Abstract
Traditionally, testosterone and estrogen have been considered to be male and female sex hormones, respectively. However, estradiol, the predominant form of estrogen, also plays a critical role in male sexual function. Estradiol in men is essential for modulating libido, erectile function, and spermatogenesis. Estrogen receptors, as well as aromatase, the enzyme that converts testosterone to estrogen, are abundant in brain, penis, and testis, organs important for sexual function. In the brain, estradiol synthesis is increased in areas related to sexual arousal. In addition, in the penis, estrogen receptors are found throughout the corpus cavernosum with high concentration around neurovascular bundles. Low testosterone and elevated estrogen increase the incidence of erectile dysfunction independently of one another. In the testes, spermatogenesis is modulated at every level by estrogen, starting with the hypothalamus-pituitary-gonadal axis, followed by the Leydig, Sertoli, and germ cells, and finishing with the ductal epithelium, epididymis, and mature sperm. Regulation of testicular cells by estradiol shows both an inhibitory and a stimulatory influence, indicating an intricate symphony of dose-dependent and temporally sensitive modulation. Our goal in this review is to elucidate the overall contribution of estradiol to male sexual function by looking at the hormone's effects on erectile function, spermatogenesis, and libido.
Keywords: estrogen, testosterone, spermatogenesis, erectile function, estrogen receptor, aromatase
ASSOCIATION BETWEEN LIBIDO AND ESTRADIOL
Role of estradiol in the brain
The effect of estradiol on libido is seen at various levels of regulation, starting with direct effects in the brain (Figure 1). Areas of the brain that control sexual behavior in mammals are thought to do so via pheromones that induce specific sexual effects on the autonomic nervous system, including changes in mood and sexual arousal. Pheromones produce increased activity in the medial preoptic area/anterior hypothalamus.1 Neurons, the most basic electrical information-transmitting cells in the central nervous system and peripheral nervous system, as well as astrocytes, star-shaped glial cells which fulfill a number of functions in the central nervous system, both convert testosterone to estrogen with aromatase. The preoptic area and anterior hypothalamus contain the highest levels of aromatase and estrogen receptors (ERs) in male rodents.2,3 Similarly, it is well known that selective serotonin reuptake inhibitors diminish libido. Serotonin receptors follow a pattern of distribution similar to that of ERs in the brain.4 However, the interaction of estradiol and serotonin is complex and will subsequently be addressed. Finally, aromatase activity is highest in the brain during development. Thus, not only does estradiol modulate sexual behavior in the adult male, it also appears to organize the early brain to program sexual behavior.3
Figure 1.
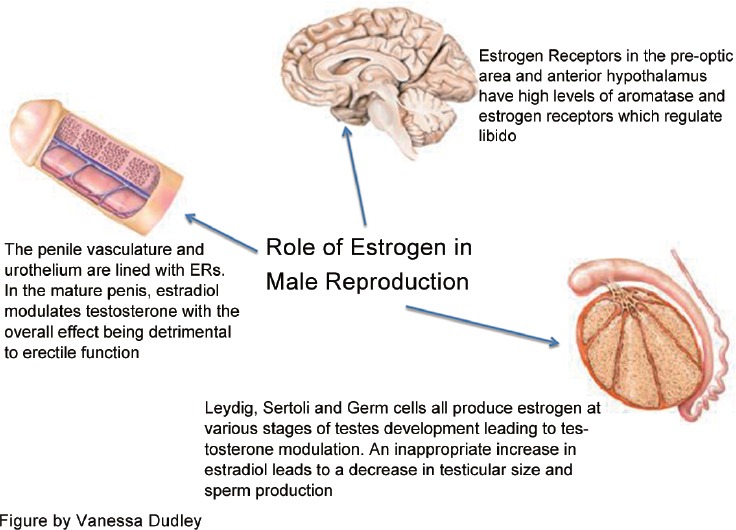
The role of estrogen in male reproduction.
Estradiol effect at low testosterone levels
To discern the effect of estradiol, it is important to evaluate its effect on libido at both low and normal levels of circulating testosterone. Decreased testosterone is clearly associated with low libido in males.5 In men with diminished testosterone, the administration of exogenous estradiol has been shown to increase libido.6 This finding is supported by rodent studies demonstrating that castrated animals given exogenous estrogen show an increase in sexual activity in a dose- and temporal-dependent manner.7 In addition, in a unique case report of a male patient with aromatase deficiency and hypogonadism, both estrogen and testosterone were required to increase libido, whereas neither hormone could achieve the effect alone suggesting that estrogen plays a necessary role in sexual desire in the setting of low testosterone.8
Similarly, patients with prostate cancer treated with androgen deprivation therapy (ADT) serve as a good model for the influence of estrogen on libido. When castrate levels of androgens were reached (T <50 ng dl-1), uniform adverse effects of hot flashes, erectile dysfunction (ED), and decrease in libido were reported.9 When comparing androgen receptor (AR) blockers versus castration, the former had better outcomes in maintaining sexual activity, presumably by increased testosterone conversion to estrogen.10 This evidence, though indirect, does perhaps suggest that elevated estrogen in men with low or absent testosterone can sustain libido. In addition, administering estradiol to men undergoing ADT for prostate cancer could possibly reduce damage to areas of the brain associated with sexual performance. Thus, an overall increase in sexual quality of life could be achieved.6
Role of estradiol in eugonadal men
While estradiol has been shown to have a positive effect on libido at low levels of testosterone, a limited number of studies have looked into the effect of estradiol supplementation in eugonadal men and reported conflicting results. One study with continuous estradiol administration in men who had normal testosterone levels showed decreases in sexual interest, fantasy, masturbation, and erections.11 In contrast, a randomized, double-blind study conducted on 50 men ages between 20 and 40 years demonstrated that sexual activity was unaffected.12
Uncontrolled case reports also have shown conflicting results. A man with aromatase deficiency was noted to have a relevant increase in sexual behavior with estrogen supplementation,13 while other aromatase-deficient men noted no change in their sexual function.14 These natural models, which have the potential to provide some clarity, along with results of the limited trials undertaken, have not provided definitive evidence one-way or the other regarding estradiol's effects on libido in the eugonadal male.
