Abstract
The persistence infection of low-risk type (type 6 or type 11) of human papillomavirus (HPV) is the main cause of genital warts. Given the high rate of recurrence after treatment, the use of a new molecular agent is certain to be of value. The aim of this study was to achieve targeted inactivation of viral E7 gene in keratinocytes using the reprogrammed clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated (Cas) 9 system. To accomplish this, a universal CRISPR-Cas9 system for targeting both HPV6/11 E7 genes was constructed by using a dual guide RNA vector. After transfection of the vector into E7-transfromed keratinocytes, the expression level of E7 protein was measured using western-blot analysis and the sequence of the E7 gene was determined using Sanger sequencing. Cell proliferation was analyzed by CCK-8 assay, and cell apoptosis was evaluated by Hoechst 33258 staining, flow cytometry analysis and ELISA assay. The results indicated that both HPV6/11 E7 genes can be inactivated by the single CRISPR-Cas9 system. Furthermore, silencing of E7 led to inhibition of cell proliferation and induction of apoptosis in E7-transfromed keratinocytes but not in normal keratinocytes. Our data suggested that the reprogrammed CRISPR-Cas9 system has the potential for the development of an adjuvant therapy for genital warts.
Keywords: apoptosis, CRISPR-Cas9, HPV E7, proliferation
INTRODUCTION
Human papillomaviruses (HPVs) are important human pathogens that cause sexually transmitted diseases.1 The most common high-risk HPV types, 16 and 18, are frequently detected in cervical and penile cancers.2 The low-risk HPV types, 6 and 11, are associated with anogenital warts and laryngeal papillomatosis.3 HPV-related lesions require continued production of the oncogenic E7 protein, which has targets in both nuclear and cytoplasm and promotes S-phase induction in differentiated human keratinocytes.4 Small molecule inhibitors targeting the E7 oncogene can be used for improving the therapeutic effects of current therapy for HPV-related genital diseases.5
DNA interference (DNAi) is a recently described natural phenomenon mediated by the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated (Cas) 9 system.6 This system can be reprogrammed to induce DNA double-strand breaks (DSBs) at specific genomic loci and to create frame shift indel mutations that result in a loss-of-function of the target gene.7 The potential of CRISPR-Cas9 to treat or prevent human genetic diseases has yet to be proven. Although it has been revealed that E7 genes of HPV16/18 can be inactivated by means of CRISPR-Cas9,8,9 there is no report about the study of the designed CRISPR-Cas9 against the E7 genes of HPV 6/11 – the pathogeny of genital warts.
In this paper, we present a reprogrammed CRISPR-Cas9 system that targets the homologous sequences of HPV6/11 E7 genes. We demonstrate that this system can destroy the HPV6/11 E7 genes in human foreskin keratinocytes which resulting in cell growth arrest and cell apoptosis. We propose that the developed CRISPR-Cas9 system represents a novel and highly effective molecular agent to treat and eliminate low-risk HPV-related diseases.
MATERIALS AND METHODS
Cell lines and cell culture
Human keratinocytes were isolated from the newborn foreskin, and the approval of this research was obtained from the Institutional Review Board of Shenzhen Second People's Hospital. In stable transfection experiments, cells were cultured in keratinocyte serum-free medium (K-SFM) (Gibco, Carlsbad, CA, USA) at 37°C in an atmosphere of 5% CO2. In transient transfection experiments, cells were grown in RPMI-1640 (1640) media (Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA) at 37°C in 5% CO2.
Construction of plasmids
To construct the reprogrammed CRISPR-Cas9 system that targets E7 genes of HPV 6/11, we designed two different sgRNA sequences (Supplemental File 1 (1.6MB, pdf) ) and inserted them into the sgRNA expression cassettes of pCRISPR-CG01 vector (Guangzhou FulenGen, Guangzhou, China; Supplemental File 2 (1.6MB, pdf) ) containing U6 promoter to drive the transcription of each sgRNA, as well as the CMV promoter to drive the expression of Cas9 protein. The same vector for expressing sgRNAs that lack the complete complementary region was also constructed and used as the negative control.
