameters and outcome of IVF/ICSI patients. Arch Gynecol Obstet. 2008;277:515–26. doi: 10.1007/s00404-007-0507-1. [DOI] [PubMed] [Google Scholar]
- 112.Petit FM, Frydman N, Benkhalifa M, Le Du A, Aboura A, et al. Could sperm aneuploidy rate determination be used as a predictive test before intracytoplasmic sperm injection? J Androl. 2005;26:235–41. doi: 10.1002/j.1939-4640.2005.tb01090.x. [DOI] [PubMed] [Google Scholar]
- 113.Aoki VW, Liu L, Jones KP, Hatasaka HH, Gibson M, et al. Sperm protamine 1/protamine 2 ratios are related to in vitro fertilization pregnancy rates and predictive of fertilization ability. Fertil Steril. 2006;86:1408–15. doi: 10.1016/j.fertnstert.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 114.Zhu Y, Wu Y, Jin K, Lu H, Liu F, et al. Differential proteomic profiling in human spermatozoa that did or did not result in pregnancy via IVF and AID. Proteomics Clin Appl. 2013;7:850–8. doi: 10.1002/prca.201200078. [DOI] [PubMed] [Google Scholar]
- 115.Azpiazu R, Amaral A, Castillo J, Estanyol JM, Guimera M, et al. High-throughput sperm differential proteomics suggests that epigenetic alterations contribute to failed assisted reproduction. Hum Reprod. 2014;29:1225–37. doi: 10.1093/humrep/deu073. [DOI] [PubMed] [Google Scholar]
- 116.Duncan MW, Thompson HS. Proteomics of semen and its constituents. Proteomics Clin Appl. 2007;1:861–75. doi: 10.1002/prca.200700228. [DOI] [PubMed] [Google Scholar]
- 117.Jodar M, Sendler E, Krawetz SA. The protein and transcript profiles of human semen. Cell Tissue Res. 2016;363:85–96. doi: 10.1007/s00441-015-2237-1. [DOI] [PubMed] [Google Scholar]
- 118.Detrick B, Hamilton RG, Folds JD. Manual of Molecular and Clinical Laboratory Immunology. 7th ed. Washington, DC: ASM Press; 2006. [Google Scholar]