Role of estradiol in hypogonadal men treated with testosterone supplementation therapy
Perhaps, most relevant to the discussion is the use of testosterone supplementation therapy (TST). The goal of TST, regardless of the method used, should be to maintain not only physiologic levels of testosterone, but also its metabolites, including estradiol which optimizes libido.15
In men with secondary hypogonadism (functioning testes and relatively low levels of luteinizing hormone [LH] and testosterone), clomiphene citrate was used to increase testosterone by acting centrally on the ER weakly. Clomiphene citrate administration raised endogenous testosterone while increasing the testosterone to estradiol (T/E) ratio.16 Also, in a later study, clomiphene citrate administered to hypogonadal men produced an increase in libido, energy, and sense of well-being.17
In 2013, Finkelstein et al. looked at the effects of testosterone and estrogen on male sexual function. They found that the administration of testosterone with and without aromatase inhibitors markedly impaired sexual function when aromatization was inhibited.18 In addition, a study by Ramasamy et al. in 2014 showed that libido was increased in men receiving TST when testosterone levels were >300 ng dl-1 and estradiol levels were >5 ng dl-1. Most compelling is the fact that in men with serum testosterone <300 ng dl-1, sexual drive was seen to be markedly higher when estradiol levels were >5 ng dl-1.19 In addition, when patients with low testosterone were treated with letrozole, a potent aromatase inhibitor, libido was decreased, suggesting that complete elimination of estradiol and decreasing the T/E ratio too severely, adversely affects sexual desire in men.20 These studies provide evidence that both estrogen and testosterone are necessary for normal libido in testosterone-deficient men. Clinically, the dependence of libido in hypogonadal men on both testosterone and estrogen indicates that a cautious approach to the use of aromatase inhibitors is warranted and that the T/E ratio has an impact. It might be reasonable that while prescribing TST one should monitor the levels of both testosterone and estrogen and their relationship to each other.
Clearly, the effect of estradiol on male sexual desire is linked to testosterone levels, as there are different outcomes when estrogen is administered at low and normal testosterone levels. Another example of this duality is seen in men with androgen resistance, where unfettered estrogen is able to stimulate subsequent breast development. However, in men with normal androgen receptor activity, estradiol is unable to stimulate breast development.21 This is thought to be due to an imbalance between the inhibitory and stimulatory effect of these hormones.22,23 Whatever the pathophysiology in breast development or libido, these hormones seem to be inextricably linked in the complicated physiology of male sexuality and development.
Finally, the effect of estradiol on mood must be considered. As mood can correlate with sexual interest, it is reasonable to consider these data when discussing the role of estradiol on libido. While cognition, well-being, and depressive symptoms improve in men whose low testosterone levels were corrected,24,25,26 higher levels of estrogen also have been associated with less depression in older patients of both sexes.27 In addition, estrogen supports serotonin levels and affects the amount of 5-HT receptors in the brain, and depending on receptor subtype, there is sexual inhibition or facilitation.28,29,30 A recent study showed a significant positive correlation between endogenous plasma estradiol levels and cortical 5-HT2A binding in men, with no independent effects on these receptors from testosterone.31 In addition, when serotonin binds to these 5-HT2A receptors in the cortex, limbic system, hypothalamus, and midbrain, sexual desire is inhibited with subsequent induction of refractoriness and sexual satiety.32 The interaction of estrogen with serotonin is complex, with overlapping influences that reaches beyond sexual desire including mood regulation and cognition.33 This fact makes its true impact on sexual desire and behavior difficult to fully elucidate.
ASSOCIATION BETWEEN ESTRADIOL AND ERECTILE FUNCTION
Erectile function is multifaceted with a necessary combination of nerve, vessel, and endocrine actions that work together to produce subsequent penile structural changes in a coordinated fashion. Smooth muscle, endothelium, and cell-to-cell communications via gap junctions are essential to erectile function, and thus pathology in any one of these can lead to ED.34 The pathophysiology and clinical role of testosterone in erectile function have been studied extensively.35,36,37 Androgens are necessary for the penis to grow and develop, and also contribute to the physiology of erections.38
Estrogen in animal models impedes normal penile development, including reduced bulk of the bulbospongiosus muscle, reduction of the spaces in corpus cavernosum, and an accumulation of fat cells within existing spaces that lead to ED in adult life. Notably, the reported exposure was limited to early development, and rats that were exposed to exogenous estradiol after day 12 of life showed no structural abnormalities.39 In addition to influence on structure, estrogen has a significant influence on penile vasculature. A case–control study of male outpatients with ED with venous leakage showed that the only difference between the men with and without ED was an increased estradiol level. The authors concluded that estradiol increases venous vascular permeability via VEGF and has a detrimental effect on erectile function through increased venous leakage.40
Estrogen also acts at the level of the brain to influence erectile function. Estrogen inhibits the hypothalamus-pituitary axis and subsequently follicle-stimulating hormone (FSH) and LH, thus reducing circulating testosterone.41,42,43 Testosterone is necessary for normal erectile function, and low testosterone produces decreased firmness, ability to maintain erections and number of erections achieved, all of which are improved with testosterone administration.44,45 The administration of exogenous estradiol has the ability to cause ED through an inhibitory effect on testosterone production. This was demonstrated in animals, where exogenous estrogen administration not only reduced testosterone levels, but also diminished the structural integrity of the corpus cavernosum with less viable smooth muscle and an increase in connective tissue.46 Similarly, in humans taking estrogen, a reduction is seen in spontaneous erections and nocturnal penile tumescence correlating with a reduction in testosterone levels.47
The effects of estrogen on erectile function discussed above largely occur as a function of its ability to decrease circulating testosterone. In addition, increased severity of ED, assessed with the International Index for Erectile Function,48 in men with low testosterone levels is worsened with high estrogen levels implying an additive effect by the two hormones.49 However, while low testosterone increases the incidence of ED, elevated estrogen levels do as well.40 This point, coupled with the fact that the corpus cavernosum vasculature and urothelium have extensive ERs, significantly more than other steroid receptors, and particularly around the neurovascular bundle, suggests mechanisms of ED separate from and in addition to central testosterone inhibition.50,51
Evidence from animal models demonstrates this independent role of estradiol in erectile function. Stimulation of ER receptors has been shown to have anti-apoptotic effects on endothelium, and the loss of this function in the crura was associated with ED.52 Similarly, when ED was induced by high estrogen levels, testosterone therapy was not helpful in restoring erections as long as the estrogen milieu was maintained.53 While animal models have not provided conclusive evidence, and the pathophysiology of erectile function is more complex than simple hormone difference, these findings provide an interesting and compelling initial step in elucidating estrogen's separate and direct effect on ED outside of its role in testosterone suppression (Figure 1).