To construct the plasmids pcDNA3.1-HPV6-E7 and pcDNA3.1-HPV11-E7, the coding sequences for HPV6 E7 (GenBank: HG793938.1) and HPV11 E7 (GenBank: KC329894.1) were chemically synthesized and inserted into pcDNA3.1(+) digested with Hind III and EcoR I, respectively.
Cell transfection
Human keratinocytes stably expressing either the HPV6 E7 gene or the HPV11 E7 gene were obtained by selecting cells with G418 after transfection with the related plasmids.10
In transient transfection experiments, cells were cultured in the plates till they reached 70% confluency and transfected with the Cas9- and dual sgRNAs expressing plasmids using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the supplier's protocols.
Western blot analysis
Cells were washed in ice-cold PBS and lysed in modified RIPA buffer. The protein concentration was calculated using the BCA protein assay. Equal amounts of whole protein extract were electrophoresed on SDS–polyacrylamide gels and transferred onto PVDF membranes (Millipore, Billerica, MA, USA). Membranes were blocked in 5% nonfat dry milk in Tris-buffered saline (TBS) and incubated overnight with the primary antibodies against HPV6 E7 (Abcam, Cambridge, MA, USA), HPV11 E7 (Abcam, Cambridge, MA, USA), and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After that, the membranes were incubated with horseradish peroxidase–conjugated secondary antibody (Amersham, Uppsala, Sweden) and the immunoblots were developed with Super-Signal chemiluminescence reagents (Pierce Chemical, Rockford, IL, USA).
Detect CRISPR/Cas9-Mediated Deletions
Cells were transfected with the plasmid expressing the CRISPR-Cas9 system as described above. Genomic DNA was extracted 48 h posttransfection and PCR was used to amplify the corresponding regions of HPV6/11 E7. Purified PCR products were then subjected to Sanger sequencing to search for the deletions.
Cell proliferation assay
Cell proliferation was examined by CCK-8 assay according to the methods provided by the company (Beyotime, Shanghai, China). 24, 48 or 72 h posttransfection, 10 μl of CCK-8 solution was added to each well of the 96-well plate, and the cells were maintained for 4 h. Absorbance value was determined at a wavelength of 450 nm using an ELISA microplate reader (Bio-Rad, Hercules, CA, USA).
Cell apoptosis assay
Morphological assessment of apoptotic cells was performed using Hoechst 33258 staining kit (Beyotime, Shanghai, China) according to the supplier's protocols. Briefly, the cells were fixed in 4% paraformaldehyde for 10 min and were washed twice with PBS. Then, the cells were stained with 0.5 ml of Hoechst 33258 staining for 5 min and were observed under a fluorescence microscope at 350 nm.
The annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) kit (BD, San Jose, CA, USA) was used to assess cell apoptosis according to the supplier's protocols. Forty-eight hours after transfection, cells were collected, counted, centrifuged, and resuspended to 5 × 105 cells in 500 μl of 1 × binding buffer. Annexin V-FITC (5 μl) and 10 μl PI were added to each tube. The samples were incubated in the dark at room temperature for 15 min. Samples were then examined immediately on a flow cytometry (Beckman, Fullerton, CA, USA).
Cell apoptosis was examined by analyzing the activity of Caspase-3 using the Enzyme-linked immunosorbent assay kit (R and D, Minneapolis, MN, USA) according to the manufacturer's instructions. The optical density (OD) values were measured using an ELISA microplate reader (Bio-Rad, Hercules, CA, USA).
Statistical analyses
Data were expressed as mean ± standard deviation (s.d.). Statistical significance was determined by Student's t-test or ANOVA. Differences were considered statistically significant at P < 0.05. All the related statistical tests were performed using SPSS version 17.0 software (SPSS Inc, Chicago, IL, USA).
