Abstract
We present the case of a 69-year-old man who was found collapsed close to a heat source and admitted to hospital for severe sepsis. He was also found to have widespread blistering and ulceration of his right leg; however, a history was unobtainable due to reduced consciousness levels. The leg lesions had the initial appearance of mixed depth burns and a management plan was made to transfer the patient to a burns unit for debridement. It was subsequently noted that the patient had a previous diagnosis of seropositive erosive rheumatoid arthritis. A biopsy of the leg lesion was performed and a diagnosis of rheumatoid vasculitis confirmed. Treatment with systemic steroids, intravenous antibiotics and intravenous immunoglobulin therapy for severe hypogammaglobulinaemia was started, and the patient was not transferred for surgical debridement. Rheumatoid vasculitis is a rare and extremely serious complication of rheumatoid arthritis that can manifest in a number of ways, occasionally mimicking other conditions. This case is essential to raise awareness of rare, severe rheumatoid vasculitis and of the potential for its misdiagnosis as a mixed depth burn.
Background
This case stresses the importance of evaluating a clinical scenario fully, in order to avoid potentially unnecessary surgical management. In this acutely unwell patient, the suspected lower leg burn was, in fact, later proven to be florid rheumatoid vasculitis requiring aggressive medical treatment.
Case presentation
We present the case of a 69-year-old man who was admitted to the intensive care unit at our hospital after being found collapsed at home next to a cooker, by his carer.
On arrival, the patient was drowsy and haemodynamically unstable with a tachypnoea of 44 (normal 12–15) and fever of 38.7°C. His blood pressure was 106/60 mm Hg, and he was tachycardic at 143 bpm with a raised C reactive protein level at 408 (normal 0–4). His white cell count was noted to be normal at 5.
No history was available of the preceding events, due to a reduced consciousness level. A chest radiograph undertaken on initial presentation demonstrated bilateral reticulonodular lower zone shadowing consistent with pneumonia. A working diagnosis of sepsis was made and, given the patient's acutely unwell state, a full-body CT was performed to rule out other potential sources of infection. Urine dipstick test was normal.
The patient was Vietnamese in origin, lived alone and, although largely independent, had once-daily carers. His medical history consisted of hepatitis B with a low viral load, emphysema, bilateral cataracts and seropositive erosive rheumatoid arthritis. He was a heavy smoker with a 40-pack year history, and was HIV negative.
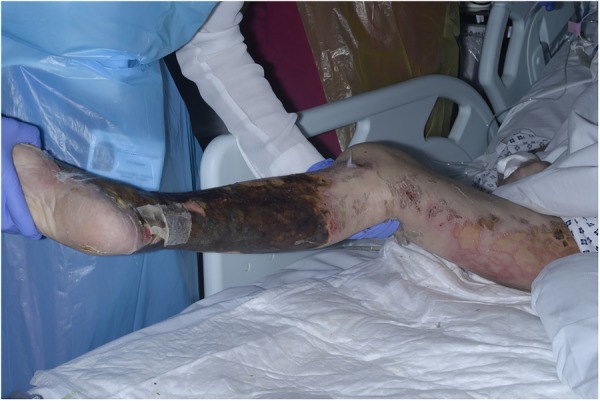
On examination, the patient had circumferential blistering and skin desquamation to 70% of his right lower leg and foot (figure 1). He had similar areas of blistering on the medial aspect of his right thigh (figure 2) and his left leg was unaffected. There were additional smaller areas of blistering and skin desquamation on the right lower leg, elbows and upper arms (<2% total body surface area). Minimal swelling accompanied the blistering and the leg remained well perfused with no tense compartments. Arterial Dopplers bilaterally were unremarkable with strong, audible pulses in the lower limbs. The impression of the attending plastic surgery team was a clinical picture of mixed depth burns sustained around the time of the patients collapse and a plan was made to transfer the patient to a burns unit for debridement.
Figure 1.