ROLE OF ESTRADIOL IN SPERMATOGENESIS
Testosterone has long been known to be the dominant sex hormone in men. However, estrogen is found at detectable levels at multiple points of development and contributes to spermatogenesis (Figure 1). In addition, an abnormal T/E ratio (<10) has been associated with decreased semen parameters, and administration of an aromatase inhibitor normalized the ratio and improved sperm concentration, motility, and morphology.20 Targeting estrogen levels has clinical value when optimizing sperm retrieval rates in men with nonobstructive azoospermia (NOA). Sperm retrieval rates were seen to increase 1.4-fold either by decreasing estradiol directly and normalizing the T/E ratios with aromatase inhibitors or indirectly blocking estradiol centrally with clomiphene citrate, in turn, increasing gonadotropin secretion.54 Although it has not been made clear if the improvement in spermatogenesis is specifically due to the reduction of estradiol in the testes when normalizing the T/E ratio, it is clear that estrogen levels play an important and modifiable clinical impact on spermatogenesis in men with NOA. In addition, varicocele has long been known to have an adverse impact on fertility and sex hormone production, and while the mechanism of this affect is unknown, estrogen has been linked.55,56 Semen analysis of “varicocele” sperm showed a marked reduction in both ER alpha and beta receptors as well as a reduced response to estradiol's necessary effects on motility and acrosin activity.56
Estradiol has been shown to exist not only in the reproductive tract of the adult male, but in the brain as well.57 In various species including humans, a more significant concentration exists in the male reproductive tract and semen than in the serum.58 While it has been shown clearly that Leydig and Sertoli cells produce estradiol, newer research has demonstrated that estradiol synthesis by germ cells within the seminiferous tubules contributes significantly to the hormonal milieu within the tubules (Figure 2).59 Aromatase has also been found in early development in all germ cells, especially during meiotic and postmeiotic stages of spermatogenesis, and in the later development of ejaculated spermatozoa.60 However, it is the presence of ERs in these cells and their precursors that provides compelling evidence that estrogen has an influence over spermatogenesis.61
Figure 2.
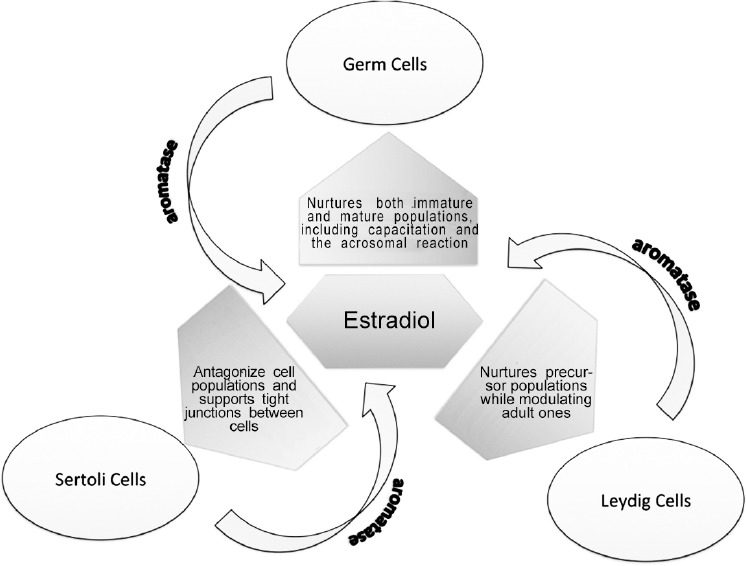
The role of estradiol in spermatogenesis.
Leydig cells – self-regulation and testosterone control via estradiol
Leydig cells, under the influence of LH, secrete testosterone, which in turn acts on Sertoli and peritubular cells, as well as vasculature, allowing them to nurture the budding spermatogonia to spermatozoa.62 At birth, fetal Leydig stem cells are not progenitors of the adult form. However, they remain in small numbers in the adult testes as a distinct cell population that is important for the generation of their adult counterparts.63 Aromatase is present largely in mature Leydig cells, producing a significant amount of the estradiol in the testes.64,65 In addition, ER mRNA has been shown to exist in both cell precursor and mature populations.66 When subjected to an alkylating agent toxic to Leydig cell populations, male rats which then had subsequent estradiol exposure during a critical stage of development experienced a blockage in the reappearance of mature Leydig cells.67 Thus, Leydig cells, at least in part, self-regulate via estrogen modulation by potentially controlling the extant population of fetal Leydig cells in a paracrine fashion. Furthermore, there is evidence suggesting that estrogen inhibits the LH effect on Leydig cells68 and that excess estrogen exposure reduces serum testosterone levels via this inhibition. The subsequent reduction of testosterone in turn reduces the number of viable sperm.69,70 Taken together, the evidence shows that estrogen plays a major role in the overall growth, development, and function of Leydig cells, in one role acting as a modulator of precursor populations and, in another role inhibiting steroidogenesis via the effect of LH on the mature Leydig cells, ultimately producing a net effect of androgen inhibition.71
Sertoli cells – contributions of estradiol to the immature testes and sperm maturation
While a major source of estrogen in the adult testes is the Leydig cell, Sertoli cells produce most of the estrogen in the immature testes.60 In multiple species, aromatase mRNA was detected in Sertoli cells at every stage of sperm development.72 In addition, it has recently been shown that ERs, specifically the beta type and G protein estrogen receptors are present in male rats’ Sertoli cells during development and are subjected to modulation by estradiol in concert with FSH.73,74,75 The overall effect of this modulation is inhibition, as shown by the antagonism of estrogen in neonatal animal testes with subsequent increase in Sertoli cell population.76,77 In addition, male neonatal rat testes exposed to estradiol displayed a subsequent dose-dependent reduction of 20%–70% of testes weight. A reduction of sperm production with poor motility was also dose-dependent, with the upper range doses of estradiol producing complete azoospermia. These changes are androgen-dependent and have been linked to a down regulation of ARs on Sertoli cells via the effect of estradiol on the coexisting ERs.78
Finally, it is known that Sertoli cells must “recognize” developing sperm, as the perpetual and cyclical topological relationship is directly related to the maturation process of spermatogonia.79 This process is essential as the immature sperm progress in the apical direction, mature, and eventually secrete into the tubules. It has been shown that FSH in combination with estradiol is necessary for the mRNA transcription of N-cadherin, the protein responsible for cell-to-cell adhesion.80,81 Thus, the dynamic process of spermatogenesis involving Sertoli cells separating and reforming tight junctions via N-cadherins is, at least in part, regulated by estrogen.