RESULTS
Design and construction of the reprogrammed CRISPR-Cas9 system
To determine if CRISPR-Cas9 can silence HPV6/11 E7 genes, we constructed a single plasmid for expressing the human codon–optimized Cas9 protein and dual sgRNAs that can be used to make two cuts simultaneously at designated sites (Figure 1a). Based on the alignment of sequences of E7 genes of HPV6/11, two highly conserved regions were selected as the targets of the dual sgRNAs (Figure 1b). A CRISPR-Cas9 system that expresses sgRNAs lacking the complementary regions was also used as the negative control.
Figure 1.
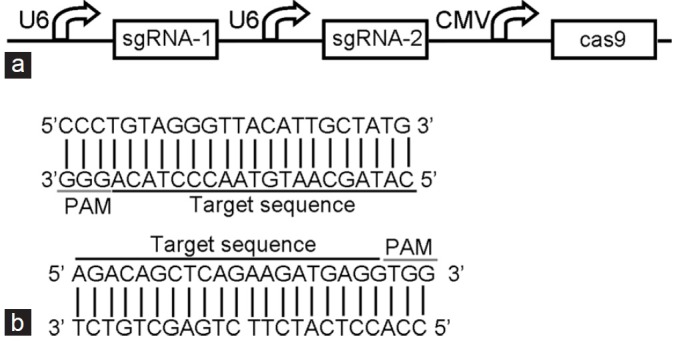
Design and construction of the reprogrammed CRISPR-Cas9 system. (a) Detailed description of the engineered vector for expressing the CRISPR-Cas9 system. (b) The two sequences targeted by sgRNAs and the related PAM sequences.
Promotion of cell growth by HPV6/11 E7 genes
We examined the potential alteration of cellular phenotypes after HPV6/11 E7 over-expression by stable transfection of the corresponding plasmids. As shown in Figure 2, consistent with the previous results,4 the E7 gene of HPV6 (P < 0.001, ANOVA) or HPV11 (P < 0.001, ANOVA) caused promotion of cell growth when compared with normal keratinocytes transfected with empty pcDNA3.1(+) vector. We then used the transformed keratinocytes as test models for analyzing the effects of reprogrammed CRISPR-Cas9 system on the HPV6/11 E7 in the following experiments.
Figure 2.
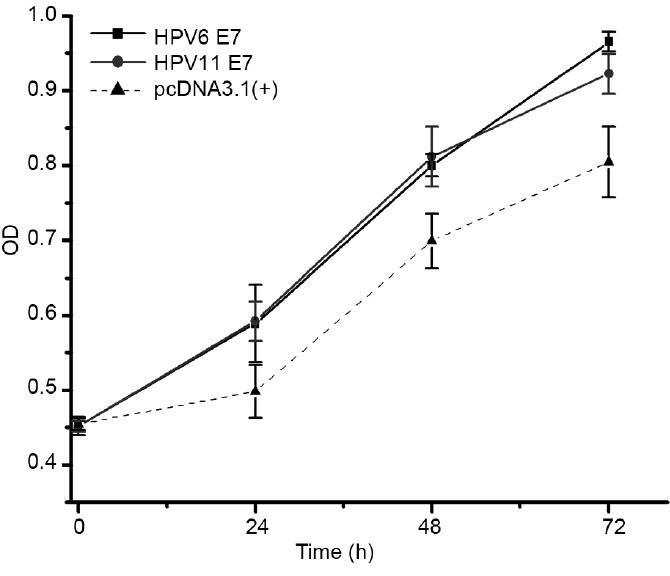
Over-expression of HPV6/11 E 7 genes in keratinocytes. Compared to keratinocytes transfected with empty pcDNA3.1(+) vector, keratinocytes transformed with HPV6 E 7 gene (P < 0.001, ANOVA) or HPV11 E 7 gene (P < 0.001, ANOVA) showed an increase in cell proliferation.
