Abstract
Purpose
A study of real-time adaptive radiotherapy systems was performed to test the hypothesis that, across delivery systems and institutions, the dosimetric accuracy is improved with adaptive treatments over non-adaptive radiotherapy in the presence of patient-measured tumor motion.
Methods and materials
Ten institutions with robotic(2), gimbaled(2), MLC(4) or couch tracking(2) used common materials including CT and structure sets, motion traces and planning protocols to create a lung and a prostate plan. For each motion trace, the plan was delivered twice to a moving dosimeter; with and without real-time adaptation. Each measurement was compared to a static measurement and the percentage of failed points for γ-tests recorded.
Results
For all lung traces all measurement sets show improved dose accuracy with a mean 2%/2 mm γ-fail rate of 1.6% with adaptation and 15.2% without adaptation (p < 0.001). For all prostate the mean 2%/2 mm γ-fail rate was 1.4% with adaptation and 17.3% without adaptation (p < 0.001). The difference between the four systems was small with an average 2%/2 mm γ-fail rate of <3% for all systems with adaptation for lung and prostate.
Conclusions
The investigated systems all accounted for realistic tumor motion accurately and performed to a similar high standard, with real-time adaptation significantly outperforming non-adaptive delivery methods.
Keywords: Robotic tracking, Gimbaled tracking, MLC tracking, Couch tracking, Organ motion
Real-time adaptive radiotherapy has been developed to account for intrafraction motion during radiotherapy treatment delivery. It allows certainty in the dose delivery and for increased dose to the target through the implementation of stereotactic body radiotherapy (SBRT).
There are three ways to account for patient motion in real-time adaptive radiotherapy; shifting the treatment source, shifting the beam or adjusting the patient position. The first commercial realizations of real-time adaptive radiotherapy were the CyberKnife Synchrony robotic tracking system (Accuray, Incorporated, Sunnyvale, CA) in 2004 [1] and the MHI Vero tracking gimbaled linac system (Mitsubishi Heavy Industries, Ltd., Japan and BrainLAB AG, Feldkirchen, Germany) in 2011 [2]. Both systems are new machine designs that shift the treatment source to track the motion of the tumors. Multi-leaf collimator (MLC) tracking is a real-time adaptation technique implemented on a conventional linac that shifts the treatment beam to follow the tumor motion. The first clinical implementation of MLC tracking was done for prostate cancer treatment in 2013 [3]. Couch tracking is another system undergoing research, development and clinical translation and relies on shifting of the patient and tumor relative to the stationary treatment beam [4], [5].
Until now, the geometric and dosimetric assessments of real-time adaptive radiotherapy systems have been performed independently with extensive pre-clinical and clinical research done into robotic tracking [6], [7], gimbaled tracking [8], [9], [10], MLC tracking [11], [12], [13] and couch tracking [4], [5], [14]. The aim of this study was to assess the dosimetric accuracy of real-time adaptive treatments over non-adaptive radiotherapy in the presence of patient-derived tumor motion using a common set of tools.