Germ cells – autocrine and paracrine effects of estradiol
Testosterone produced by Leydig cells and FSH from the anterior pituitary are necessary for Sertoli cells to transduce signals and produce factors that nurture germ cells.82,83 In addition, when testosterone is withdrawn from germ cells by administering ethane 1,2-dimethanesulfonate, an alkylating agent that selectively kills Leydig cells in adult rat testes, apoptosis is induced via the Fas/Bcl-2 system.84 While this requirement of testosterone for germ cell survival is well established, evidence of estrogen's effect on germ cells is a more recent discovery. Aromatase has been confirmed in the cytoplasm surrounding elongated spermatids, as well as ejaculated sperm, and a link exists between estradiol production in sperm and capacitation and the acrosome reaction. When placed in a noncapacitating medium, only estradiol and aromatizable steroids were able to increase sperm motility and migration, making estradiol necessary for sperm maturation and successful fertilization.85
In the neonatal period, the major portion of estradiol is synthesized by Sertoli cells, and germ cell precursors are stimulated through plasma membrane ERs.86 In addition, as germ cells multiply and begin producing estradiol, the hormone inhibits aromatase in Sertoli cells in a paracrine fashion to allow them subsequently to proliferate and nurture the spermatogonia to maturity.87 Along with estradiol from Leydig cells, the estrogens produced by germ cells allow for autocrine self-stimulation, thus creating a positive feedback loop promoting germ cell and subsequent paracrine Sertoli cell propagation.88
Unlike estrogen's inhibitory influences on Leydig and Sertoli cells, as described above, a stimulatory effect is seen with germ cells. Like Sertoli and Leydig cells, ERs and aromatase are found in germ cells, again suggesting the production of as well as modulation by estradiol.86,89,90 It was once thought that Leydig cells produced most of the estrogen in the adult testes, but new evidence shows that germ cells produce anywhere from 50% to 60% of the hormone.60,91 Most recently, it has been demonstrated that estradiol, along with platelet-derived growth factor (PDGF), stimulates germ cell proliferation that is dose-dependent and nonadditive, giving Sertoli cells a way to regulate the germ cells’ entry into mitosis via estradiol production.92,93 When incubating mature human seminiferous tubules without survival factors, even low concentrations of estradiol effectively enhanced germ cell production by the inhibition of apoptosis. Thus, estrogen has proven to be a potent hormone necessary for germ cell survival.94 Seemingly, contradictory to this is the fact that excess estrogen acts as an inhibitor of spermatogenesis in human95 as well as rat models.70,78,96 However, it is likely the inhibitory effect of estrogen on Sertoli cells, rather than on germ cells, that causes the overall inhibition of spermatogenesis.
An interesting case report from the New England Journal of Medicine describing a man with an estrogen receptor mutation with normal sexual development and relatively normal semen parameters warrants discussion. While the patient had a low normal sperm density, a seemingly contradictory fact to the evidence presented above, his sperm viability was well below normal at 18%, and fertility could not be assessed.97 In male mouse models, the targeted disruption of estrogen receptors led to alteration in spermatogenesis and infertility; however, in human models, many different novel mutations in ERs have been identified, some in infertile men and some in men with normal fertility.98,99 Perhaps, this means relatively normal sexual parameters can be explained by a mutation or receptor type that does not confer infertility. The authors did not address that, however this point once again addresses the complexity of estradiol on male sexual function and demonstrates why further study is needed.
CONCLUSIONS
The exact role of estradiol in each area of male sexual function including libido, erectile function, and spermatogenesis, is difficult to determine. A complex balance of testosterone, estradiol, aromatase, and ERs in the testes, penis, and brain confirms an indispensable and highly regulated hormonal interaction of estrogen in the male. ERs and aromatase share topographic locations with pheromones in the brain, making it clear that estrogen contributes to early sexual development as well as sexual behavior in adulthood. Estrogen can sustain libido as well as affect the amount of serotonin receptors in the brain modulating mood, mental state, cognition, and emotion. Erectile function is adversely affected by estrogen exposure in early penile development, and exposure to estradiol in the mature penis leads to increased vascular permeability with increased ED. ED from increased estradiol exposure is independent of testosterone level. In addition, spermatogenesis is dependent upon estradiol to some extent, as all cells involved in the process of sperm production contain aromatase and express ERs. Finally, estradiol levels should be considered when treating men with TST, as estradiol levels below 5 ng dl-1 correlate to a decrease in libido. Considering the complexity and taking into account some conflicting data, more research is necessary so that when better understood, estradiol can become clinically useful in treating diminished libido, ED, and perhaps even oligospermia.