Inhibition of HPV6/11 E7 expression by the reprogrammed CRISPR-Cas9
We next asked if we could inactivate the endogenous HPV6/11 E7 genes in the transformed keratinocytes using the reprogrammed CRISPR-Cas9 system. Plasmid expressing specific CRISPR-Cas9 or the negative control was transient transfected into keratinocytes. We then monitored the expression levels of E7 proteins at 48 h posttransfection using western-blot analysis. Interestingly, expression of the single CRISPR-Cas9 system resulted in the loss of both HPV6 E7 protein (Figure 3a) and HPV11 E7 protein (Figure 3b). To further confirm the efficacy of the CRISPR-Cas9 system, we sequenced HPV6/11 E7 genes in cells treated with CRISPR-Cas9 and the results revealed that the intervening DNA segment can be deleted by Cas9 (Figure 4a and 4b, Supplemental File 3 (1.6MB, pdf) ). We therefore concluded that the reprogrammed CRISPR-Cas9 system can effectively silence the expression of HPV6/11 E7 genes.
Figure 3.
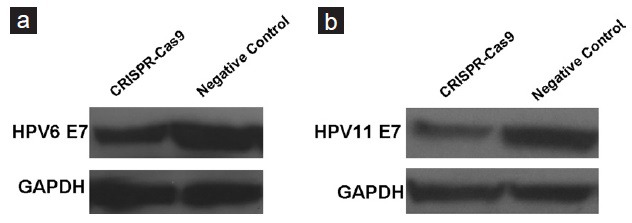
Detection of expression level of E 7 in keratinocytes treated with the reprogrammed CRISPR-Cas9 system. Western-blot analysis was done to detect the expression level of HPV6 E 7 (a) or HPV11 E 7 (b) in keratinocytes transfected with either the reprogrammed CRISPR-Cas9 system or negative control.
Figure 4.
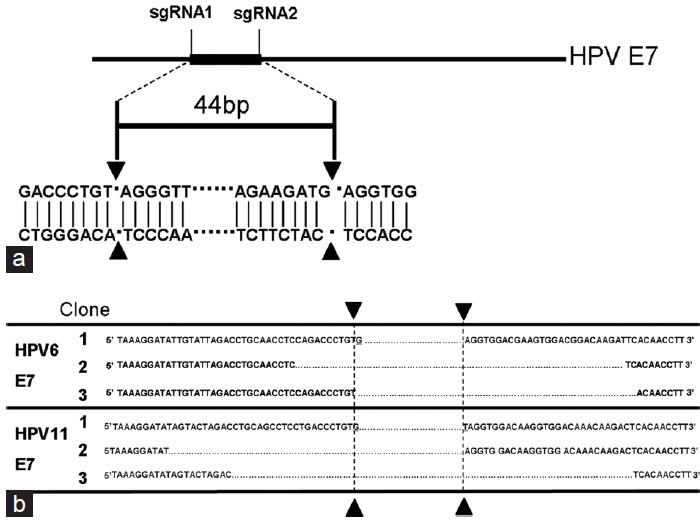
Detect deletions of E 7 in keratinocytes treated with the reprogrammed CRISPR-Cas9 system. (a) Schematic of the deleted region in HPV E 7 gene. (b) DNA sequences for the PCR products from different clones.
Inhibition of proliferation of the E7-transformed keratinocytes by the reprogrammed CRISPR-Cas9
E7 expression is known to be required for the growth and survival of HPV-transformed cells,4 and we therefore next examined whether the proliferation of E7-transformed keratinocytes can be affected by the reprogrammed CRISPR-Cas9.
