Abstract
We present a case of intracranial arteriovenous fistula with perimedullary venous drainage presenting with acute myelopathy, which is an unusual presentation of this uncommon condition. Subsequent catheter angiogram defined the arterial feeders from the meningohypophyseal trunk and petrosal branch of the middle meningeal artery. The patient was successfully embolised, resulting in complete obliteration of the fistula, and significant resolution of brainstem and cervical cord changes along with clinical improvement.
Background
Dural arteriovenous fistulas (DAVFs) comprise a heterogeneous collection of conditions, involving presence of shunts between dural arteries and dural venous drainage; they can occur either intracranially or in the spinal dura.1 Intracranial DAVFs account for 15% of all cerebrovascular malformations. Cranial DAVFs with perimedullary venous drainage are uncommon among these, but more likely to have an aggressive neurological course, and present with myelopathy and brainstem changes. Only a few isolated case reports and small case series have been described, with the majority of those cases involving the dura within the posterior cranial fossa or at the foramen magnum.2 We present a case of DAVF with perimedullary venous drainage, clinically presenting as rapid onset myelopathy, and discuss some of the characteristics and issues involved with pathophysiology and management.
Case presentation
A 65-year-old woman, while on a cruise in the Mediterranean, developed weakness in her right hand grip that soon progressed to involve the rest of the right-sided upper and lower limbs. Within the next 3 h, she developed dizziness, unsteadiness of gait, frequency of urine, vomiting and fluctuating consciousness. She was attended to by the ship's doctor; her symptoms were attributed to sea-sickness and low serum potassium, for which she was started on replacement therapy. She had a background history of hypertension, stage II chronic kidney disease (ex-kidney donor), and no history of trauma or craniotomy. Her symptoms persisted and, on the following day, she was noted to have developed weakness in the left lower limb. She was therefore transferred to the nearest hospital, in Puglia, Italy.
Investigations
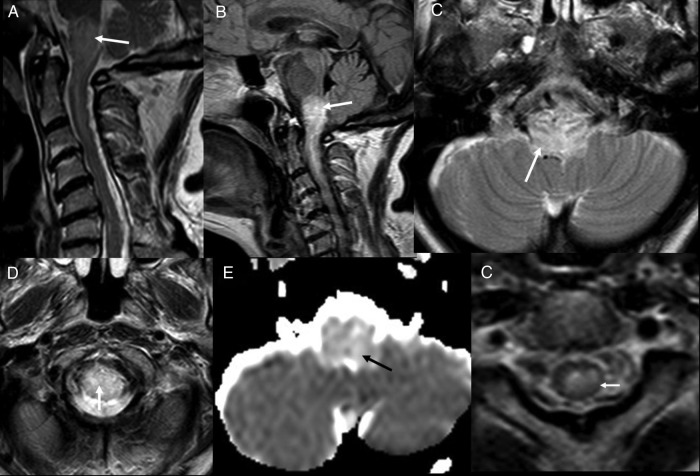
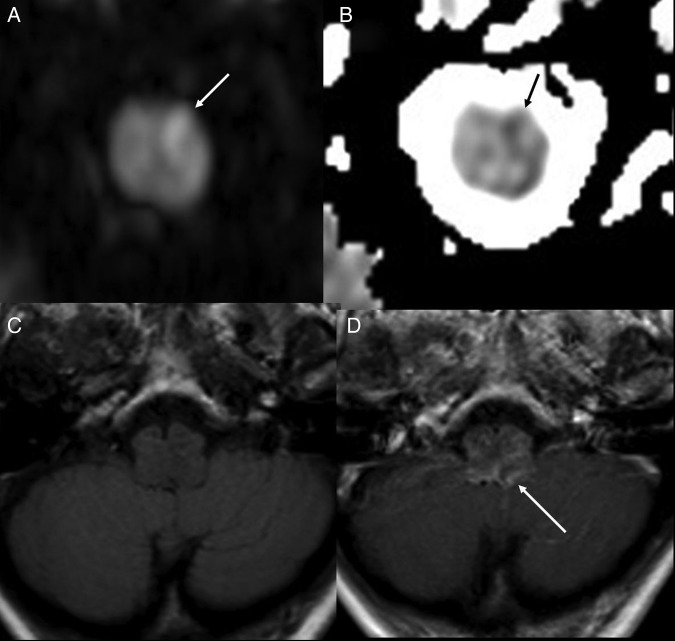