REFERENCES
- 1.Savic I, Berglund H, Lindstrom P. Brain response to putative pheromones in homosexual men. Proc Natl Acad Sci U S A. 2005;102:7356–61. doi: 10.1073/pnas.0407998102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol rev. 2010;62:155–98. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCarthy MM. The two faces of estradiol: effects on the developing brain. Neuroscientist. 2009;15:599–610. doi: 10.1177/1073858409340924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson ER, Davis SR. Another role highlighted for estrogens in the male: sexual behavior. Proc Natl Acad Sci U S A. 2000;97:14038–40. doi: 10.1073/pnas.011526097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Travison TG, Morley JE, Araujo AB, O’Donnell AB, McKinlay JB. The relationship between libido and testosterone levels in aging men. J Clin Endocrinol Metab. 2006;91:2509–13. doi: 10.1210/jc.2005-2508. [DOI] [PubMed] [Google Scholar]
- 6.Wibowo E, Schellhammer P, Wassersug RJ. Role of estrogen in normal male function: clinical implications for patients with prostate cancer on androgen deprivation therapy. J Urol. 2011;185:17–23. doi: 10.1016/j.juro.2010.08.094. [DOI] [PubMed] [Google Scholar]
- 7.Davidson JM, Allinson PA. Effects of estrogen on the sexual behavior of male rats. Endocrinology. 1969;84:1365–72. doi: 10.1210/endo-84-6-1365. [DOI] [PubMed] [Google Scholar]
- 8.Carani C, Granata AR, Rochira V, Caffagni G, Aranda C, et al. Sex steroids and sexual desire in a man with a novel mutation of aromatase gene and hypogonadism. Psychoneuroendocrinology. 2005;30:413–7. doi: 10.1016/j.psyneuen.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Perlmutter MA, Lepor H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev Urol. 2007;9(Suppl 1):S3–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Penson DF. Quality of life following prostate cancer treatments. Curr Urol Rep. 2000;1:71–7. doi: 10.1007/s11934-000-0038-9. [DOI] [PubMed] [Google Scholar]
- 11.Bancroft J, Tennent G, Loucas K, Cass J. The control of deviant sexual behaviour by drugs I. Behavioural changes following oestrogens and anti-androgens. Br J Psychiatry. 1974;125:310–5. doi: 10.1192/bjp.125.3.310. [DOI] [PubMed] [Google Scholar]
- 12.Bagatell CJ, Heiman JR, Rivier JE, Bremner WJ. Effects of endogenous testosterone and estradiol on sexual behavior in normal young men. J Clin Endocrinol Metab. 1994;78:711–6. doi: 10.1210/jcem.78.3.8126146. [DOI] [PubMed] [Google Scholar]
- 13.Carani C, Rochira V, Faustini-Fustini M, Balestrieri A, Granata AR. Role of oestrogen in male sexual behaviour: insights from the natural model of aromatase deficiency. Clin Endocrinol. 1999;51:517–24. doi: 10.1046/j.1365-2265.1999.00849.x. [DOI] [PubMed] [Google Scholar]
- 14.Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, et al. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med. 1997;337:91–5. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 15.Basson R. Re: andropause: a misnomer for a true clinical entity. J Urol. 2000;164:1319. [PubMed] [Google Scholar]
- 16.Shabsigh A, Kang Y, Shabsign R, Gonzalez M, Liberson G, et al. Clomiphene citrate effects on testosterone/estrogen ratio in male hypogonadism. J Sex Med. 2005;2:716–21. doi: 10.1111/j.1743-6109.2005.00075.x. [DOI] [PubMed] [Google Scholar]
- 17.Katz DJ, Nabulsi O, Tal R, Mulhall JP. Outcomes of clomiphene citrate treatment in young hypogonadal men. BJU Int. 2012;110:573–8. doi: 10.1111/j.1464-410X.2011.10702.x. [DOI] [PubMed] [Google Scholar]
- 18.Finkelstein JS, Yu EW, Burnett-Bowie SA. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369:2457. doi: 10.1056/NEJMc1313169. [DOI] [PubMed] [Google Scholar]
- 19.Ramasamy R, Scovell JM, Kovac JR, Lipshultz LI. Elevated serum estradiol is associated with higher libido in men on testosterone supplementation therapy. Eur Urol. 2014;65:1224–5. doi: 10.1016/j.eururo.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Schlegel PN. Aromatase inhibitors for male infertility. Fertil Steril. 2012;98:1359–62. doi: 10.1016/j.fertnstert.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 21.Hughes IA, Davies JD, Bunch TI, Pasterski V, Mastroyannopoulou K, et al. Androgen insensitivity syndrome. Lancet. 2012;380:1419–28. doi: 10.1016/S0140-6736(12)60071-3. [DOI] [PubMed] [Google Scholar]
- 22.Johnson RE, Murad MH. Gynecomastia: pathophysiology, evaluation, and management. Mayo Clin Proc. 2009;84:1010–5. doi: 10.1016/S0025-6196(11)60671-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rochefort H, Garcia M. The estrogenic and antiestrogenic activities of androgens in female target tissues. Pharmacol Ther. 1983;23:193–216. doi: 10.1016/0163-7258(83)90013-x. [DOI] [PubMed] [Google Scholar]
- 24.Wang C, Alexander G, Berman N, Salehian B, Davidson T, et al. Testosterone replacement therapy improves mood in hypogonadal men – A clinical research center study. J Clin Endocrinol Metab. 1996;81:3578–83. doi: 10.1210/jcem.81.10.8855804. [DOI] [PubMed] [Google Scholar]
- 25.Khera M. Patients with testosterone deficit syndrome and depression. Arch Esp Urol. 2013;66:729–36. [PubMed] [Google Scholar]
