Abstract
The role of adjuvant hormonal therapy and optimized regimens for high-risk localized prostate cancer after radical prostatectomy remains controversial. Herein, the clinical trial CU1005 prospectively evaluated two regimens of maximum androgen blockage or bicalutamide 150 mg daily as immediate adjuvant therapy for high-risk localized prostate cancer. Overall, 209 consecutive patients were recruited in this study, 107 of whom received 9 months of adjuvant maximum androgen blockage, whereas 102 received 9 months of adjuvant bicalutamide 150 mg. The median postoperative follow-up time was 27.0 months. The primary endpoint was biochemical recurrence. Of the 209 patients, 59 patients developed biochemical recurrence. There was no difference between the two groups with respect to clinical characteristics, including age, pretreatment prostate-specific antigen, Gleason score, surgical margin status, or pathological stages. The maximum androgen blockage group experienced longer biochemical recurrence-free survival (P = 0.004) compared with the bicalutamide 150 mg group. Side-effects in the two groups were similar and could be moderately tolerated in all patients. In conclusion, immediate, 9-month maximum androgen blockage should be considered as an alternative to bicalutamide 150 mg as adjuvant treatment for high-risk localized prostate cancer patients after radical prostatectomy.
Keywords: adjuvant, bicalutamide, high-risk, maximum androgen blockage, prostate cancer, radical prostatectomy
INTRODUCTION
Prostate cancer (PCa) is the second most common cancer in men worldwide. An estimated 1.1 million men worldwide were diagnosed with PCa in 2012, accounting for 15% of all cancers diagnosed in men. With an estimated 307 000 deaths in 2012, PCa was the fifth leading cause of death from cancer in men (6.6% of total men deaths). In the People's Republic of China, although PCa incidence and mortality rates remain low (age-standardized incidence and mortality rates, 5.3/100 000 and 2.5/100 000, respectively in 2012), PCa is the most common and the most lethal male urogenital system cancer, as it is worldwide.1
Radical prostatectomy (RP) is regarded as a curative treatment for localized PCa. Unfortunately, for high-risk patients, some experience prostate-specific antigen (PSA) recurrence in a relatively short time after RP.2,3,4,5,6,7 Adjuvant treatments, including hormonal therapy, radiation, or chemotherapy, are usually required to treat these patients.8 A previously published clinical trial has already proven that early adjuvant hormonal therapy can benefit patients with nodal metastases who have undergone RP.9,10 In a randomized clinical trial, 309 patients with locally advanced, lymph node-negative PCa (stage pT3-4pN0) after RP were included. After a median follow-up of 6.1 years, patients in the adjuvant flutamide 750-mg group experienced improved recurrence-free survival compared with the observation group though no difference was noted for overall survival between the two groups.11 In a clinical analysis of the efficacy and tolerability of bicalutamide 150 mg once daily as an adjuvant treatment in addition to standard care for locally advanced, nonmetastatic PCa, Mcleod et al. also concluded that adjuvant bicalutamide could improve the objective progression-free survival versus standard care alone, but did not improve overall survival.12
In clinical practice, some physicians advocate maximum androgen deprivation (MAB) therapy while others prefer bicalutamide 150 mg daily as an adjuvant treatment. However, optimal adjuvant hormonal therapy treatment regimens for high-risk patients after RP remain uncertain. Thus, the goal of this randomized clinical trial was to evaluate the treatment effect between MAB and bicalutamide 150 mg as immediate adjuvant hormonal therapy for localized high-risk PCa.
