Abstract
Angiosarcomas are rare and aggressive malignant tumours of vascular or endothelial origin that can originate in the breast. They can be classified as primary or secondary, with the latter most commonly due to postoperative radiotherapy as part of breast conservation therapy (wide local excision and adjuvant radiotherapy) for breast cancer. We report a case of postirradiation secondary angiosarcoma in a 56-year-old woman, alongside a review of the current literature, to inform clinicians of its clinical presentation and characteristics as a high index of clinical suspicion is required for an accurate diagnosis.
Background
Angiosarcomas, a subtype of soft-tissue sarcomas, are rare and aggressive malignant tumours of vascular or endothelial origin; with the most common histological appearance described as a vasoformative pattern of growth.1 Angiosarcomas can originate in a variety of places (liver, spleen, bone or heart), and approximately 8% arise in the breast.2 3 Indeed, they account for 0.04% of malignant neoplasms of the breast and have a poor long-term prognosis.4
Angiosarcomas can be classified as primary or secondary. Primary angiosarcomas (PAs) typically occur in women aged 30–50 years with no identifiable risk factors and are more likely to present with a palpable mass.5 Secondary angiosarcomas (SAs) may occur from chronic lymphoedema (known as the eponymous Stewart-Treves syndrome) or from radiotherapy. With breast conservation therapy (wide local excision and adjuvant radiotherapy) being undertaken more widely, there is a growing number of reports of postradiotherapy-induced SA (PRSA).5 A median latency period from radiation to development of SA is reported to be 7 years (range: 3–19 years).6 Perhaps a 5-year follow-up after breast conservation therapy is too short a time period.
We aim to describe a case of PRSA, with a review of the current literature, to inform clinicians of its clinical presentation and characteristics.
Case presentation
A 56-year-old woman originally presented in 2007 with a poorly mobile, firm lump in the lower quadrant of her breast. She had no significant medical or family history. On further examination, there was neither skin tethering, nipple inversion, discharge, nor palpable lymphadenopathy. Histology revealed an invasive ductal carcinoma (oestrogen and progesterone receptor positive).
Various surgical options were discussed and the patient decided to undergo breast conservation therapy with postoperative radiotherapy of 40 Gy delivered in 15 fractions of radiation given over 15 days, and additional antihormonal therapy (tamoxifen) to reduce the risk of recurrence. She was consequently followed up for 5 years with surveillance mammography, which revealed only postradiation changes.
In 2013, she presented again with left-sided mastalgia and a violaceous, red discolouration with bruising in the left lower quadrant of the breast, adjacent to the previous surgical scar (figures 1 and 2). Triple assessment (history and examination, ultrasound scan and core biopsy) was performed. Ultrasound imaging revealed non-specific skin thickening adjacent to the scar site and the subsequent core biopsy came back as a high-grade angiosarcoma, positive for CD34, composed of spindle cells, vascular and cystic spaces, and prominent areas of haemorrhage. A subsequent staging CT and bone scan did not show evidence of metastases.
Figure 1.
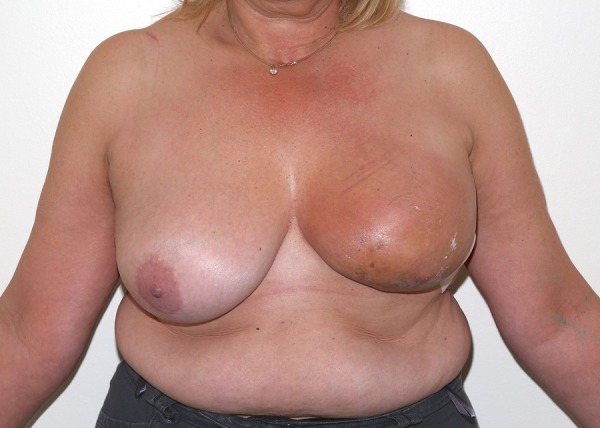
Violaceous red discolouration of the left breast.
Figure 2.