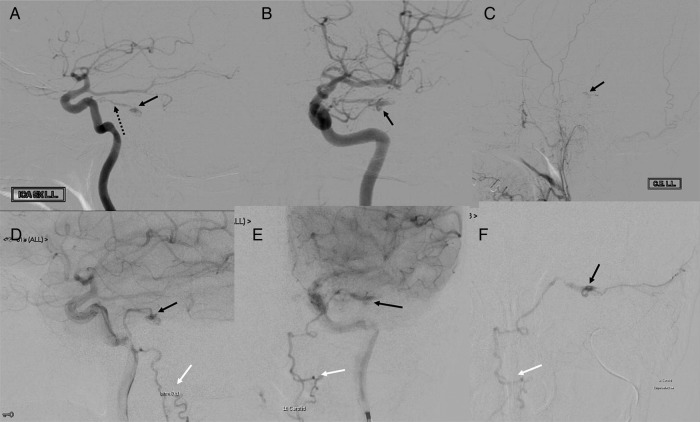
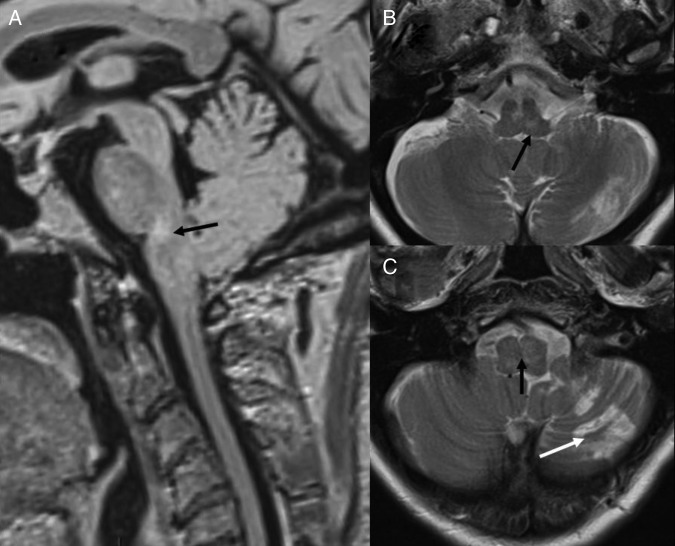
The patient had a CT scan of the brain, which was reported to be normal. Subsequent brain MRI showed evidence of brainstem swelling, and high signal in T2 images was detected in the lower medulla oblongata and upper cervical cord (figure 1). There was a thin rim of peripheral low T2 signal around the upper cervical cord. On diffusion imaging, there was a predominantly high ADC signal suggesting vasogenic oedema, although there was suggestion of a small area of restricted diffusion in the medulla on the left side, suggesting a small infarct (figure 2). On intravenous contrast, there was patchy enhancement, with vascular congestion in the lower brainstem and upper cervical cord (figure 2). The patient underwent cerebral digital subtraction angiography (DSA), which revealed a DAVF in the posterior fossa on the left side (figure 3). The arterial supply was from the tentorial branch of the meningohypophyseal trunk (MHT), with a very small feeder arising from the petrosal branch of the middle meningeal artery (MMA). The venous drainage was to an isolated segment of the left superior petrosal sinus (SPS), and from there via a communicating superior petrosal vein to the pontomedullary venous plexus and upper cervical perimedullary plexus. Small radiculomedullary communicating veins draining to the paravertebral venous plexus were also noted. The vein of Rosenthal was noted to be absent. There was a sluggish flow in the vein of Galen, and a stenosis of a short segment of the left transverse sinus.
Figure 1.
Initial MRI. T2 sagittal (A), FLAIR sagittal (B) and T2 axials (C and D) show presence of oedema in medulla and upper cervical cord (white arrow). ADC map (E) shows high ADC value suggestive of vasogenic oedema (black arrow). T2 axial (F) though upper cervical cord showing low peripheral T2 signal (white arrow). FLAIR, fluid attentuated inversion recovery; ADC, apparent diffusion coefficient.
Figure 2.
DWI (A) and ADC map (B), showing a probable small area of restricted diffusion (arrows) suggesting small recent infarct. T1 axial (C) and T1 axial post contrast (D) showing vascular congestion and enhancement (white arrow). DWI, diffusion weighted images; ICA, internal carotid artery.
Figure 3.