MATERIALS AND METHODS
Trial design
The trial CU1005 (ChiCTR-TRC-10001866) is a prospective, single-center, open, randomized, noninferiority phase II study. From June 2010 to January 2013, 209 high-risk localized PCa patients over 18 years old who underwent RP within 1 month were consecutively recruited in this study. Before the operation, patients were required to have no metastases on preoperative emission computed tomography and chest radiographs. Patients recruited in our clinical trial were of relatively good healthy condition with a life expectancy >12 weeks and graded as 0–1 based on the World Health Organization health status evaluation system. Patients were not eligible if they received neoadjuvant hormonal therapy or took part in another clinical trial within 30 days of enrollment. All patients had histologically confirmed prostatic adenocarcinoma with either advanced pathological stage (≥T3) or positive surgical margins or regional lymph node metastasis or localized PCa with preoperative PSA ≥20 ng ml−1 or Gleason score ≥8. All patients’ PSA should have decreased to <0.2 ng ml−1 within 4 weeks of RP. All subjects provided written informed consent before enrollment. Eligible patients were randomly assigned in a 1:1 ratio to receive 9 months of adjuvant bicalutamide 150 mg orally once daily or 9 months of MAB (goserelin 3.6 mg [Zoladex, AstraZeneca, London, UK], triptorelin 3.75 mg [Diphereline, Ipsen Pharma Biotech, Paris, France] or leuprorelin 3.6 mg [Enantone, Takeda, Osaka, Japan] every month plus bicalutamide 50 mg [Casodex, AstraZeneca, London, UK] daily) until biochemical recurrence (BCR) after RP. Treatment commenced within 2 weeks of randomization. Second-line treatment was started if the treatment failed (PSA rose above 0.2 ng ml−1).
Postoperative evaluation and endpoints
During the follow-up period, emission computed tomography and pelvic magnetic resonance imaging were performed at the appearance of biochemical progression or symptom manifestation. Blood chemistry tests, including PSA levels were assessed every 4 weeks. Baseline was considered the time of randomization. BCR was defined as two consecutive PSA values of 0.2 ng ml−1 or greater. The primary efficacy endpoint was time to BCR. Adverse events occurring during the randomized therapy were classified using National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (NCI-CTCAE v3.0).
Statistical analysis
The Chi-square test was used to compare differences between patient groups with respect to demographic, clinical, and pathological characteristics and adverse events. To study differences in time to BCR between the two groups, Kaplan–Meier survival curves and the log-rank test were calculated using Statistical Package for the Social Sciences (SPSS) 19.0 software version 16.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered as statistically significant.
RESULTS
Patients
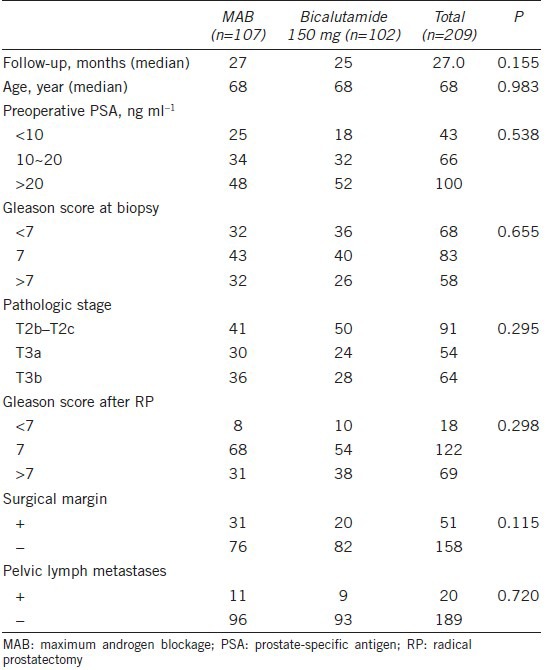
A total of 209 consecutive patients were randomized to receive bicalutamide 150 mg orally once daily (n = 102) or MAB (goserelin 3.6 mg, triptorelin 3.75 mg, or leuprorelin 3.6 mg every month plus bicalutamide 50 mg daily; n = 107). The two treatment groups were well balanced with respect to patient demographics and baseline characteristics, including time of follow-up, age, preoperative PSA, Gleason score at biopsy and after RP, pathologic stage, surgical margins, and pelvic lymph metastases (Table 1). The median follow-up time of the analysis was 27 months and the median age of all participants was 68 years.
Table 1.