Methods and materials
Common materials
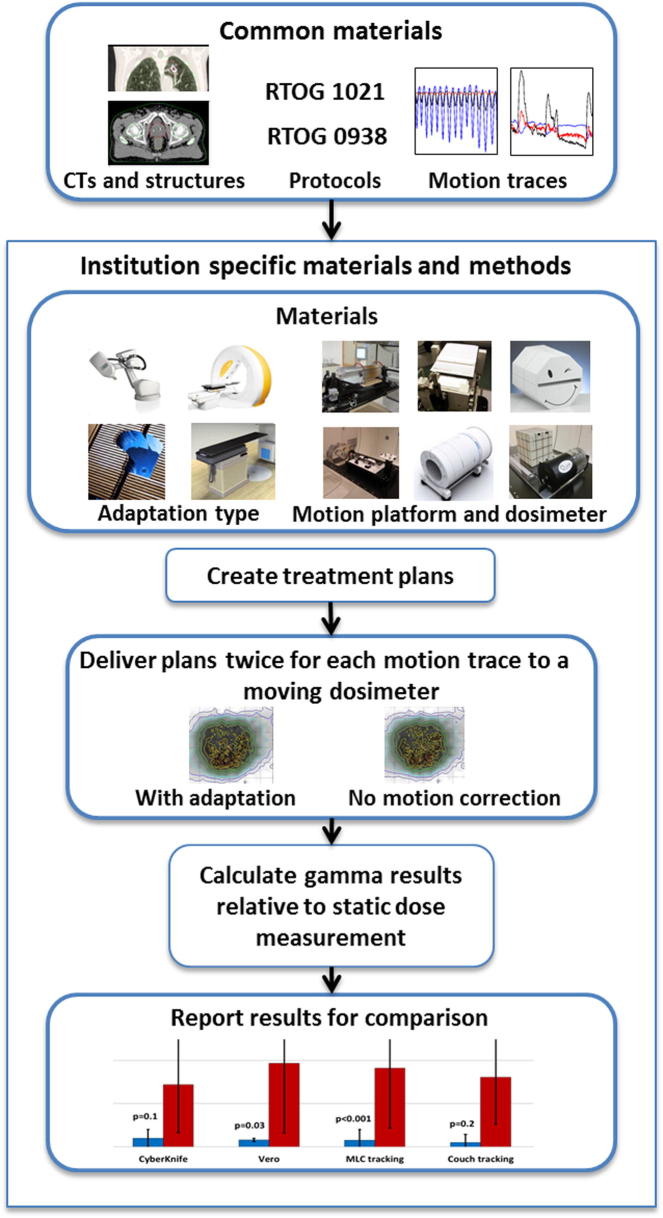
The philosophy of the study was to keep as much of the equipment and technique in common as possible, such as patient CT images, structures (e.g. target and organ-at-risk contours and CTV-PTV margins), planning dosimetric constraints, patient motion files and analysis metrics. An overview of the study is presented in Fig. 1.
Fig. 1.
Overview of the materials and methods of the study.
Contours
The lung contours were obtained from a stage I non-small cell lung cancer patient. The tumor volume was 7.2 cm3, which was the median size tumor of the 21 patients in the series [15]. The contour definitions and margins were per Radiation Therapy Oncology Group (RTOG) 1021. The CTV (=GTV) to PTV margins were 5 mm in all directions. This margin deviated from the RTOG guidelines of 5 mm laterally and 10 mm in the superior–inferior direction as the tumor motion is explicitly accounted for. For similarity across all platforms, only a single phase (end-exhale) of the 4DCT scan was used, simulating a single phase or breath hold scan for clinical use. The use of a single phase did not account for target or normal tissue deformation, however for the case chosen there was minimal deformation of the target from phase to phase.
The prostate contours were acquired from a patient with an average prostate volume (55.3 cm3) as determined from a previous study [16]. The contour definitions were per RTOG 0938. Note that 0938 explicitly included and stratified for both robotic tracking and linac-based treatments. The CTV to PTV margins were 3 mm posteriorly and 5 mm elsewhere. All data sets were anonymized for this study.
Dose prescription
The lung stereotactic dose was 54 Gy in 3 fractions, prescribed to 95% of the PTV volume (PTV D95) as per RTOG 1021. The dose constraints used were from the SBRT arm of RTOG 1021.
The prostate stereotactic dose prescription was 36.25 Gy in 5 fractions, prescribed to the PTV D95 as per RTOG 0938. The dose constraints used were from the five-fraction arm of RTOG 0938.