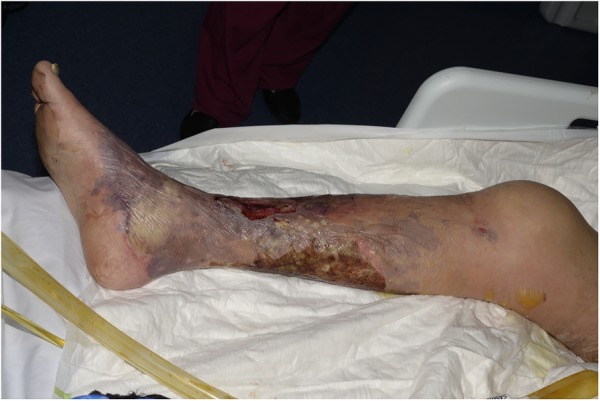
Appearance of mixed depth burns on the lower leg on initial presentation.
Figure 2.
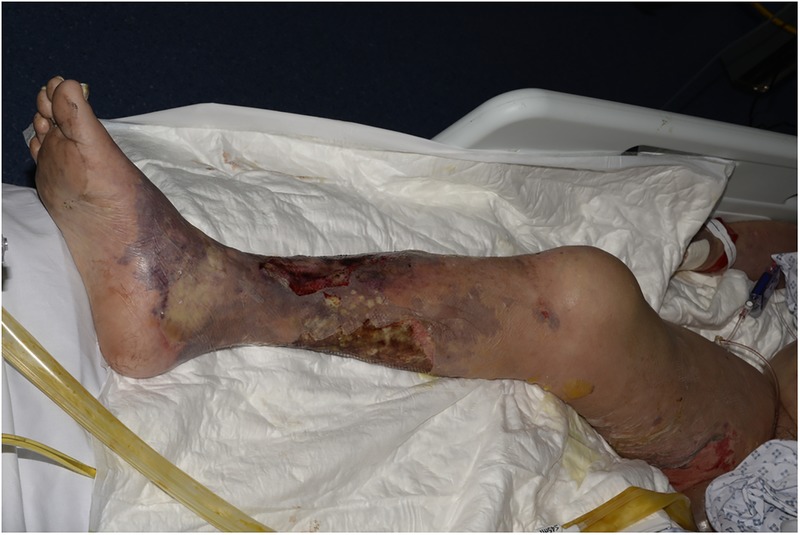
Appearance of mixed depth burns on the lower leg, with a misleading ‘splash effect’ of burns on the upper medial thigh on initial presentation.
The patient's legs did not appear to be infected on clinical assessment, with no evidence of spreading cellulitis, pus or superficial collections. The full-body CT confirmed no subcutaneous gas and no collections in the legs but did highlight a significant right middle lobe consolidation in the lungs, accompanied by bibasal consolidation on a background of severe emphysema.
Previous medical notes revealed that the patient had failed to attend a number of rheumatology outpatient appointments in the last year and therefore input from the rheumatology team was sought. A collateral history from his wife suggested that he had not been taking the leflunomide and hydroxychloroquine prescribed to manage his condition. The rheumatology team concluded that the clinical signs on the right leg and smaller vasculitic lesions found over the elbow and upper arms could be a manifestation of undertreated, severe rheumatoid arthritis, cryoglobulinaemia or polyarteritis nodosa (PAN). Under these conditions, surgical debridement of non-infected tissue would not be the correct management and therefore a vasculitic work up and skin biopsy of the affected leg were undertaken. Initial treatment with 100 mg hydrocortisone three times a day was started.
Investigations
Blood test investigations demonstrated a rheumatoid factor level of 21 (normal 0–19) and anti-cyclic citrullinated peptide level of 9 (normal 0–7). Antinuclear antibody (including Hep-2 antinuclear antibody) and extractable nuclear antigen antibody tests were negative. Tests for lupus anticoagulant, anticardiolipin antibodies and antineutrophil cytoplasmic antibodies were all negative. Warm testing for cryoglobulins and an antiphospholipid screen were also found to be negative. Complement levels of C3 levels were mildly low at 0.68 (0.75–1.65) but C4 levels were normal.
Given the patient's background of hepatitis B, PAN was considered as a differential diagnosis. However, his hepatitis B was well controlled and, on abdominal CT, there was no evidence of the typical wedge-shaped organ infarctions of PAN. Electromyography was performed, which did not show the vasculitic neuropathy commonly associated with PAN.