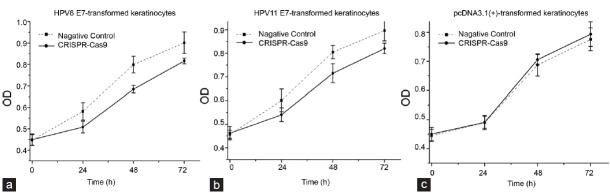
The keratinocytes transformed with HPV6/11 E7 genes were transiently transfected with the reprogrammed CRISPR-Cas9 or negative control in 96-well plates. In CCK-8 assays, we demonstrated that the proliferations of keratinocytes were decreased (P < 0.001 for each cell line, ANOVA) when cells were treated with the reprogrammed CRISPR-Cas9 (Figures 5a and 5b). In addition, such effects were not observed in keratinocytes stably transfected with empty pcDNA3.1(+) vector (Figure 5c), indicating that the reprogrammed CRISPR-Cas9 may have no off-target effects.
Figure 5.
The CRISPR-Cas9 system induced anti-growth effects in HPV6/11 E 7-transfromed keratinocytes. Obvious difference between the control group and the CRISPR-Cas9 group was observed in HPV6 E 7-transformed keratinocytes (a) (P < 0.001, ANOVA) and HPV 11 E 7-transformed keratinocytes (b) (P < 0.001, ANOVA). Such difference was not observed in keratinocytes transfected with pcDNA3.1(+) empty vector (c) (P > 0.05, ANOVA). Results were shown as mean ± s.d.
Induction of apoptosis of the E7-transformed keratinocytes by the reprogrammed CRISPR-Cas9
Finally, we examined whether the apoptosis of E7-transformed keratinocytes can be affected by the reprogrammed CRISPR-Cas9. 48 h after transfection of the reprogrammed CRISPR-Cas9 or negative control, the cell apoptosis changes of E7-transformed keratinocytes were determined by Hoechst 33258 staining, flow cytometry analysis, and ELISA. Cells transfected with the reprogrammed CRISPR-Cas9 exhibited strong blue fluorescence, revealing the typical apoptosis characteristics (Figure 6a). As revealed by the flow cytometry analysis, the percentage of apoptotic cells was indeed elevated in the reprogrammed CRISPR-Cas9–treated cells (Figure 6b). An increase of caspase 3 (P < 0.001 for each cell line, t-test) was also observed in these cells (Figure 6c). By contrast, the reprogrammed CRISPR-Cas9 did not induce apoptosis in keratinocytes stably transfected with the empty plasmid (Figure 6a–6c).
Figure 6.
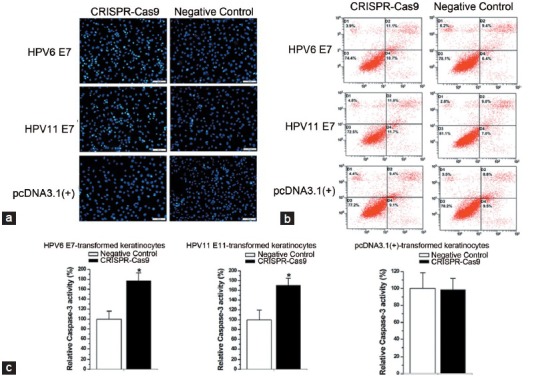
The CRISPR-Cas 9 system induced cell apoptosis in HPV6/11 E 7-transfromed keratinocytes. Cell apoptosis was measured by Hoechst 33258 staining (a), flow cytometry analysis (b) and ELISA assay (c). Results were shown as mean ± s.d. *P < 0.001. Scale bar = 100 μm in each figure.
DISCUSSION
The current treatments for genital warts usually include trichloroacetic acid, cryotherapy, and surgical removal.11 Although there are many approaches for treating this disease, the rate of recurrence after successful clearance is still very high.11 Further improvements in treatment outcome may derive from a combination of conventional therapy with novel molecular agents.5 The expression of HPV E7 protein is positively associated with uncontrolled proliferation of keratinocytes in the development of genital warts.5,12,13 With these features in mind, E7 gene is an ideal target for molecular reagents against HPV6/11. In the previous works, antisense oligodeoxynucleotides,14 ribozymes,15 and short interfering RNA (siRNA)16 have already been constructed to inhibit the expression level of E7 mRNA/protein. However, these approaches have only achieved limited success due to the low accessibility of most sites on HPV6/11 E7 mRNAs.14 Therefore, treatments based on new molecular agents are still to be developed.