Motion traces
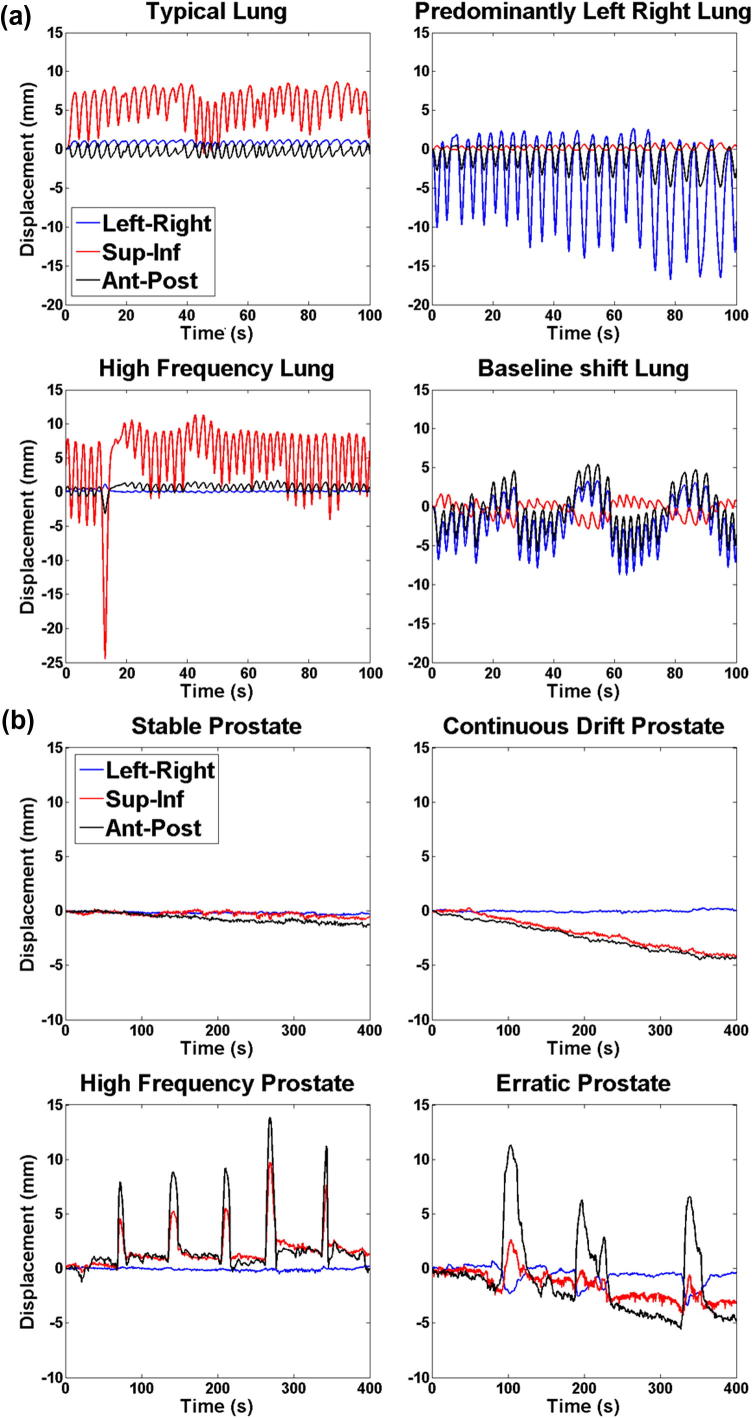
Four lung tumor and four prostate tumor motion traces were selected from large databases of patients measured with the CyberKnife Synchrony [17] (25 Hz sampling frequency) and the Calypso [18] (Varian Medical Systems, Palo Alto, CA, USA) (10 Hz sampling frequency) systems, respectively (Fig. 2). The traces were selected because they represented a variety of observed categories of motion for lung and prostate tumors. For lung a typical lung motion trace was selected representative of the majority of lung motion during treatments with the motion predominantly in the superior–inferior direction, three other more atypical traces were selected including motion predominantly in the left–right direction, high frequency motion and a trace with baseline shifts. A stable trace was selected for the prostate which represents the majority of prostate motions and three atypical motion traces were also selected including continuous drift, high frequency and erratic traces. Additionally, for the lung a one-dimensional (superior–inferior) sinusoidal motion trace was included with 1.5 cm range and 4 s period. The lung tumor traces also had an observed external surrogate motion for use by systems that rely on combined internal and external motion monitoring. When three degrees of freedom were not available to use for motion correction, the superior–inferior motion trace was used and the same external surrogate motion used. Each trace was 400 s in length, and it was repeated from the beginning, until the end of the delivery if the delivery was longer than 400 s.
Fig. 2.