A skin biopsy of the right calf lesion demonstrated acute leucocytoclastic vasculitis with overlying epidermal necrosis (figures 3–5). This finding was in keeping with the diagnosis of rheumatoid vasculitis as it is a process that involves superficial postcapillary venules and deeper dermal vessels. No convincing thrombi were identified and there was no evidence of involvement of the medium-sized vessels in the deep dermis commonly associated with PAN. The biopsy indicated that thermal burns were unlikely to be the cause of the injury as areas of basal necrosis with overlying viable epidermis were observed, which is not traditionally a pattern observed in thermal injuries.
Figure 3.
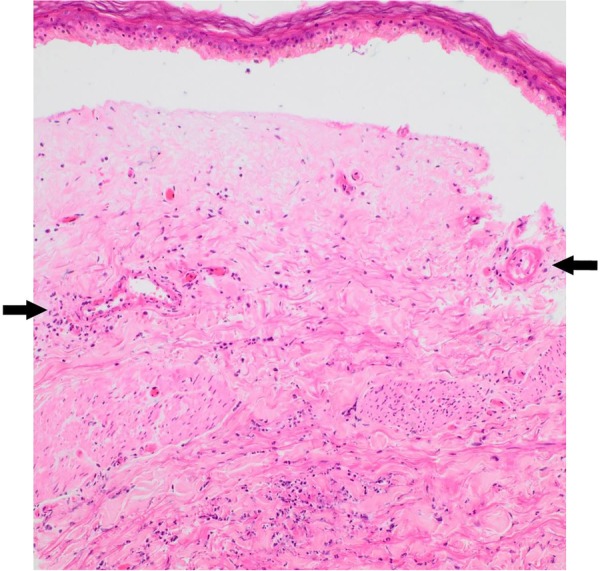
Dermal–epidermal separation. Superficial epidermis intact with basal infarction only. Thin-walled vessels in mid-dermis showing prominent fibrinoid necrosis of vessel walls associated with leucocytoclasia and neutrophilic infiltrate, consistent with leucocytoclastic vasculitis (H&E ×40) (see arrows).
Figure 4.
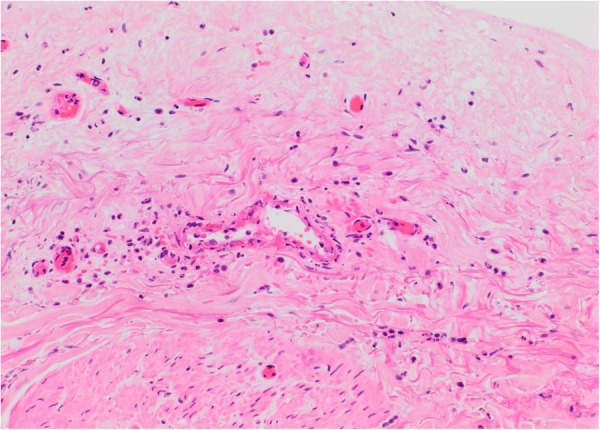
High power magnification of vessel from first image (H&E ×200).
Figure 5.
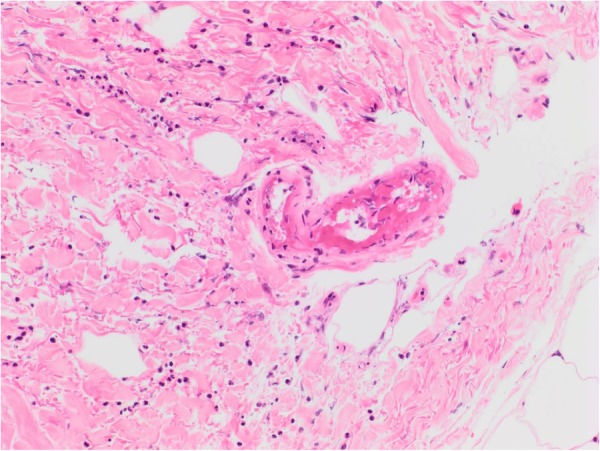
Features of leucocytoclastic vasculitis: prominent fibrinoid necrosis of thin-walled vessel walls in reticular dermis associated with nuclear dust and neutrophilic infiltrate.