DSA. Left ICA injection. Lateral views (A and D) and AP views (B and E) showing MHT (broken arrow in A), site of fistula/nidus (black arrows) and perimedullary venous drainage (white arrows). Left ECA injection, lateral view (C), showing nidus (black arrow). Superselective MMA injection (F) showing site of fistula/nidus (black arrow) and perimedullary venous drainage (white arrow). AP, anteroposterior; DSA, digital subtraction angiography; ECA, external carotid artery; MHT, meningohypophyseal trunk; MMA, middle meningeal artery.
Differential diagnosis
Based on MRI findings, there can be a potential differential of encephalitis, neuromyelitis optica (NMO), tumour, sarcoid and vascular aetiologies. There were no febrile changes nor was the enhancement pattern on MRI particularly suggestive of encephalitis. The overall clinical picture and MRI enhancement pattern were also inconsistent with NMO, sarcoid or tumour. The large area of abnormality was difficult to attribute entirely to acute infarct both on a clinical basis and on diffusion MRI. Due to vascular congestion (figure 2D) and changes in the upper cervical cord (figure 1F), the possibility of a DAVF was raised; although the site was difficult to exactly guess on MRI, it could conceivably have been the posterior fossa. On subsequent DSA, the diagnosis was confirmed and no differential was considered.
Treatment
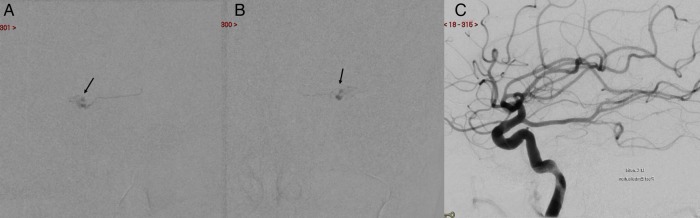
It was decided to embolise the DAVF. A microcatheter was fed via the MMA feeder and the tip was positioned within the nidal network. PHIL 25% (precipitating hydrophobic injectable liquid) was used to occlude the nidus and the arterialised segment of the SPS. Complete angiographic occlusion at completion was obtained (figure 4).
Figure 4.
Embolisation. PHIL cast seen (black arrows in A and B). Postembolisation left ICA injection, lateral view (C), shows absence of fistula. PHIL, precipitating hydrophobic injectable liquid.
Outcome and follow-up
The intraoperative and postoperative courses were uneventful. The patient made a very good recovery and was discharged in good condition 2 weeks later. Six weeks after discharge, the patient was free of symptoms apart from minimal remaining weakness (grade 4+) in the right lower limb. She was followed up in the neurovascular outpatient clinic 3 months following discharge: she was walking independently, with complete resolution of her symptoms. Her repeat MRI showed significant resolution of brainstem and upper cervical cord changes with minor residual signal changes (figure 5). However, there was an infarct on the inferior aspect of the left cerebellar hemisphere.
Figure 5.
Follow-up MRI. FLAIR sagittal (A) and T2 axials (B and C) show significant resolution of oedema with some residual changes (black arrow) and left cerebellar infarct (white arrow in C).
Discussion
The majority of DAVFs are idiopathic, while a minority are associated with preceded events such as trauma, cranial surgery and dural sinus thrombosis.3 Intracranial DAVFs represent about 6% of supratentorial and 35% of infratentorial vascular malformations.4 On review of the literature, we could identify 54 cases of DAVF with perimedullary venous drainage. In the majority of the reported cases, the fistula was located on the dura mater of the posterior fossa; of these cases, 18 were situated in the tentorium cerebilli, 11 in the petrosal sinus and petrous apex, 9 in the foramen magnum, 4 at the torcula, 7 at transverse/sigmoid sinuses, 1 each described in the occipital region and base of posterior fossa, while no exact description of the site of the fistula was given in three cases.2 5 6 The only reported site outside the posterior cranial fossa was in the cavernous sinus.2 With regard to the laterality of the shunt site, of the 54 cases reported, only 38 cases described the laterality, 76% were left sided, 21% were right sided and in 2.6% (single case), the fistula was situated at the torcula and received bilateral blood supply from both occipital arteries.7 There was a clear male preponderance with 66% males and 34% females (where gender was identifiable).2 Mean age was 52.8 and 55.4 years for females and males, respectively.