Patient-measured (a) lung and (b) prostate tumor motion traces used for this study. The fifth lung trace (not pictured) is a 1D (superior–inferior) sinusoidal motion trace with range 1.5 cm and 4 s period.
Institution specific materials and methods
Overall 10 institutions with real-time adaptive radiotherapy systems participated in the study; two robotic tracking, two gimbaled tracking, four MLC tracking and two couch tracking institutions. The different institutions had different planning systems, dosimetry phantoms and motion platforms. The preferred dosimetry equipment was a volumetric-type detector and a motion platform to allow programable motion in at least one dimension (Table 1). For consistency, all treatments were performed with 6MV treatment beams.
Table 1.
Institution specific materials and methods for each of the ten measurement sets; including planning and delivery systems, experimental set ups and measurements obtained.
| Adaption Type | Institution | System version | Planning system | Treatment type | Motion guidance | Motion platform | Dosimetry phantom | Degrees of freedom (Lung/prostate) |
|---|---|---|---|---|---|---|---|---|
| Robotic tracking | University Clinic Schleswig-Holstein | CyberKnife | Multiplan | Robotic | kV and optical imaging | Custom | Octavius | 3D/3D |
| Stanford University | CyberKnife | Multiplan | Robotic | kV and optical imaging | CIRS dynamic phantom | Stereotactic Dose Verification Phantom | 1D/1D | |
| Gimbaled tracking | Kyoto University Hospital | Vero | iPLAN | IMRT | kV and optical imaging | Quasar (prostate) Custom (lung) | I’mRT | 4D/1D |
| University Hospital Brussels | Vero | iPLAN | Conformal | kV and optical imaging | BrainLab gating phantom | Quasar respiratory phantom | 1D/– | |
| MLC tracking | Northern Sydney Cancer Centre | Varian Trilogy Millennium MLC | Eclipse | VMAT | Calypso | HexaMotion | Delta4 | 3D/3D |
| Aarhus University Hospital | Varian Truebeam Millennium MLC | Eclipse | VMAT | Calypso | HexaMotion | Delta4 | 3D/3D | |
| Rigshospitalet Copenhagen | Varian Novalis Tx HD MLC | Eclipse | VMAT | Optical ExacTrac | HexaMotion | Delta4 | 3D/3D | |
| Institute of Cancer Research, London | Elekta Synergy Agility MLC | Pinnacle | IMRT | Motion platform | Custom | Delta4 | 3D/3D | |
| Couch tracking | University Hospital Zurich | Varian Trilogy Protura couch | Eclipse | VMAT | Optical | HexaMotion | Delta4 | 4D/2D |
| University Hospital of Würzburg | Elekta Synergy HexaPod couch | Pinnacle | VMAT | Optical | HexaPod | ArcCHECK | 3D/3D | |
IMRT = Intensity modulated radiation therapy.
VMAT = Volumetric modulated arc therapy.
kV = kilovoltage.
D = Degrees of freedom.
4D = Three degrees of freedom of dosimeter with 1D external surrogate motion.
The systems used by the ten institutions are shown in Table 1. The two robotic tracking institutions both used a CyberKnife Robotic Radiosurgery System and the multiplan planning system version 3.5 and 4.6 and the Synchrony system, kV and optical imaging was used as the motion guidance system. The institutions using gimbaled tracking both implemented the technique using the Vero system and iPlan 4.5.3 planning system (BrainLAB AG, Feldkirchen, Germany). kV only and combined kV and optical imaging was for motion guidance. MLC tracking was performed at four institutions; using a Varian Trilogy linac with Millennium MLC, a Varian Truebeam linac with millennium MLC, an Varian Novalis Tx linac with HD MLC (Varian Medical Systems, Palo Alto, CA) and an Elekta Synergy linac with Agility MLC (Elekta AB, Stockholm, Sweden). All used Eclipse planning system (Varian Medical Systems, Palo Alto, CA, USA) except for the last which used Pinnacle 9.8 (Philips Radiation Oncology Systems, Milpitas, CA). The two institutions implementing MLC tracking on the Varian platform used a Calypso input signal. The MLC tracking implemented on the Novalis used ExacTrac optical tracking (BrainLAB AG, Feldkirchen, Germany) and on the Synergy a motion signal from the motion platform was used with additional latency and noise (100 ± 10 ms). Couch tracking was implemented on a Varian trilogy linac using a Protura couch (Civco Medical Solutions) and Eclipse planning system and an Elekta Synergy using a HexaPOD evo RT system couch (Elekta AB, Stockholm, Sweden) and Pinnacle planning system. Both institutions used optical tracking for motion guidance.