- 26.Thilers PP, Macdonald SW, Herlitz A. The association between endogenous free testosterone and cognitive performance: a population-based study in 35 to 90 year-old men and women. Psychoneuroendocrinology. 2006;31:565–76. doi: 10.1016/j.psyneuen.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Kulkarni J, Gavrilidis E, Worsley R, Van Rheenen T, Hayes E. The role of estrogen in the treatment of men with schizophrenia. Int J Endocrinol Metab. 2013;11:129. doi: 10.5812/ijem.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fink G, Sumner B, Rosie R, Wilson H, McQueen J. Androgen actions on central serotonin neurotransmission: relevance for mood, mental state and memory. Behav Brain Res. 1999;105:53–68. doi: 10.1016/s0166-4328(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 29.Meston CM, Gorzalka BB. Psychoactive drugs and human sexual behavior: the role of serotonergic activity. J Psychoactive Drugs. 1992;24:1–40. doi: 10.1080/02791072.1992.10471616. [DOI] [PubMed] [Google Scholar]
- 30.Luine V, Rhodes J. Gonadal hormone regulation of MAO and other enzymes in hypothalamic areas. Neuroendocrinology. 1983;36:235–41. doi: 10.1159/000123461. [DOI] [PubMed] [Google Scholar]
- 31.Frokjaer VG, Erritzoe D, Juul A, Nielsen FÅ, Holst K, et al. Endogenous plasma estradiol in healthy men is positively correlated with cerebral cortical serotonin 2A receptor binding. Psychoneuroendocrinology. 2010;35:1311–20. doi: 10.1016/j.psyneuen.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Pfaus JG. Reviews: pathways of sexual desire. J Sex Med. 2009;6:1506–33. doi: 10.1111/j.1743-6109.2009.01309.x. [DOI] [PubMed] [Google Scholar]
- 33.Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;4:43–58. doi: 10.1177/1534582305277152. [DOI] [PubMed] [Google Scholar]
- 34.Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32:379–95, v. doi: 10.1016/j.ucl.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain P, Rademaker AW, McVary KT. Testosterone supplementation for erectile dysfunction: results of a meta-analysis. J Urol. 2000;164:371–5. [PubMed] [Google Scholar]
- 36.Corona G, Isidori AM, Buvat J, Aversa A, Rastrelli G, et al. Testosterone supplementation and sexual function: a meta-analysis study. J Sex Med. 2014;11:1577–92. doi: 10.1111/jsm.12536. [DOI] [PubMed] [Google Scholar]
- 37.Isidori AM, Buvat J, Corona G, Goldstein I, Jannini EA, et al. A critical analysis of the role of testosterone in erectile function: from pathophysiology to treatment – A systematic review. Eur Urol. 2014;65:99–112. doi: 10.1016/j.eururo.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 38.Traish AM, Goldstein I, Kim NN. Testosterone and erectile function: from basic research to a new clinical paradigm for managing men with androgen insufficiency and erectile dysfunction. Eur Urol. 2007;52:54–70. doi: 10.1016/j.eururo.2007.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goyal H, Braden T, Williams C, Dalvi P, Mansour M, et al. Estrogen-induced abnormal accumulation of fat cells in the rat penis and associated loss of fertility depends upon estrogen exposure during critical period of penile development. J Toxicol Sci. 2005;87:242–54. doi: 10.1093/toxsci/kfi233. [DOI] [PubMed] [Google Scholar]
- 40.Mancini A, Milardi D, Bianchi A, Summaria V, De Marinis L. Increased estradiol levels in venous occlusive disorder: a possible functional mechanism of venous leakage. Int J Impot Res. 2005;17:239–42. doi: 10.1038/sj.ijir.3901287. [DOI] [PubMed] [Google Scholar]
- 41.Santen RJ. Is aromatization of testosterone to estradiol required for inhibition of luteinizing hormone secretion in men? J Clin Invest. 1975;56:1555–63. doi: 10.1172/JCI108237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marshall JC, Case GD, Valk TW, Corley KP, Sauder SE, et al. Selective inhibition of follicle-stimulating hormone secretion by estradiol. Mechanism for modulation of gonadotropin responses to low dose pulses of gonadotropin-releasing hormone. J Clin Invest. 1983;71:248–57. doi: 10.1172/JCI110765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bagatell CJ, Dahl KD, Bremner WJ. The direct pituitary effect of testosterone to inhibit gonadotropin secretion in men is partially mediated by aromatization to estradiol. J Androl. 1994;15:15–21. [PubMed] [Google Scholar]
- 44.Chiang HS, Cho SL, Lin YC, Hwang TI. Testosterone gel monotherapy improves sexual function of hypogonadal men mainly through restoring erection: evaluation by IIEF score. Urology. 2009;73:762–6. doi: 10.1016/j.urology.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 45.Mills TM, Wiedmeier VT, Stopper VS. Androgen maintenance of erectile function in the rat penis. Biol Reprod. 1992;46:342–8. doi: 10.1095/biolreprod46.3.342. [DOI] [PubMed] [Google Scholar]
- 46.Srilatha B, Adaikan PG. Estrogen and phytoestrogen predispose to erectile dysfunction: do ER-alpha and ER-beta in the cavernosum play a role? Urology. 2004;63:382–6. doi: 10.1016/j.urology.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 47.Kwan M, VanMaasdam J, Davidson JM. Effects of estrogen treatment on sexual behavior in male-to-female transsexuals: experimental and clinical observations. Arch Sex behav. 1985;14:29–40. doi: 10.1007/BF01541350. [DOI] [PubMed] [Google Scholar]
- 48.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 49.El-Sakka AI. Impact of the association between elevated oestradiol and low testosterone levels on erectile dysfunction severity. Asian J Androl. 2013;15:492–6. doi: 10.1038/aja.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dietrich W, Haitel A, Huber JC, Reiter WJ. Expression of estrogen receptors in human corpus cavernosum and male urethra. J Histochem Cytochem. 2004;52:355–60. doi: 10.1177/002215540405200306. [DOI] [PubMed] [Google Scholar]
- 51.Jesmin S, Mowa CN, Matsuda N, Salah-Eldin AE, Togashi H, et al. Evidence for a potential role of estrogen in the penis: detection of estrogen receptor-alpha and -beta messenger ribonucleic acid and protein. Endocrinology. 2002;143:4764–74. doi: 10.1210/en.2002-220628. [DOI] [PubMed] [Google Scholar]