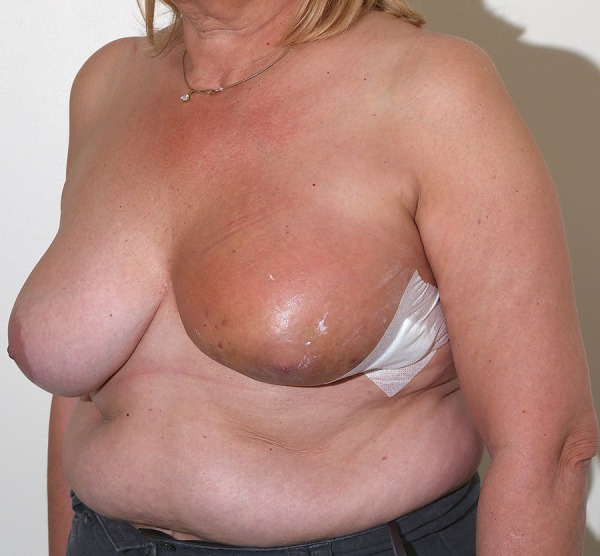
Violaceous red discolouration of the left breast.
Treatment
The patient was referred to the Regional Sarcoma Unit, where she agreed to a left mastectomy with an immediate latissimus dorsi reconstruction; no adjuvant therapy was given.
Outcome and follow-up
The patient was treated successfully: histology confirmed a complete excision with sufficient clear margins. She is currently on routine follow-up after having no signs of recurrence 18 months after the operation.
Discussion
PRSA was first defined by Cahan et al8 and further expanded on by Arlen et al7 as malignancy occurring in the location of the previous field of radiation, with latency of years after radiotherapy; histologically different from the primary neoplasm. In 1981, the first PRSA in the skin overlying an irradiated breast was reported.9 Indeed, an adjusted OR of 11.6 (95% CIs 4.3 to 26.1) for PRSA development in women with previous breast radiation has been reported.3 Clearly, with such a high OR and an increase in breast conservation therapy, the early recognition of PRSA is key to adequate treatment.
A retrospective study at the Mayo clinic (n=41) reported that SAs of the breast typically presented later in life compared with PA (median age: 73 vs 43 years, respectively, p<0.0001) and were more likely to be high grade (p=0.02): 33% (6/18 patients) with PA had high-grade features compared with 82% (9/11 patients) of patients with SA.5 Interestingly, despite a clear difference in grading, there was no difference in overall survival between the two forms for angiosarcoma: median time from diagnosis to death for PA and SA was 2.3 (range: 1–13.5) and 5.4 (range: 0.4–7.1) years, respectively (p=0.9); with a calculated 5-year overall survival of 46% and 69%, respectively (p=0.8).5 However, the small sample size requires that the data be interpreted cautiously as the results may not be applicable externally. In addition, the details of morbidity outcomes were not reported.
For a raised index of clinical suspicion, one must have an appreciation of the clinical characteristics allowing accurate recognition. SAs primarily tend to present with cutaneous discolouration (most commonly described as a violaceous, red rash) commonly with associated bruising (perhaps due to the vascular nature of the malignancy), as in our case.5 Initial presentations have been additionally described as an eczematous rash, haematoma like in appearance, or as a breast swelling.3 In a small proportion of people (7%), a palpable mass has been reported as the presenting symptom.5 One possible explanation for this is that surgery and adjuvant radiotherapy make it more difficult to palpate masses.
The imaging features of SA are highly variable and may delay the correct diagnosis. Mammography often reveals postsurgical changes with non-specific skin thickening—easily mistakable for postradiotherapy changes. It should be noted that such changes are likely to decrease in prominence over time.10 Furthermore, ill-defined, asymmetric masses have been reported in a subset of patients.11 Ultrasonography, similarly, may exhibit non-specific skin thickening, as noted in our case; associated masses range from circumscribed to ill-defined with accompanying hypervascularity, and mixed hyperechoic and hypoechoic regions.4 MRI of the breast (with gadolinium) may reveal a small foci of enhancement with haemorrhage within the thickened skin and seems to be the most promising imaging modality.3 4 12 Clearly, imaging only plays a complementary role in SA diagnosis and it remains a predominantly clinical diagnosis requiring a high index of suspicion.
In our case, postsurgical radiotherapy was given according to the guidelines set up the Royal College of Radiologists, based on grade B evidence.13 Despite this, there seems to be a growing incidence of PRSA. Perhaps further work is required to find a more suitable regime for radiotherapy to reduce PRSA. It is important to note that the increase in risk of developing PRSA does not displace the benefit of radiotherapy in breast conservation therapy.
Treatment of SA is primarily surgical (mastectomy with clear margins), as seen in our case. Adjuvant chemotherapy with doxorubicin or paclitaxel-based agents may be considered in patients with advanced disease; additional radiotherapy remains controversial.3 An overall survival of 18–36 months is reported although few report average survival to be longer than 3 years.5 Randomised trials are required to determine the role of adjuvant chemotherapy and radiotherapy on local recurrence and disease-specific survival.