All motion platforms used in this study had sub-millimeter accuracy with eight of the ten institutions using 3D or 4D (3D target motion plus external surrogate) motion for the lung measurements and two institutions using 1D programable motion platforms for lung measurements.
Five of the institutions used the Delta4 detector (ScandiDos AB, Uppsala, Sweden) which is comprised of two crossed panel diode arrays and has a spatial resolution of 5 mm centrally and 10 mm on the periphery. One institution used the arcCHECK (Sun Nuclear Corp., Melbourne, FL) dosimeter which is a cylindrical diode array with a detector spacing of 10 mm. One institution used the Octavius (PTW, Freiburg) which uses 2D ion chamber array with detector spacing of 2.5 mm. The other three institutions used film phantoms including a stereotactic Dose Verification Phantom (Standard Imaging Inc., Middleton, WI, USA), the I’mRT phantom (IBA, Schwarzenbruck, German) and the Quasar Respiratory Phantom (Modus Medical, Ontario, Canada) with Gafchromic® EBT3film (ISP, Wayne, NJ) with a positional accuracy of 0.5 mm and absolute dosimetric accuracy of 1.5% based the recommended analysis protocol for Gafchromic® EBT3.
Data collected
All participating institutions used standardized forms to report results. Data reported for the lung plan were the PTV homogeneity index (HI) , PTV conformity index (CI) (CI = the volume receiving the prescription isodose divided by the volume of the PTV), and mean lung dose. For prostate planning the PTV HI, PTV CI, mean rectal dose, and mean bladder dose were reported. The dosimetric results reported by each institution were the percentage of points passing 3%/3 mm, 2%/2 mm and 1%/1 mm global γ-tests (normalized to the maximum dose of the static reference and using 10% low dose cutoff) for motion with and without real-time adaptation for all four prostate motion traces and five lung traces. Institutions using film measurements used a 20% low dose cut off rather than 10% for the γ analysis due to a restriction in film size. Treatment delivery time was also reported by the institutions.
All institutions reported planning and dosimetric results for lung and nine of the ten institutions reported planning and dosimetric prostate results. As clinical prostate treatments have not been performed using real-time adaptation on the gimbaled tracking system to date, one institution did not report prostate planning and dosimetric results. The remaining gimbaled tracking institution reported 3%/3 mm γ-tests only, due to measurement device accuracy.
Statistical analysis
The Wilcoxon signed-ranked test was used to test the hypothesis, that real-time adaptive radiotherapy improves the dosimetric accuracy over non-adaptive radiotherapy in the presence of realistic tumor motion, between the sets of motion traces and between each adaptation type and the total collective adaptation and no motion adaptation sets. A p-value of less than 0.05 was considered to be statistically significant.
Results
The planning results showing the mean and range of CI, HI and mean organ at risk doses (mean lung, rectal and bladder doses), along with the treatment delivery times are shown in Table 2.
Table 2.
Mean and range of the reported planning values and average delivery time for the lung and prostate plans for all ten institutions measurement sets.