Baseline demographics of the patient population
Biochemical recurrence-free survival
At a similar median follow-up of 27 months for the MAB group versus 25 months for the bicalutamide 150-mg daily group (P = 0.155), 19.6% (21/107) of the patients in the MAB group developed BCR and 37.3% (38/102) of the patients in the bicalutamide 150-mg daily group developed BCR. Therefore, MAB prolonged biochemical recurrence-free survival over bicalutamide 150 mg after RP in the overall study population (P = 0.004; Figure 1).
Figure 1.
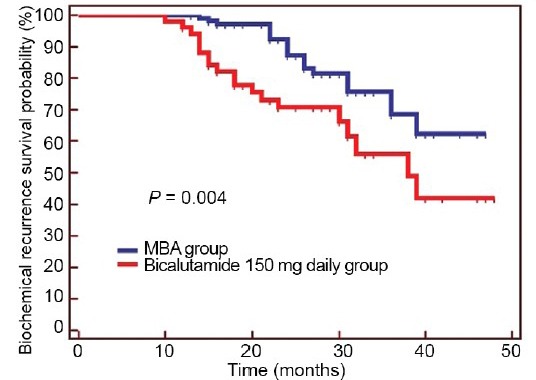
Kaplan–Meier curve of biochemical recurrence-free survival: MAB group versus bicalutamide 150-mg daily group (P = 0.004).
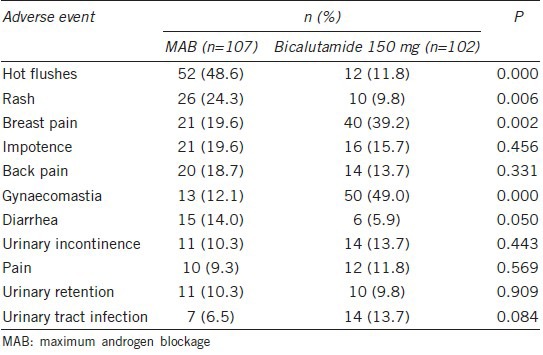
Toxicity
All of the side effects were moderately acceptable for both groups. The most common adverse event was the pharmacological side effects of hot flashes in the MAB group (52/107, 48.6%; P = 0.000) and gynecomastia in the bicalutamide 150-mg daily group (50/102, 49.0%; P = 0.000; Table 2). Moreover, the incidences of rash in the MAB group (26/107, 24.3%, P = 0.006) and breast pain in the bicalutamide 150-mg daily group (40/102, 39.2%; P = 0.002) were also relatively high (Table 2). Other adverse events, such as diarrhea, urinary incontinence, pain, urinary retention, and urinary tract infection were similarly infrequent in both groups (Table 2). During the follow-up period, two patients in the bicalutamide 150-mg daily group temporarily stopped the treatment as a result of hepatic dysfunction and breast pain, respectively. Because of diarrhea or hepatic dysfunction, three patients in the MAB group, stopped treatment for no more than 2 months. However, all these five patients recovered from the side effects and eventually finished the 9-month treatment regimen. As a result, no one withdrew from the study because of severe pharmacological side effects during the follow-up.
Table 2.
All adverse effects with an incidence ≥10% in either treatment group
DISCUSSION
The CU1005 clinical trial compared the 9-month treatment effects of MAB with bicalutamide 150 mg as adjuvant therapy after RP in high-risk PCa patients. In a previous study, both MAB and bicalutamide 150 mg daily were shown to be effective as adjuvant therapy for PCa patients. In the Dorff et al. clinical trial, although the final primary treatment comparison results are not ready for publication, 2 years of adjuvant MAB after RP resulted in an extremely low rate of disease recurrence and PCa-specific death for high-risk patients in S9921 – the estimated 5-year biochemical failure-free survival is 92.5% (95% [confidence interval] CI, 90–95), and the 5-year overall survival is 95.9% [95% CI, 93.9–97.9]).13 In 2000, See et al. reported that bicalutamide 150 mg in addition to standard care reduced the risk of PSA progression by 59% compared with standard care alone, irrespective of whether patients received RP or radiotherapy as standard care (hazard ratio [HR] 0.41; 95% CI, 0.38–0.45; P < 0.0001). Furthermore, significant reductions were also observed following RP (51%; HR 0.49; 95% CI, 0.43–0.56; P < 0.0001).14 In 2005, McLeod et al. investigated the ongoing Early Prostate Cancer (EPC) trial program and the combination of Trials 23, 24, and 25. They found that adjuvant bicalutamide 150 mg prolonged objective progression-free survival versus standard care alone (P = 0.004) for locally advanced patients after RP.12 Therefore, although the clinical benefit has already been proven in previous studies, the best choice for adjuvant hormonal therapy remains uncertain. In this study, we compared the treatment efficacy between the two therapies regarding time to BCR. We concluded that patients who underwent 9 months of MAB treatment experienced longer BCR-free survival than patients in the bicalutamide 150-mg group with the moderately acceptable toxicity of both groups.