Differential diagnosis
Mixed depth burns
Rheumatoid vasculitis
Cryoglobulinaemia
PAN
Treatment
The patient was treated for acute chest sepsis with intravenous co-amoxiclav and clarithromycin, as recommended by the microbiology department. Currently, there are no official guidelines for the management of rheumatoid vasculitis, as the condition is so rare.1 Accordingly, this patient was treated with intravenous immunoglobulin (0.4 g/kg/day for 5 days) for his severe hypogammaglobulinaemia, along with hydrocortisone 50 mg three times a day. Definitive treatment for the rheumatoid arthritis was deferred until his recovery from the acute sepsis.
Outcome and follow-up
The patient remains stable and has been extubated and stepped down from the intensive care unit onto the ward. As ongoing treatment for the rheumatoid vasculitis, rituximab has now been started. The right leg wounds are improving and remain dry and non-infected (figures 6 and 7).
Figure 6.
Deeper eschar appearance of lower leg and desquamation of thigh 10 days after initial presentation.
Figure 7.
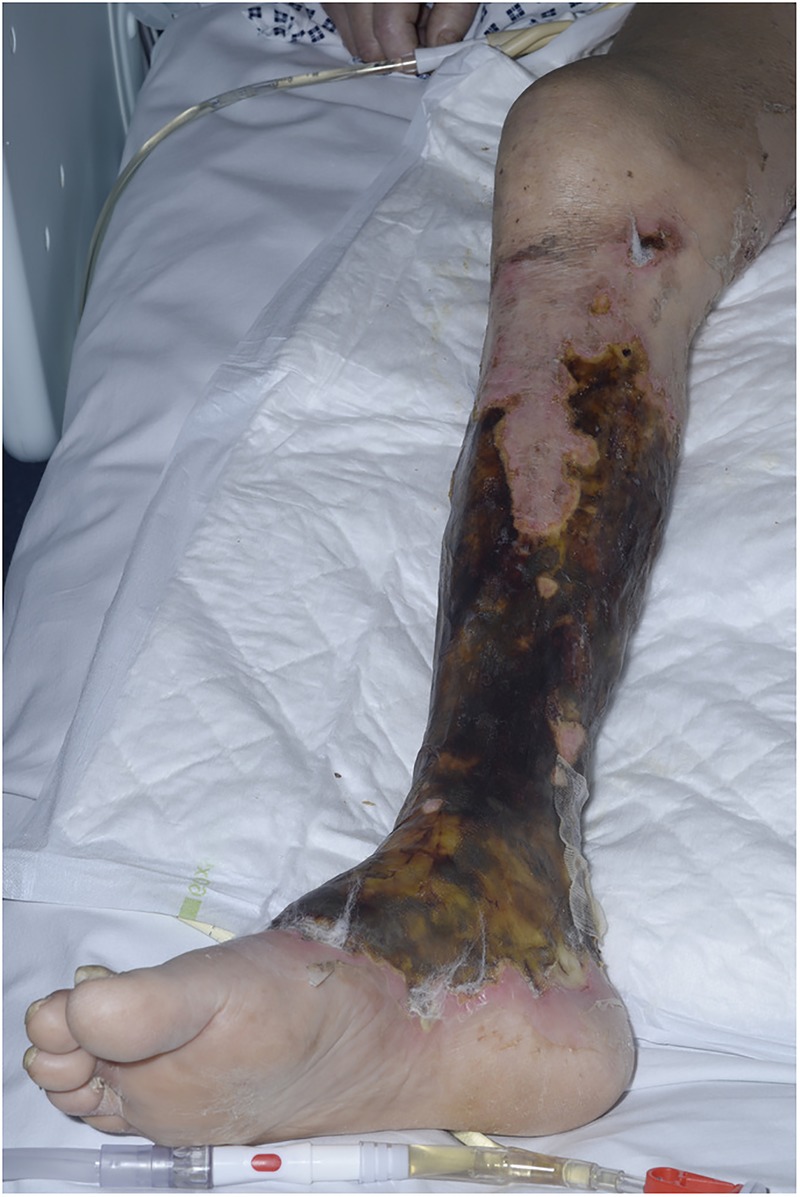
Deeper eschar appearance of lower leg lesion 10 days after initial presentation.
Discussion
To the best of our knowledge, this is the first case to be reported where severe, untreated rheumatoid vasculitis has manifested with signs and symptoms similar to those of a severe mixed depth burn and thus it is an essential contribution to the current literature.