| Adaptation type |
||||
|---|---|---|---|---|
| Robotic tracking | Gimbaled tracking | MLC tracking | Couch tracking | |
| Lung | ||||
| CI | 1.15 (1.11–1.19) | 1.15 (1.11–1.2) | 0.99 (0.95–1.09) | 0.98 (0.97–0.98) |
| HI | 0.41 (0.39–0.42) | 0.58 (0.5–0.65) | 0.31 (0.17–0.51) | 0.41 (0.38–0.43) |
| Mean lung dose (Gy) | 3.9 (3.7–4.2) | 2.8 (2.7–2.99) | 2.9 (2.6–3.2) | 2.9 (2.6–3.2) |
| Treatment time (min) | 36.5 (30–43) | 18 (16–19.5) | 8.2 (6.2–11) | 11.5 (8–15) |
| Prostate | ||||
| CI | 1.13 (1.05–1.21) | 1.1 | 1.07 (0.97–1.16) | 1.02 (0.99–1.04) |
| HI | 0.17 (0.16–0.17) | 0.04 | 0.05 (0.03–0.06) | 0.04 (0.02–0.05) |
| Mean rectal dose (Gy) | 14.3 (12.0–16.7) | 13.3 | 12.6 (7.89–16.93) | 9.2 (8.4-10) |
| Mean bladder dose (Gy) | 12.8 (11.4–14.3) | 7.5 | 7.3 (5.9–8.9) | 7.0 (6.5–7.5) |
| Treatment time (min) | 37 (30–43.5) | 10 | 4.5 (3–6) | 4 (3–4.5) |
CI = Conformity index.
HI = Homogeneity index.
When comparing real-time adaptation to without adaptation for all ten institution sets combined there was a significant difference (p < 0.001) for both lung and prostate, for 3%/3 mm, 2%/2 mm, and 1%/1 mm γ-fail rates. For all lung traces (combined) all institution measurement sets showed improved dose accuracy with a mean 2%/2 mm γ-fail rate of 1.6% with adaptation and 15.2% without adaptation (p < 0.001). All prostate traces (combined) also had improved accuracy, the mean 2%/2 mm γ-fail rate was 1.4% with adaptation and 17.3% without adaptation (p < 0.001). The mean (±1 SD) percentage of points that failed the 3%/3 mm, 2%/2 mm and 1%/1 mm γ-tests for each trajectory with all institution sets combined and the γ-fail rates for each real-time adaptation system when all motion trajectory results were combined are available in the Supplemental material.
For both lung and prostate the high frequency motion traces resulted in the highest γ-fail rates while both the typical lung and the stable prostate traces resulted in the lowest γ-fail rates. The mean of the institution γ-fail rates for the 3%/3 mm γ criteria without adaptation was 19.0% and 8.5% for the high frequency lung and prostate traces respectively. These γ-fail rates were reduced with adaptation to 0.1% for both lung and prostate. Similarly for the 2%/2 mm and 1%/1 mm γ-tests the high frequency for both lung and prostate had the highest γ-fail rates without adaptation and with adaptation these γ-fail rates were significantly reduced (p < 0.001).
All four real-time adaptation systems performed similarly for both lung and prostate using the 3%/3 mm and 2%/2 mm test γ criteria. Larger differences were seen in the results of the 1%/1 mm γ-tests particularly for lung where the gimbaled tracking with adaptation did not result in a significant change from without adaptation. MLC tracking had four sets of measurements while the other adaptation systems had at most two sets, only one gimbaled tracking set for prostate was reported.
Discussion
Across four delivery systems and for a range of prostate and lung motions we have shown that real-time adaptation offers superior dose conformality to current delivery techniques. Small differences in the dosimetric fidelity of the four real-time adaptation systems in this study were observed for 3%/3 mm γ-tests. A larger dosimetric difference was observed between using real-time adaptive radiotherapy and no adaptation, the most common current treatment. It is worth noting that when the dosimetric differences between real-time adaptation and no adaptation are put in the context of other dosimetric errors in radiotherapy, we can conclude that motion is a leading order dosimetric error. The addition of motion (without adaptation) results in mean 3%/3 mm γ-fail rates much higher than the 2.1% mean fail rates seen for conventional IMRT commissioning where no motion is considered [19]. Random errors were not modeled for the conventional arm of study, but are implicitly accounted for within the adaptive arm. These results indicate that independent of the method used to account for motion in real-time, substantial treatment quality gains can be made by implementing real-time adaptive radiotherapy.