In this clinical trial, we performed immediate adjuvant hormonal therapy after RP for patients based on previous study results. Previous studies support the hypothesis that early adjuvant hormonal therapy should be adopted for high-risk patients compared with deferred treatment. In 2007, the clinical study from the Mayo Clinic evaluated the treatment effects of hormonal therapy applied at five different time points (1: adjuvant androgen deprivation, 2: androgen deprivation therapy started at a postoperative PSA of 0.4 ng ml−1 or greater, 3: at prostate-specific antigen 1.0 ng ml−1 or greater, 4: at PSA 2.0 ng ml−1 or greater, and 5: at systemic progression) for 8290 patients after RP. A significant benefit in the 10-year systemic progression-free-survival and cancer specific survival was observed in the adjuvant hormonal treatment group. However, the results suggested that men who started hormonal therapy at a postoperative PSA of 0.4 ng ml−1 or greater, or 1.0 or 2.0 ng ml−1 did not experience the same benefits.15 Kowalczyk and colleagues also evaluated the treatment effect between early (<4 months after RP, n = 419) and delayed (4–12 months after RP, n = 544). They found that initiating adjuvant hormonal therapy <5 months after RP for pT3 is associated with improved PCa-specific mortality. In their study, they also found that early adjuvant hormonal therapy is also associated with fewer bone-related events and salvage hormonal therapy was used less.16 In a randomized study composed of patients with lymph node metastases after RP, Messing et al. compared the treatment effect between immediate and deferred (hormonal therapy to be given upon detection of distant metastases or symptomatic recurrence) androgen deprivation therapy. They found that men assigned immediate androgen deprivation therapy had a significant improvement in overall survival (HR 1.84 [95% CI, 1.01–3.35], P = 0.04), PCa-specific survival (HR 4.09 [95% CI, 1.76–9.49], P = 0.0004), and progression-free survival (HR 3.42 [95% CI, 1.96–5.98], P < 0.0001).9 Based on these results, we administered immediate adjuvant hormonal therapy for the patients.
Although adjuvant radiation therapy is currently deemed the best recommended treatment after RP for pT3pN0 patients with a high-risk of local failure after RP (owing to positive margins according to the 2013 National Comprehensive Cancer Network guidelines Asia consensus statement on PCa), a number of these patients are not currently receiving radiation therapy after RP.17 The reasons for this discrepancy may be because of the latter's impact on postoperative recovery and serious toxicity caused by radiation. Suardi et al. evaluated the impact of adjuvant radiation therapy on urinary continence recovery after RP. They found that the 1- and 3-year urinary continence recovery was only 51% and 59% for patients who underwent adjuvant radiation therapy versus 81% and 87% for patients not receiving this treatment, respectively (P < 0.001).18 Other serious long-term urinary complications, such as bladder neck contracture and urethral stricture, and bowel symptoms were also frequently reported.19,20 To improve oncologic control and avoid long-term complications, doctors would rather choose adjuvant hormonal therapy after RP for these high-risk patients. Tsurumaki et al. reported the long-term results of RP with immediate adjuvant androgen deprivation therapy for pT3N0 PCa patients. After the median follow-up of 98.7 months, the 10-year hormone-refractory biochemical progression-free survival and cancer-specific survival rates were 88.3% and 96.3%, respectively, which suggests that immediate adjuvant androgen deprivation therapy is a valid treatment option for patients with pT3N0M0 PCa.21 However, this does not mean that it is always safe to treat patients with hormonal therapy. As previous studies have proven that long-term adjuvant hormonal therapy may cause serious insomnia, fatigue, hot flashes, and inferior social function, sexual interest, and activity compared with short-term adjuvant hormonal therapy,22,23 we chose a 9-month treatment regimen to balance oncologic control and side effects.