- 52.Shirai M, Yamanaka M, Shiina H, Igawa M, Ogishima T, et al. Androgen, estrogen, and progesterone receptor gene regulation during diabetic erectile dysfunction and insulin treatment. Urology. 2004;64:1244–9. doi: 10.1016/j.urology.2004.06.062. [DOI] [PubMed] [Google Scholar]
- 53.Kataoka T, Hotta Y, Ohno M, Maeda Y, Kimura K. Limited effect of testosterone treatment for erectile dysfunction caused by high-estrogen levels in rats. Int J Impot Res. 2013;25:201–5. doi: 10.1038/ijir.2013.21. [DOI] [PubMed] [Google Scholar]
- 54.Ramasamy R, Ricci JA, Palermo GD, Gosden LV, Rosenwaks Z, et al. Successful fertility treatment for Klinefelter's syndrome. J Urol. 2009;182:1108–13. doi: 10.1016/j.juro.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 55.Guido C, Santoro M, De Amicis F, Perrotta I, Panza S, et al. Human sperm anatomy and endocrinology in varicocele: role of androgen receptor. Reproduction. 2014;147:589–98. doi: 10.1530/REP-13-0542. [DOI] [PubMed] [Google Scholar]
- 56.Guido C, Perrotta I, Panza S, Middea E, Avena P, et al. Human sperm physiology: estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) influence sperm metabolism and may be involved in the pathophysiology of varicocele-associated male infertility. J Cell Physiol. 2011;226:3403–12. doi: 10.1002/jcp.22703. [DOI] [PubMed] [Google Scholar]
- 57.Roselli CE, Abdelgadir SE, Resko JA. Regulation of aromatase gene expression in the adult rat brain. Brain Res Bull. 1997;44:351–7. doi: 10.1016/s0361-9230(97)00214-1. [DOI] [PubMed] [Google Scholar]
- 58.Hess RA. Oestrogen in fluid transport in efferent ducts of the male reproductive tract. Rev Reprod. 2000;5:84–92. doi: 10.1530/ror.0.0050084. [DOI] [PubMed] [Google Scholar]
- 59.Carreau S, Bouraima-Lelong H, Delalande C. Estrogen, a female hormone involved in spermatogenesis. Adv Med Sci. 2012;57:31–6. doi: 10.2478/v10039-012-0005-y. [DOI] [PubMed] [Google Scholar]
- 60.Carreau S, Bouraima-Lelong H, Delalande C. Role of estrogens in spermatogenesis. Front Biosci (Elite Ed) 2012;4:1–11. doi: 10.2741/e356. [DOI] [PubMed] [Google Scholar]
- 61.Carreau S, Bourguiba S, Lambard S, Galeraud-Denis I, Genissel C, et al. Reproductive system: aromatase and estrogens. Mol Cell Endocrinol. 2002;193:137–43. doi: 10.1016/s0303-7207(02)00107-7. [DOI] [PubMed] [Google Scholar]
- 62.Sharpe RM, Maddocks S, Kerr JB. Cell-cell interactions in the control of spermatogenesis as studied using Leydig cell destruction and testosterone replacement. Am J Anat. 1990;188:3–20. doi: 10.1002/aja.1001880103. [DOI] [PubMed] [Google Scholar]
- 63.Kerr JB, Knell CM. The fate of fetal Leydig cells during the development of the fetal and postnatal rat testis. Development. 1988;103:535–44. doi: 10.1242/dev.103.3.535. [DOI] [PubMed] [Google Scholar]
- 64.Genissel C, Carreau S. Regulation of the aromatase gene expression in mature rat Leydig cells. Mol Cell Endocrinol. 2001;178:141–6. doi: 10.1016/s0303-7207(01)00409-9. [DOI] [PubMed] [Google Scholar]
- 65.Genissel C, Levallet J, Carreau S. Regulation of cytochrome P450 aromatase gene expression in adult rat Leydig cells: comparison with estradiol production. J Endocrinol. 2001;168:95–105. doi: 10.1677/joe.0.1680095. [DOI] [PubMed] [Google Scholar]
- 66.Zhai J, Lanclos KD, Abney TO. Estrogen receptor messenger ribonucleic acid changes during Leydig cell development. Biol Reprod. 1996;55:782–8. doi: 10.1095/biolreprod55.4.782. [DOI] [PubMed] [Google Scholar]
- 67.Abney TO, Myers RB. 17 beta-estradiol inhibition of Leydig cell regeneration in the ethane dimethylsulfonate-treated mature rat. J Androl. 1991;12:295–304. [PubMed] [Google Scholar]
- 68.van Beurden WM, Roodnat B, Mulder E, van der Molen HJ. Further characterization of the effects of hypophysectomy, FSH and estrogen on LH stimulation of testosterone production in Leydig cells isolated from immature rats. Steroids. 1978;31:83–98. doi: 10.1016/0039-128x(78)90021-1. [DOI] [PubMed] [Google Scholar]
- 69.Cigorraga SB, Sorrell S, Bator J, Catt KJ, Dufau ML. Estrogen dependence of a gonadotropin-induced steroidogenic lesion in rat testicular Leydig cells. J Clin Invest. 1980;65:699–705. doi: 10.1172/JCI109716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Atanassova N, McKinnell C, Walker M, Turner KJ, Fisher JS, et al. Permanent effects of neonatal estrogen exposure in rats on reproductive hormone levels, Sertoli cell number, and the efficiency of spermatogenesis in adulthood. Endocrinology. 1999;140:5364–73. doi: 10.1210/endo.140.11.7108. [DOI] [PubMed] [Google Scholar]
- 71.Abney TO. The potential roles of estrogens in regulating Leydig cell development and function: a review. Steroids. 1999;64:610–7. doi: 10.1016/s0039-128x(99)00041-0. [DOI] [PubMed] [Google Scholar]
- 72.Haverfield JT, Ham S, Brown KA, Simpson ER, Meachem SJ. Teasing out the role of aromatase in the healthy and diseased testis. Spermatogenesis. 2011;1:240–9. doi: 10.4161/spmg.1.3.18037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lucas TF, Lazari MF, Porto CS. Differential role of the estrogen receptors ESR1 and ESR2 on the regulation of proteins involved with proliferation and differentiation of Sertoli cells from 15-day-old rats. Mol Cell Endocrinol. 2014;382:84–96. doi: 10.1016/j.mce.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 74.Pearl CA, Mason H, Roser JF. Immunolocalization of estrogen receptor alpha, estrogen receptor beta and androgen receptor in the pre-, peri- and post-pubertal stallion testis. Anim Reprod Sci. 2011;125:103–11. doi: 10.1016/j.anireprosci.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 75.Royer C, Lucas TF, Lazari MF, Porto CS. 17 beta-estradiol signaling and regulation of proliferation and apoptosis of rat Sertoli cells. Biol Reprod. 2012;86:108. doi: 10.1095/biolreprod.111.096891. [DOI] [PubMed] [Google Scholar]