DAVFs may present incidentally or with symptoms related to the location and pattern of venous drainage.8 In 40 of the reported 54 cases, there were symptoms and/or signs of progressive myelopathy during initial presentation (74%), while eight patients had had no symptoms or signs of myelopathy. They presented with meningeal irritation and subarachnoid haemorrhage (SAH)—(4), aphasia (1), coma secondary to intraventricular haemorrhage associated with SAH (2) and headache, ataxia and cranial nerve palsies (1).7 9 In those presenting with myelopathy, most commonly, the symptoms were slowly progressive, starting with sensory disturbance and motor loss in the lower limbs, and extending to the upper limbs. Sphincter function was usually impaired, while the brainstem symptoms were the last to appear.10 Acute onset of symptoms has been reported in 25%.2
The pathophysiology of myelopathy has been a matter of debate.1 There are three hypotheses described: venous hypertension, arterial steal and direct compression by enlarged veins, clot or varicose vessels. Venous hypertension is more widely accepted; the fistula causes increased pressure in the perimedullary venous system with stagnation of the blood flow in intramedullary veins, leading to reduction in perfusion in microcirculation, and vasogenic oedema.11 However, there has been the occasional case of high-flow caroticocavernous fistula presenting with cervical myelopathy due to compression of the cervical spinal cord by dilated perimedullary veins.12 We wonder if there are altered patterns of venous drainage that can predispose to developing myelopathy in these patients. Interestingly, in our case, the vein of Rosenthal was absent, with a sluggish flow in the vein of Galen and stenosis of a short segment of the left transverse sinus. While some of these could be related to high-flow venopathy from the DAVF, it is possible that absence or paucity of alternate venous drainage pathways may result in predominant drainage through the perimedullary venous plexus, predisposing to myelopathy and other complications such as venous infarcts. Rarely, these patients can present exclusively with cerebral signs and symptoms without myelopathy. In such patients, working drainage for the venous blood from the congested perimedullary vessels into the epidural plexus via a medullary-radicular vein has been described, which would explain the missing myelopathy.7
The cause of rapid progression is not always certain but, generally, it is believed to be presence of thrombosis in vascular channels, or infarct or haemorrhage. In our case, the patient presented first with right sided rapidly progressive weakness, associated with some brainstem symptoms and later the left lower limb weakness that rapidly evolved within 24 h. There was suggestion of a small recent infarct in the left side of the medulla that may have caused sudden onset and deterioration.
The initial imaging diagnosis of such patients presenting with myelopathy is often challenging, since presenting symptoms are related to the spine, and they usually have spinal imaging in the first instance. It is important to identify the MRI changes, which primarily tend to be oedema in the brainstem and cervical cord. A low peripheral signal on T2-weighted images has been described.13 On DWI, there is usually high ADC signal due to vasogenic oedema, although it can vary if it is complicated by infarcts. Presence of abnormal vascular channels in the subarachnoid space is helpful and points towards a vascular aetiology. As expected, no parenchymal nidus is seen. Complications can include venous infarction, parenchymal and subdural haematomas, and cerebral vasogenic oedema.14
The definite diagnosis is by catheter DSA, which would include all six vessels. All of the reported cases in the literature had more than a single arterial feeder, the reported main arterial suppliers, however, were the MMA, occipital artery, ascending pharyngeal artery, MHT, vertebral artery, posterior meningeal artery, artery of the foramen rotundum, and meningeal branches of the internal and external carotid arteries. Based on their pattern of venous drainage, DAVFs draining to perimedullary veins are classified as type V based on Cognard classification, which signifies a quite aggressive clinical course.15
In the management of cranial DAVF, the pattern of venous drainage is determinant. As in all dural fistulas, treatment is directed towards closure of the draining vein, either by surgical or endovascular techniques (transvenous or transarterial).1 In the literature, the management was declared in 34 of 54 cases, embolisation (15), surgery (8) and embolisation followed by surgery (11). In view of potential serious complications, the goal of treatment is to obtain a rapid, complete anatomical cure of the lesion. Generally, all treatment options (embolisation, surgery or embolisation followed by surgery) remain justifiable, there is no evidence to consider any one treatment the rest, yet it depends on the patient presentation, lesion characteristics, surgeon preference and the availability of equipment and expertise. Complications can occur, including irreversible brainstem damage.16 Cortical venous reflux, which is associated with a mortality rate of 10.4%, may persist after treatment in some patients.17
We have presented an uncommon case of a patient with a DAVF with perimedullary venous drainage, who presented with progressive myelopathy, which was uncommon as it was rapid in onset, probably due to acute infarct. The patient was treated with transarterial embolisation, which completely obliterated the fistula, showing improvement in imaging and the clinical picture. This condition, being rare, can be difficult to clinically suspect and diagnose quickly, and will often need specialised centres for final diagnosis. DAVFs can be treated with vascular intervention or surgery, depending on available expertise, and overall anatomy and accessibility of the fistula.
Learning points.