Our study has several limitations. First, it included only a relatively small number of patients from a single institution, which may introduce selection bias. Second, results found in Chinese populations may not be directly applied to other populations. Larger cohorts from multiple centers are needed to validate our results, and we welcome international collaborations to achieve this goal. Moreover, a longer follow-up is needed to assess the impact of adjuvant therapy on clinical progression-free survival, cancer-specific survival, and overall survival.
CONCLUSION
Patients experienced longer BCR-free survival in the MAB group while the side effects were moderately acceptable in both groups. As adjuvant hormonal therapy is recommended for high-risk localized PCa after RP, immediate MAB may be the preferential option for patients reluctant to receive adjuvant radiation therapy.
AUTHORS’ CONTRIBUTIONS
KC and XJQ designed the study, collected, analyzed, and interpreted the clinical data, and wrote and revised the manuscript. HLZ, BD, and YZ helped collect the clinical data. GHS, YJS, and YPZ revised the manuscript. DWY supervised the project and revised the manuscript. All authors approved the final version and agreed to publish the manuscript.
COMPETING FINANCIAL INTERESTS
All authors declare no competing financial interests.
ACKNOWLEDGMENTS
None.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, Forman D, Bray F, et al. Lyon, France: International Agency for Research on Cancer; 2013. [Last accessed on 2014 Nov 11]. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 Internet. Available from: http://www.globocan.iarc.fr . [Google Scholar]
- 2.Carver BS, Bianco FJ, Scardino PT, Eastham JA. Long-term outcome following radical prostatectomy in men with clinical stage T3 prostate cancer. J Urol. 2006;176:564–8. doi: 10.1016/j.juro.2006.03.093. [DOI] [PubMed] [Google Scholar]
- 3.Petrovich Z, Lieskovsky G, Stein JP, Huberman M, Skinner DG. Comparison of surgery alone with surgery and adjuvant radiotherapy for pT3N0 prostate cancer. BJU Int. 2002;89:604–11. doi: 10.1046/j.1464-410x.2002.02698.x. [DOI] [PubMed] [Google Scholar]
- 4.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910–4. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen CT, Reuther AM, Stephenson AJ, Klein EA, Jones JS. The specific definition of high risk prostate cancer has minimal impact on biochemical relapse-free survival. J Urol. 2009;181:75–80. doi: 10.1016/j.juro.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 6.Yossepowitch O, Eggener SE, Bianco FJ, Carver BS, Serio A, et al. Radical prostatectomy for clinically localized, high risk prostate cancer: critical analysis of risk assessment methods. J Urol. 2007;178:493–9. doi: 10.1016/j.juro.2007.03.105. 499. [DOI] [PubMed] [Google Scholar]
- 7.Namiki M, Konaka H. What is appropriate neoadjuvant/adjuvant androgen deprivation for high-risk/locally advanced prostate cancer? Asian J Androl. 2011;13:624–5. doi: 10.1038/aja.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito T, Kitamura Y, Komatsubara S, Matsumoto Y, Sugita T, et al. Outcomes of locally advanced prostate cancer: a single institution study of 209 patients in Japan. Asian J Androl. 2006;8:555–61. doi: 10.1111/j.1745-7262.2006.00175.x. [DOI] [PubMed] [Google Scholar]
- 9.Messing EM, Manola J, Yao J, Kiernan M, Crawford D, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–9. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 10.Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, et al. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999;341:1781–8. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]