- 76.Berger T, Conley AJ, Van Klompenberg M, Roser JF, Hovey RC. Increased testicular Sertoli cell population induced by an estrogen receptor antagonist. Mol Cell Endocrinol. 2013;366:53–8. doi: 10.1016/j.mce.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 77.Berger T, Conley A. Reduced endogenous estrogen and hemicastration interact synergistically to increase porcine Sertoli cell proliferation. Biol Reprod. 2014;90:114. doi: 10.1095/biolreprod.114.117770. [DOI] [PubMed] [Google Scholar]
- 78.Gill-Sharma MK, Dsouza S, Padwal V, Balasinor N, Aleem M, et al. Antifertility effects of estradiol in adult male rats. J Endocrinol Invest. 2001;24:598–607. doi: 10.1007/BF03343900. [DOI] [PubMed] [Google Scholar]
- 79.Newton SC, Blaschuk OW, Millette CF. N-cadherin mediates Sertoli cell-spermatogenic cell adhesion. Dev Dyn. 1993;197:1–13. doi: 10.1002/aja.1001970102. [DOI] [PubMed] [Google Scholar]
- 80.MacCalman CD, Getsios S, Farookhi R, Blaschuk OW. Estrogens potentiate the stimulatory effects of follicle-stimulating hormone on N-cadherin messenger ribonucleic acid levels in cultured mouse Sertoli cells. Endocrinology. 1997;138:41–8. doi: 10.1210/endo.138.1.4831. [DOI] [PubMed] [Google Scholar]
- 81.MacCalman CD, Farookhi R, Blaschuk OW. Estradiol regulates E-cadherin mRNA levels in the surface epithelium of the mouse ovary. Clin Exp Metastasis. 1994;12:276–82. doi: 10.1007/BF01753834. [DOI] [PubMed] [Google Scholar]
- 82.Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol. 1998;9:411–6. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- 83.Warren DW, Huhtaniemi IT, Tapanainen J, Dufau ML, Catt KJ. Ontogeny of gonadotropin receptors in the fetal and neonatal rat testis. Endocrinology. 1984;114:470–6. doi: 10.1210/endo-114-2-470. [DOI] [PubMed] [Google Scholar]
- 84.Nandi S, Banerjee PP, Zirkin BR. Germ cell apoptosis in the testes of Sprague Dawley rats following testosterone withdrawal by ethane 1,2-dimethanesulfonate administration: relationship to fas? Biol Reprod. 1999;61:70–5. doi: 10.1095/biolreprod61.1.70. [DOI] [PubMed] [Google Scholar]
- 85.Aquila S, Sisci D, Gentile M, Carpino A, Middea E, et al. Towards a physiological role for cytochrome P450 aromatase in ejaculated human sperm. Hum Reprod. 2003;18:1650–9. doi: 10.1093/humrep/deg340. [DOI] [PubMed] [Google Scholar]
- 86.Zhou Q, Nie R, Prins GS, Saunders PT, Katzenellenbogen BS, et al. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl. 2002;23:870–81. [PubMed] [Google Scholar]
- 87.Boitani C, Ritzen EM, Parvinen M. Inhibition of rat Sertoli cell aromatase by factor (s) secreted specifically at spermatogenic stages VII and VIII. Mol Cell Endocrinol. 1981;23:11–22. doi: 10.1016/0303-7207(81)90113-1. [DOI] [PubMed] [Google Scholar]
- 88.Meccariello R, Chianese R, Chioccarelli T, Ciaramella V, Fasano S, et al. Intra-testicular signals regulate germ cell progression and production of qualitatively mature spermatozoa in vertebrates. Front Endocrinol. 2014;5:69. doi: 10.3389/fendo.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hess RA, Gist DH, Bunick D, Lubahn DB, Farrell A, et al. Estrogen receptor (alpha and beta) expression in the excurrent ducts of the adult male rat reproductive tract. J Androl. 1997;18:602–11. [PubMed] [Google Scholar]
- 90.Saunders PT, Fisher JS, Sharpe RM, Millar MR. Expression of oestrogen receptor beta (ER beta) occurs in multiple cell types, including some germ cells, in the rat testis. J Endocrinol. 1998;156:R13–7. doi: 10.1677/joe.0.156r013. [DOI] [PubMed] [Google Scholar]
- 91.Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1517–35. doi: 10.1098/rstb.2009.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li H, Papadopoulos V, Vidic B, Dym M, Culty M. Regulation of rat testis gonocyte proliferation by platelet-derived growth factor and estradiol: identification of signaling mechanisms involved. Endocrinology. 1997;138:1289–98. doi: 10.1210/endo.138.3.5021. [DOI] [PubMed] [Google Scholar]
- 93.Thuillier R, Mazer M, Manku G, Boisvert A, Wang Y, et al. Interdependence of platelet-derived growth factor and estrogen-signaling pathways in inducing neonatal rat testicular gonocytes proliferation. Biol Reprod. 2010;82:825–36. doi: 10.1095/biolreprod.109.081729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pentikainen V, Erkkila K, Suomalainen L, Parvinen M, Dunkel L. Estradiol acts as a germ cell survival factor in the human testis in vitro . J Clin Endocrinol Metab. 2000;85:2057–67. doi: 10.1210/jcem.85.5.6600. [DOI] [PubMed] [Google Scholar]
- 95.Handelsman DJ, Wishart S, Conway AJ. Oestradiol enhances testosterone-induced suppression of human spermatogenesis. Hum Reprod. 2000;15:672–9. doi: 10.1093/humrep/15.3.672. [DOI] [PubMed] [Google Scholar]
- 96.Kalla NR. Demonstration of direct effect of estrogen on rat spermatogenesis. Acta Eur Fertil. 1987;18:293–302. [PubMed] [Google Scholar]
- 97.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–61. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 98.Khattri A, Pandey R, Gupta N, Chakravarty B, Deenadayal M, et al. Estrogen receptor β gene mutations in Indian infertile men. Mol Hum Reprod. 2009;15:513–20. doi: 10.1093/molehr/gap044. [DOI] [PubMed] [Google Scholar]
- 99.Eddy E, Washburn T, Bunch D, Goulding E, Gladen B, et al. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology. 1996;137:4796–805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]