In conclusion, PRSA is a growing phenomenon with the increase in breast conservation therapy and suffers a poor prognosis. Surgery with clear resection margins is currently the mainstay of treatment. Radiotherapy should continue to be offered as part of breast conservation therapy as its benefit far outweighs the risk of PRSA development. Breast cancer survivors who have undergone radiotherapy should be aware of the risk of PRSA and be educated about suspicious skin lesions. In addition, clinicians should have a high index of suspicion for any skin lesions developing after breast conservation therapy and should seek appropriate investigative measures.
Learning points.
Secondary angiosarcoma (SA) is a rare and aggressive malignancy with a rising incidence due to an increase in breast conservation therapy (wide local excision and adjuvant radiotherapy).
The risk of developing SA after radiotherapy should not offset the decision to deliver radiotherapy as part of breast conservation therapy for breast cancer.
SA is a clinical diagnosis that requires a high index of suspicion. Skin lesions presenting as a violaceous, red rash with associated bruising; an eczematous rash; a haematoma-like appearance; breast swelling or breast lump should seek further referral and biopsy.
Patients should be informed of the potential risk of SA after radiotherapy and be educated on seeking urgent medical opinion appropriately.
Surgery (mastectomy with clear margins) remains the mainstay treatment of SA.
Footnotes
Twitter: Follow Fahad Iqbal at @fmiqbal786
Contributors: FMI drafted the manuscript. BA and RV made suitable changes to the manuscript. All the authors agreed on the final draft before submission.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Eppelheimer CN, Marti JL, Eisenberg A et al. A case of secondary angiosarcoma of the breast after breast-conserving surgery and radiation: review of radiological and pathological findings. J Clin Imaging Sci 2015;5:45 10.4103/2156-7514.163989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldblum JR, Weiss SW, Folpe AL. Enzinger and Weiss's soft tissue tumors. St Louis: Mosby, 2001. [Google Scholar]
- 3.Zemanova M, Machalekova K, Sandorova M et al. Clinical management of secondary angiosarcoma after breast conservation therapy. Rep Pract Oncol Radiother 2014;19:37–46. 10.1016/j.rpor.2013.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang WT, Hennessy BTJ, Dryden MJ et al. Mammary angiosarcomas: imaging findings in 24 patients. Radiology 2007;242:725–34. 10.1148/radiol.2423060163 [DOI] [PubMed] [Google Scholar]
- 5.Scow JS, Reynolds CA, Degnim AC et al. Primary and secondary angiosarcoma of the breast: the Mayo Clinic experience. J Surg Oncol 2010;101:401–7. 10.1002/jso.21497 [DOI] [PubMed] [Google Scholar]
- 6.D'Angelo SP, Antonescu CR, Kuk D et al. High-risk features in radiation-associated breast angiosarcomas. Br J Cancer 2013;109:2340–6. 10.1038/bjc.2013.590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arlen M, Higinbotham NL, Huvos AG et al. Radiation-induced sarcoma of bone. Cancer 1971;28:1087–99. [DOI] [PubMed] [Google Scholar]
- 8.Cahan WG, Woodard HQ, Higinbotham NL et al. Sarcoma in irradiated bone. Report of eleven cases. Cancer 1948;1:3–29. [DOI] [PubMed] [Google Scholar]
- 9.Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer 1981;48:1907–21. [DOI] [PubMed] [Google Scholar]
- 10.Moore A, Hendon A, Hester M et al. Secondary angiosarcoma of the breast: can imaging findings aid in the diagnosis? Breast J 2008;14:293–8. 10.1111/j.1524-4741.2008.00577.x [DOI] [PubMed] [Google Scholar]
- 11.Glazebrook KN, Magut MJ, Reynolds C. Angiosarcoma of the breast. Am J Roentgenol 2008;190:533–8. 10.2214/AJR.07.2909 [DOI] [PubMed] [Google Scholar]
- 12.Sanders LM, Groves AC, Schaefer S. Cutaneous angiosarcoma of the breast on MRI. Am J Roentgenol 2006;187:W143–6. 10.2214/AJR.05.1940 [DOI] [PubMed] [Google Scholar]
- 13.The Royal College of Radiologists. Radiotherapy dose-fractionation. London, 2006. https://www.rcr.ac.uk/sites/default/files/publication/Dose-Fractionation_Final.pdf [Google Scholar]