All four real-time adaptation systems were comparable when assessed by 3%/3 mm and 2%/2 mm γ-tests. The main difference was seen with the stricter 1%/1 mm criterion and is likely due to planning and measurement rather than a difference in dose delivery as many different treatment planning systems, motion platforms and detectors were used. In particular, the gimbaled tracking 1%/1 mm γ-test results in this study (measured using film dosimetry) do not mirror the results of previous geometric accuracy studies [9], [20] that demonstrate high geometric accuracy comparable with other real-time adaptation techniques. The outcome of the statistical analysis may have been affected by the sample size as MLC tracking had four sets of measurements while the other adaptation systems had at most two sets, only one gimbaled tracking set for prostate was reported.
The dosimetric failures were observed to be both inside the high dose target volumes and in the low dose areas toward the edges of the dose distributions for both the lung and prostate cases, with lung results showing a greater number of failures near the edge of the target volumes potentially because of the larger motion trajectories and steeper dose gradients. The cause of the failures is a complex interaction between the plan complexity, delivery time with respect to motion, dose delivery angle and position with respect to motion and various real-time adaptation technique specific factors.
Planning using different treatment planning systems for different treatment techniques and platforms means that there are differences in the treatment plans used by the institutions in this study. These differences in plans, such as the steepness of dose gradients, could have an effect on the γ-test results. The ranges of both the CI and HI (Table 2) of the plans for the different real-time adaptation systems have overlapped in this study but are not exactly the same and this will have carried through to the dosimetric results.
The delivery systems have some key differences including motion detection, prediction and correction strategies, however the overall results are similar in that they effectively account for intrafraction motion during dose delivery as evident in the 3%/3 mm results in this study.
A notable difference in the results is the delivery times, with robotic tracking taking the longest time to deliver a plan and both robotic tracking and gimbaled tracking having significantly longer treatment times than those of the MLC tracking and couch tracking deliveries. The different planning and delivery techniques used by each of the institutions also contribute to a range of treatment times for all four real-time adaptation techniques.
The difference in measurement devices likely had a large effect on the dose result comparison between the institutions in this study. Dosimeters used in this study included diode devices Delta4 (five institutions) and ArcCHECK (one institution), the Octavius ion chamber detector (one institution) and film dosimetry devices (four institutions). Each of the dosimeters has different limitations and advantages including different spatial and dosimetric accuracies. Future studies could use consistent dosimetry equipment however trade-offs would still need to be made, for example the dosimetric accuracy of ion chambers or diodes vs. the spatial resolution of film.
In this study a 5 mm CTV to PTV margin was used for our lung contour rather than the RTOG 1021 protocol of 5 mm with a 1 cm margin in the superior–inferior direction or the use of an Internal Target Volume (ITV) because the motion was explicitly accounted for. Treatment margins are comprised of various components including contouring uncertainties and tumor deformation as well as potential motion related factors of specific real-time adaptation techniques such as surrogacy uncertainties and residual motion due to tracking and delivery accuracy. The results presented here in the form of gamma failure rates cannot directly be translated into margin reductions but could allow for increased confidence in the delivery of existing plans.
Real-time adaptive technologies will evolve and improve further in their geometric and dosimetric fidelity as localization methods improve, latency times are reduced, prediction algorithms improve, and hardware limitations, such as velocity and acceleration constraints, are overcome. The results presented here can be considered as a snapshot in time and can be used as a benchmark for future system improvements or for yet-to-be-developed real-time adaptive radiotherapy systems. The real-time adaptive results will continue to get closer to results that would be achieved if no motion were present; the no motion compensation results will stay the same. Therefore the dosimetric difference between real-time adaptive radiotherapy and no motion compensation will continue to increase.
Conclusion
A multi-institutional comparison study encompassing robotic, gimbaled, multileaf collimator and couch tracking was performed between real-time adaptive radiotherapy systems and non-adaptive radiotherapy. The results show that the systems all account for realistic tumor motion accurately and performed to a similar high standard, with real-time adaptation significantly outperforming non-adaptive methods. To enable the performance of this study at any radiotherapy center, the input data, method and report forms can be downloaded from http://sydney.edu.au/medicine/radiation-physics/data/real-time-benchmarking-data.php
Conflicts of interest
Prof. Keall and Dr. Booth report grants from Varian Medical Systems. The Institute of Cancer Research acknowledge support from Elekta AB under a research agreement and were also supported by Cancer Research UK under Programme C33589/A19727 and NHS funding to the NIHR Biomedical Research Centre at The Royal Marsden and The Institute of Cancer Research. Dr. Poulsen and Dr. Worm reports grants and non-financial support from Varian Medical Systems. Dr. Ravkilde reports non-financial support from Scandidos. Dr. Pommer reports grants from Niels Bohr Institute, University of Copenhagen and grants from Varian Medical Systems.