Cranial dural arteriovenous fistulas (DAVF) is an important though rare cause of acute myelopathy.
Diagnosis is often difficult on cross-sectional imaging and digital subtraction angiography is essential for final diagnosis.
Depending on anatomy, treatment is often successful and complete.
The clinical outcome is good, if complete obliteration of the DAVF is achieved.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.van Rooij WJ, Sluzewski M, Beute GN. Intracranial dural fistulas with exclusive perimedullary drainage: the need for complete cerebral angiography for diagnosis and treatment planning. AJNR Am J Neuroradiol 2007; 28:348–51. [PMC free article] [PubMed] [Google Scholar]
- 2.Ricolfi F, Manelfe C, Meder JF et al. . Intracranial dural arteriovenous fistulae with perimedullary venous drainage. Anatomical, clinical and therapeutic considerations. Neuroradiology 1999;41:803–12. [DOI] [PubMed] [Google Scholar]
- 3.Chung SJ, Kim JS, Kim JC et al. . Intracranial dural arteriovenous fistulas: analysis of 60 patients. Cerebrovasc Dis 2002;13:79–88. [DOI] [PubMed] [Google Scholar]
- 4.Newton TH, Cronqvist S. Involvement of dural arteries in intracranial arteriovenous malformations. Radiology 1969;93:1071–8. [DOI] [PubMed] [Google Scholar]
- 5.Mahagne MH, Rogopoulos A, Paquis PH et al. . Fistules artérioveineuses durales intracraniennes a drainage veineux médullaire. Rev Neurol 1992;148:789–92. [PubMed] [Google Scholar]
- 6.Wiesmann M, Padovan CS, Pfister HW et al. . Intracranial dural arteriovenous fistula with spinal medullary venous drainage. Eur Radiol 2000;10:1606–9. 10.1007/s003300000382 [DOI] [PubMed] [Google Scholar]
- 7.Brunereau L, Gobin YP, Meder JF et al. . Intracranial dural arteriovenous fistulas with spinal venous drainage: relation between clinical presentation and angiographic findings. AJNR Am J Neuroradiol 1996;17:1549–54. [PMC free article] [PubMed] [Google Scholar]
- 8.Hurst RW, Bagley LJ, Galetta S et al. . Dementia resulting from dural arteriovenous fistulas: the pathologic findings of venous hypertensive encephalopathy. AJNR Am J Neuroradiol 1998;19:1267–73. [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou LF, Chen L, Song DL et al. . Tentorial dural arteriovenous fistulas. Surg Neurol 2007;67:472–81. 10.1016/j.surneu.2006.08.078 [DOI] [PubMed] [Google Scholar]
- 10.Jun L. Intracranial dural arteriovenous fistula with venous reflux to the brainstem and spinal cord mimicking brainstem infarction—case report. Neurol Med Chir 2004;44:24–8. [DOI] [PubMed] [Google Scholar]
- 11.Lagares A, Perez-Nunez A, Alday R et al. . Brief Report of Special Case Dural arteriovenous fistula presenting as brainstem ischaemia. Acta Neurochir (Wien) 2007;149:965–7. [DOI] [PubMed] [Google Scholar]
- 12.Narita Y, Watanabe Y, Hoshino T et al. . Myelopathy due to large veins draining recurrent spontaneous caroticocavernous fistula. Neuroradiology 1992;34:433–5. 10.1007/BF00596510 [DOI] [PubMed] [Google Scholar]
- 13.Hurst RW, Grossman I. Peripheral spinal cord hypointensity on T2-weighted MR images: a reliable imaging sign of venous hypertensive myelopathy. AJNR Am J Neuroradiol 2000;21:781–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Hähnel S, Jansen O, Geletneky K. MR appearance of an intracranial dural arteriovenous fistula leading to cervical myelopathy. Neurology 1998;51:1131–5. 10.1212/WNL.51.4.1131 [DOI] [PubMed] [Google Scholar]
- 15.Cognard C, Gobin YP, Pierot L et al. . Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology 1995;194:671–80. 10.1148/radiology.194.3.7862961 [DOI] [PubMed] [Google Scholar]
- 16.Takaaki M, Hara T, Inoue M et al. . Pontine venous congestion due to dural arteriovenous fistula of the cavernous sinus: case report and review of the literature. Surg Neurol Int 2012;3:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Dijk JM, terBrugge KG, Willinsky RA et al. . Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke 2002;33:1233–6. 10.1161/01.STR.0000014772.02908.44 [DOI] [PubMed] [Google Scholar]