Abstract
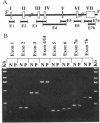
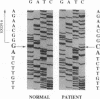
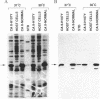
Deficiency of carbonic anhydrase II (carbonate hydro-lyase, EC 4.2.1.1) is the primary defect in the syndrome of osteopetrosis, renal tubular acidosis, and cerebral calcification. In this report we describe the molecular basis for carbonic anhydrase II deficiency in the American family in which the association of carbonic anhydrase II deficiency with this syndrome was first recognized. The three affected siblings from this family are compound heterozygotes, each having inherited two different mutations in the structural gene for carbonic anhydrase II. The paternal mutation is a splice acceptor site mutation at the 3' end of intron 5. The maternal mutation is a missense mutation in exon 3 that substitutes a tyrosine for histidine-107. We show that the mutant enzyme expressed in bacteria from the cDNA containing the His-107----Tyr mutation has detectable, though greatly reduced, activity. We suggest that residual activity of the His-107----Tyr mutant enzyme may explain the absence of mental retardation and the relatively mild phenotype of carbonic anhydrase II deficiency in affected members of this family.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cochat P., Loras-Duclaux I., Guibaud P. Déficit en anhydrase carbonique II: ostéopétrose, acidose rénale tubulaire et calcifications intrâcraniennes. Revue de la littérature à partir de trois observations. Pediatrie. 1987;42(2):121–128. [PubMed] [Google Scholar]
- Guibaud P., Larbre F., Freycon M. T., Genoud J. Ostéopétrose et acidose rénale tubulaire. Deux cas de cette association dans une fratrie. Arch Fr Pediatr. 1972 Mar;29(3):269–286. [PubMed] [Google Scholar]
- Hung T., Mak K., Fong K. A specificity enhancer for polymerase chain reaction. Nucleic Acids Res. 1990 Aug 25;18(16):4953–4953. doi: 10.1093/nar/18.16.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MAREN T. H. A simplified micromethod for the determination of carbonic anhydrase and its inhibitors. J Pharmacol Exp Ther. 1960 Sep;130:26–29. [PubMed] [Google Scholar]
- McConlogue L., Brow M. A., Innis M. A. Structure-independent DNA amplification by PCR using 7-deaza-2'-deoxyguanosine. Nucleic Acids Res. 1988 Oct 25;16(20):9869–9869. doi: 10.1093/nar/16.20.9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J. C., Venta P. J., Tashian R. E., Hewett-Emmett D. Nucleotide sequence of human liver carbonic anhydrase II cDNA. Nucleic Acids Res. 1987 Jun 11;15(11):4687–4687. doi: 10.1093/nar/15.11.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H., Marelich G. P., Grubb J. H., Kyle J. W., Sly W. S. Cloning, expression, and sequence homologies of cDNA for human carbonic anhydrase II. Genomics. 1987 Oct;1(2):159–166. doi: 10.1016/0888-7543(87)90008-5. [DOI] [PubMed] [Google Scholar]
- Nair S. K., Calderone T. L., Christianson D. W., Fierke C. A. Altering the mouth of a hydrophobic pocket. Structure and kinetics of human carbonic anhydrase II mutants at residue Val-121. J Biol Chem. 1991 Sep 15;266(26):17320–17325. [PubMed] [Google Scholar]
- Osborne W. R., Tashian R. E. An improved method for the purification of carbonic anhydrase isozymes by affinity chromatography. Anal Biochem. 1975 Mar;64(1):297–303. doi: 10.1016/0003-2697(75)90434-0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Zhu X. L., Sly W. S. Carbonic anhydrase isozymes IV and II in urinary membranes from carbonic anhydrase II-deficient patients. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6073–6076. doi: 10.1073/pnas.87.16.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly W. S., Hewett-Emmett D., Whyte M. P., Yu Y. S., Tashian R. E. Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc Natl Acad Sci U S A. 1983 May;80(9):2752–2756. doi: 10.1073/pnas.80.9.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly W. S., Whyte M. P., Sundaram V., Tashian R. E., Hewett-Emmett D., Guibaud P., Vainsel M., Baluarte H. J., Gruskin A., Al-Mosawi M. Carbonic anhydrase II deficiency in 12 families with the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. N Engl J Med. 1985 Jul 18;313(3):139–145. doi: 10.1056/NEJM198507183130302. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Su T. S., Lin L. H. Analysis of a splice acceptor site mutation which produces multiple splicing abnormalities in the human argininosuccinate synthetase locus. J Biol Chem. 1990 Nov 15;265(32):19716–19720. [PubMed] [Google Scholar]
- Sundaram V., Rumbolo P., Grubb J., Strisciuglio P., Sly W. S. Carbonic anhydrase II deficiency: diagnosis and carrier detection using differential enzyme inhibition and inactivation. Am J Hum Genet. 1986 Feb;38(2):125–136. [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., D'Amico D., Chiba I., Buchhagen D. L., Minna J. D. Identification of intronic point mutations as an alternative mechanism for p53 inactivation in lung cancer. J Clin Invest. 1990 Jul;86(1):363–369. doi: 10.1172/JCI114710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashian R. E. The carbonic anhydrases: widening perspectives on their evolution, expression and function. Bioessays. 1989 Jun;10(6):186–192. doi: 10.1002/bies.950100603. [DOI] [PubMed] [Google Scholar]
- Vainsel M., Fondu P., Cadranel S., Rocmans C., Gepts W. Osteopetrosis associated with proximal and distal tubular acidosis. Acta Paediatr Scand. 1972 Jul;61(4):429–434. doi: 10.1111/j.1651-2227.1972.tb15859.x. [DOI] [PubMed] [Google Scholar]
- Venta P. J., Welty R. J., Johnson T. M., Sly W. S., Tashian R. E. Carbonic anhydrase II deficiency syndrome in a Belgian family is caused by a point mutation at an invariant histidine residue (107 His----Tyr): complete structure of the normal human CA II gene. Am J Hum Genet. 1991 Nov;49(5):1082–1090. [PMC free article] [PubMed] [Google Scholar]
- Whyte M. P., Murphy W. A., Fallon M. D., Sly W. S., Teitelbaum S. L., McAlister W. H., Avioli L. V. Osteopetrosis, renal tubular acidosis and basal ganglia calcification in three sisters. Am J Med. 1980 Jul;69(1):64–74. doi: 10.1016/0002-9343(80)90501-x. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X. L., Sly W. S. Carbonic anhydrase IV from human lung. Purification, characterization, and comparison with membrane carbonic anhydrase from human kidney. J Biol Chem. 1990 May 25;265(15):8795–8801. [PubMed] [Google Scholar]