- 11.Wirth MP, Weissbach L, Marx FJ, Heckl W, Jellinghaus W, et al. Prospective randomized trial comparing flutamide as adjuvant treatment versus observation after radical prostatectomy for locally advanced, lymph node-negative prostate cancer. Eur Urol. 2004;45:267–70. doi: 10.1016/j.eururo.2003.10.013. 270. [DOI] [PubMed] [Google Scholar]
- 12.McLeod DG, Iversen P, See WA, Morris T, Armstrong J, et al. Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. BJU Int. 2006;97:247–54. doi: 10.1111/j.1464-410X.2005.06051.x. [DOI] [PubMed] [Google Scholar]
- 13.Dorff TB, Flaig TW, Tangen CM, Hussain MH, Swanson GP, et al. Adjuvant androgen deprivation for high-risk prostate cancer after radical prostatectomy: SWOG S9921 study. J Clin Oncol. 2011;29:2040–5. doi: 10.1200/JCO.2010.32.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.See W, Iversen P, Wirth M, McLeod D, Garside L, et al. Immediate treatment with bicalutamide 150mg as adjuvant therapy significantly reduces the risk of PSA progression in early prostate cancer. Eur Urol. 2003;44:512. doi: 10.1016/s0302-2838(03)00366-x. [DOI] [PubMed] [Google Scholar]
- 15.Siddiqui SA, Boorjian SA, Inman B, Bagniewski S, Bergstralh EJ, et al. Timing of androgen deprivation therapy and its impact on survival after radical prostatectomy: a matched cohort study. J Urol. 2008;179:1830–7. doi: 10.1016/j.juro.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Kowalczyk KJ, Gu X, Nguyen PL, Lipsitz SR, Trinh QD, et al. Optimal timing of early versus delayed adjuvant radiotherapy following radical prostatectomy for locally advanced prostate cancer. Urol Oncol. 2014;32:303–8. doi: 10.1016/j.urolonc.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Ghia AJ, Shrieve DC, Tward JD. Adjuvant radiotherapy use and patterns of care analysis for margin-positive prostate adenocarcinoma with extracapsular extension: postprostatectomy adjuvant radiotherapy: a SEER analysis. Urology. 2010;76:1169–74. doi: 10.1016/j.urology.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 18.Suardi N, Gallina A, Lista G, Gandaglia G, Abdollah F, et al. Impact of adjuvant radiation therapy on urinary continence recovery after radical prostatectomy. Eur Urol. 2014;65:546–51. doi: 10.1016/j.eururo.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 19.Sowerby RJ, Gani J, Yim H, Radomski SB, Catton C. Long-term complications in men who have early or late radiotherapy after radical prostatectomy. Can Urol Assoc J. 2014;8:253–8. doi: 10.5489/cuaj.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofmann T, Gaensheimer S, Buchner A, Rohloff R, Schilling A. An unrandomized prospective comparison of urinary continence, bowel symptoms and the need for further procedures in patients with and with no adjuvant radiation after radical prostatectomy. BJU Int. 2003;92:360–4. doi: 10.1046/j.1464-410x.2003.04365.x. [DOI] [PubMed] [Google Scholar]
- 21.Tsurumaki SY, Fukuhara H, Suzuki M, Fujimura T, Nakagawa T, et al. Long-term results of radical prostatectomy with immediate adjuvant androgen deprivation therapy for pT3N0 prostate cancer. BMC Urol. 2014;14:13. doi: 10.1186/1471-2490-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolla M, de Reijke TM, Van Tienhoven G, Van den Bergh AC, Oddens J, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–27. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 23.Denham JW, Wilcox C, Joseph D, Spry NA, Lamb DS, et al. Quality of life in men with locally advanced prostate cancer treated with leuprorelin and radiotherapy with or without zoledronic acid (TROG 03.04 RADAR): secondary endpoints from a randomised phase 3 factorial trial. Lancet Oncol. 2012;13:1260–70. doi: 10.1016/S1470-2045(12)70423-0. [DOI] [PubMed] [Google Scholar]