Rheumatoid vasculitis was first identified in 1898 in the work of Bannatyne,2 who described an inflammatory process within the vasa nervorum of a patient suffering from neuritis. It is now recognised to be a highly heterogeneous condition that primarily affects small-sized and medium-sized vessels and that can manifest in a number of body systems.
The presenting signs and symptoms of rheumatoid vasculitis are notoriously varied. Cutaneously, it can manifest as purpura, nail fold infarcts, digital ischaemia of the fingertips, gangrene, and, as in this case, ulceration and blistering of the upper or lower extremities.3 Severe rheumatoid vasculitis almost invariably presents with bilateral signs, therefore, diagnostically, the unilateral distribution seen in this patient combined with the severity of the lesions, was highly unusual.
The last few decades have seen a significant improvement in the management of rheumatoid arthritis, both in terms of early aggressive treatment with disease modifying drugs and with the introduction of biological response modifying drugs.4 This improvement of management has, in turn, greatly reduced the incidence of rheumatoid vasculitis, as rheumatoid vasculitis tends to only present when the rheumatoid arthritis is poorly controlled. This decline in incidence of rheumatoid vasculitis may also be attributable to a reduction in smoking, which is its main environmental risk factor.5 Other known risk factors for developing rheumatoid vasculitis are male sex, long-standing seropositive nodular erosive disease and certain human leucocyte antigen (HLA) haplotypes.6 Although the HLA status of this patient is unknown, he had all the other risk factors, which provided additional weight to the diagnosis of severe rheumatoid vasculitis.
Owing to this increased rarity of rheumatoid vasculitis, clinicians have become less aware of it as a potential diagnosis and thus it is infrequently considered by non-rheumatological specialties.
This case is an essential contribution to the literature to highlight the importance of obtaining a thorough history and multidisciplinary teamwork. Clinicians should be vigilant with regards to diagnoses of patients and in ensuring that patients are not subject to unnecessary treatment.
Patient's perspective.
It was very frightening being so unwell and I feel lucky to have made a good recovery. I am looking forward to being back home as I can be more comfortable there. I am going to need more support in the future.
Learning points.
Improved treatment of rheumatoid arthritis has led to a decreased awareness of the rare but life-threatening complications of the disease.
Increased awareness of rheumatoid vasculitis, as a differential diagnosis for burns, is needed in order to prevent misdiagnosis and incorrect surgical management.
Even when a clinical situation appears clear, all of the available information must be carefully reviewed in order to avoid potentially harmful misdiagnoses.
Acknowledgments
The authors thank Dr William Rickaby, FRCPath (St John's Institute of Dermatology, St Thomas’ Hospital), for his kind provision and interpretation of the biopsy images.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Genta MS, Genta RM, Gabay C. Systemic rheumatoid vasculitis: a Review. Semin Arthritis Rheum 2006;36:88–98. 10.1016/j.semarthrit.2006.04.006 [DOI] [PubMed] [Google Scholar]
- 2.Bannatyne GA. Rheumatoid arthritis: its pathology, morbid anatomy, and treatment. London: Forgotten Books, 2013:73 (Original work published 1896). [Google Scholar]
- 3.Makol A, Matteson EL, Warrington KJ. Rheumatoid vasculitis: an update. Curr Opin Rheumatol 2015;27:63–70. 10.1097/BOR.0000000000000126 [DOI] [PubMed] [Google Scholar]
- 4.Ntatsaki E, Mooney J, Scott DG et al. . Systemic rheumatoid vasculitis in the era of modern immunosuppressive therapy. Rheumatology (Oxford) 2014;53:145–52. 10.1093/rheumatology/ket326 [DOI] [PubMed] [Google Scholar]
- 5.Makol A, Crowson CS, Wetter DA et al. . Vasculitis associated with rheumatoid arthritis: a case–control study. Rheumatology (Oxford) 2014;53:890–9. 10.1093/rheumatology/ket475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voskuyl AE, Zwinderman AH, Westedt ML et al. . Factors associated with the development of vasculitis in rheumatoid arthritis: results of a case-control study. Ann Rheum Dis 1996;55:190–2. 10.1136/ard.55.3.190 [DOI] [PMC free article] [PubMed] [Google Scholar]