Just in recently, there are some papers in which the reprogrammed CRISPR-Cas9 systems are used to destroy or rebuild some other virus, such as HBV,17 EBV,18 HSV,19 and HIV.20 In this work, we tested the possibility of using the reprogrammed CRISPR-Cas9 system as a novel molecular agent for against E7 genes of low-risk HPV types (HPV6/11). In theory, the constructed CRISPR/Cas9 system can simultaneously break two gene loci by co-expressing a single Cas9 protein with two sgRNAs.21 Our data have shown that sgRNAs directed to the highly conserved regions could inactive both HPV-11 E7 and HPV-16 E7. These results highlighted the considerable potential of the reprogrammed CRISPR-Cas9 for treating related viruses across species. In examining cellular phenotypes, we found that the reprogrammed CRISPR-Cas9 inhibits cell proliferation and promotes cell apoptosis in E7-transformed keratinocytes. These results demonstrated the efficacy of the CRISPR-Cas9 in controlling the deregulated phenotypes induced by HPV6/11 E7. Because the HPV E7 genes share no sequence homology to any human genes, knockout of this gene can never lead to the death of normal cells. It should be noted that the transfection of cells with a plasmid expressing the reprogrammed CRISPR-Cas9 only led to incomplete knockdown of HPV6/11 E7. To further improve the efficacy of gene knockout by CRISPR-Cas9 and to develop the reprogrammed system as a novel molecular drug for treating condyloma acuminatum, lentiviral vector or nanotechnology can be used for gene transfer in the future work.
In conclusion, we have developed a kind of potent CRISPR-Cas9 that suppresses cell growth and induces apoptosis in HPV E7-transformed cells while having minimal effect in E7-negative human cells. The reprogrammed CRISPR-Cas9 may be further developed as an adjuvant therapy for genital warts.
AUTHOR CONTRIBUTIONS
YCL designed the project, carried out the experiments, performed the statistical analysis and wrote the paper; ZMC and XJZ conceived the study. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
The authors are indebted to the donors, whose names were not included in the author list, but who participated in this program. This work was funded by the National Key Basic Research Programme of China (973 Programme) (2014CB745201) and the National Natural Science Foundation of China (81402103).
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Remschmidt C, Fesenfeld M, Kaufmann AM, Deleré Y. Sexual behavior and factors associated with young age at first intercourse and HPV vaccine uptake among young women in Germany: implications for HPV vaccination policies. BMC Public Health. 2014;14:1248–54. doi: 10.1186/1471-2458-14-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghosh I, Mittal S, Banerjee D, Singh P, Dasgupta S, et al. Study of accuracy of colposcopy in VIA and HPV detection-based cervical cancer screening program. Aust N Z J Obstet Gynaecol. 2014;54:570–5. doi: 10.1111/ajo.12282. [DOI] [PubMed] [Google Scholar]
- 3.Muñoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010;102:325–39. doi: 10.1093/jnci/djp534. [DOI] [PubMed] [Google Scholar]
- 4.McKee CH, Onder Z, Ashok A, Cardoso R, Moroianu J. Characterization of the transport signals that mediate the nucleocytoplasmic traffic of low risk HPV11 E7. Virology. 2013;443:113–22. doi: 10.1016/j.virol.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stern PL, van der Burg SH, Hampson IN, Broker TR, Fiander A, et al. Therapy of human papillomavirus-related disease. Vaccine. 2012;30:71–82. doi: 10.1016/j.vaccine.2012.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, et al. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–7. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhen S, Hua L, Takahashi Y, Narita S, Liu YH, et al. In vitro and in vivo growth suppression of human papillomavirus 16-positive cervical cancer cells by CRISPR/Cas9. Biochem Biophys Res Commun. 2014;450:1422–6. doi: 10.1016/j.bbrc.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy EM, Kornepati AV, Goldstein M, Bogerd HP, Poling BC, et al. Inactivation of the human papillomavirus E6 or E7 gene in cervical carcinoma cells by using a bacterial CRISPR/Cas RNA-guided endonuclease. J Virol. 2014;88:11965–72. doi: 10.1128/JVI.01879-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan D, Pecoraro G, Rosenberg I, Defendi V. Transformation of C127 mouse fibroblasts by human papillomavirus 16. Cancer Res. 1988;48:2505–11. [PubMed] [Google Scholar]
- 11.Lacey CJ, Woodhall SC, Wikstrom A, Ross J. 2012 European guideline for the management of anogenital warts. J Eur Acad Dermatol Venereol. 2013;27:e263–70. doi: 10.1111/j.1468-3083.2012.04493.x. [DOI] [PubMed] [Google Scholar]
- 12.Genovese NJ, Banerjee NS, Broker TR, Chow LT. Casein kinase II motif-dependent phosphorylation of human papillomavirus E7 protein promotes p130 degradation and S-phase induction in differentiated human keratinocytes. J Virol. 2008;82:4862–73. doi: 10.1128/JVI.01202-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang B, Chen W, Roman A. The E7 proteins of low- and high-risk human papillomaviruses share the ability to target the pRB family member p130 for degradation. Proc Natl Acad Sci U S A. 2006;103:437–42. doi: 10.1073/pnas.0510012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clawson GA, Miranda GQ, Sivarajah A, Xin P, Pan W, et al. Inhibition of papilloma progression by antisense oligonucleotides targeted to HPV11 E6/E7 RNA. Gene Ther. 2004;11:1331–41. doi: 10.1038/sj.gt.3302303. [DOI] [PubMed] [Google Scholar]
- 15.Liu DZ, Jin YX, Hou H, Huang YZ, Yang GC, et al. Preparation and identification of activity of anti-HPV-6b/11E1 universal ribozyme–Rz1198 in vitro . Asian J Androl. 1999;1:195–201. [PubMed] [Google Scholar]
- 16.Chen XZ, Zhu KJ, Xu Y, Tang XY, Cai XZ, et al. RNA interference silences the human papillomavirus 6b/11 early gene E7 in vitro and in vivo . Clin Exp Dermatol. 2010;35:509–15. doi: 10.1111/j.1365-2230.2009.03624.x. [DOI] [PubMed] [Google Scholar]
- 17.Seeger C, Sohn JA. Targeting Hepatitis B Virus with CRISPR/Cas9. Mol Ther Nucleic Acids. 2014;3:e216–22. doi: 10.1038/mtna.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuen KS, Chan CP, Wong NH, Ho CH, Ho TH, et al. CRISPR/Cas9-mediated genome editing of Epstein-Barr virus in human cells. J Gen Virol. 2015;96:626–36. doi: 10.1099/jgv.0.000012. [DOI] [PubMed] [Google Scholar]
- 19.Russell TA, Stefanovic T, Tscharke DC. Engineering herpes simplex viruses by infection-transfection methods including recombination site targeting by CRISPR/Cas9 nucleases. J Virol Methods. 2014;213C:18–25. doi: 10.1016/j.jviromet.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Hu W, Kaminski R, Yang F, Zhang Y, Cosentino L, et al. RNA-directed gene editing specifically eradicates latent and prevents new HIV-1 infection. Proc Natl Acad Sci U S A. 2014;111:11461–6. doi: 10.1073/pnas.1405186111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabadi AM, Ousterout DG, Hilton IB, Gersbach CA. Multiplex CRISPR/Cas9-based genome engineering from a single lentiviral vector. Nucleic Acids Res. 2014;42:e147–57. doi: 10.1093/nar/gku749. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.