Acknowledgments
Christian Rønn Hansen and Carsten Brink for the lung dataset, Thomas Eade for the prostate dataset, Deborah Whalley for contouring the datasets per protocol.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.radonc.2016.03.006.
Appendix A. Supplementary data
Mean (mean ± 1 SD) percentage of points that failed the 3%/3 mm, 2%/2 mm and 1%/1 mm γ-tests with and without adaptation for each of the motion traces (all adaptation type results combined). * indicate Wilcoxon sign rank p-values < 0.05 for the adaptation to no motion correction pairs.
Mean (mean ± 1 SD) percentage of points that failed the 3%/3 mm, 2%/2 mm and 1%/1 mm γ-tests with and without adaptation for each of the adaptation systems (all motion trace results combined). * indicate Wilcoxon sign rank p-values < 0.05 for the adaptation to no motion correction pairs.
References
- 1.Koong A.C., Le Q.T., Ho A., Fong B., Fisher G., Cho C. Phase i study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017–1021. doi: 10.1016/j.ijrobp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Depuydt T., Poels K., Verellen D., Engels B., Collen C., Buleteanu M. Treating patients with real-time tumor tracking using the vero gimbaled linac system: implementation and first review. Radiother Oncol. 2014;112:343–351. doi: 10.1016/j.radonc.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Keall P.J., Colvill E., O’Brien R., Ng J.A., Poulsen P.R., Eade T. The first clinical implementation of electromagnetic transponder-guided mlc tracking. Med Phys. 2014;41:020702. doi: 10.1118/1.4862509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang S., Zeimetz J., Ochsner G., Schmid Daners M., Riesterer O., Klöck S. Development and evaluation of a prototype tracking system using the treatment couch. Med Phys. 2014;41:021720. doi: 10.1118/1.4862077. [DOI] [PubMed] [Google Scholar]
- 5.Wilbert J., Baier K., Hermann C., Flentje M., Guckenberger M. Accuracy of real-time couch tracking during 3-dimensional conformal radiation therapy, intensity modulated radiation therapy, and volumetric modulated arc therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2013;85:237–242. doi: 10.1016/j.ijrobp.2012.01.095. [DOI] [PubMed] [Google Scholar]
- 6.Dieterich S., Pawlicki T. Cyberknife image-guided delivery and quality assurance. Int J Radiat Oncol Biol Phys. 2008;71:S126–S130. doi: 10.1016/j.ijrobp.2007.08.081. [DOI] [PubMed] [Google Scholar]
- 7.Poels K., Depuydt T., Verellen D., Gevaert T., Dhont J., Duchateau M. Improving the intra-fraction update efficiency of a correlation model used for internal motion estimation during real-time tumor tracking for sbrt patients: fast update or no update? Radiother Oncol. 2014;112:352–359. doi: 10.1016/j.radonc.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Depuydt T., Verellen D., Haas O., Gevaert T., Linthout N., Duchateau M. Geometric accuracy of a novel gimbals based radiation therapy tumor tracking system. Radiother Oncol. 2011;98:365–372. doi: 10.1016/j.radonc.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Poels K., Depuydt T., Verellen D., Engels B., Collen C., Heinrich S. A complementary dual-modality verification for tumor tracking on a gimbaled linac system. Radiother Oncol. 2013;109:469–474. doi: 10.1016/j.radonc.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Mukumoto N., Nakamura M., Sawada A., Takahashi K., Miyabe Y., Takayama K. Positional accuracy of novel x-ray-image-based dynamic tumor-tracking irradiation using a gimbaled mv x-ray head of a vero4drt (mhi-tm2000) Med Phys. 2012;39:6287–6296. doi: 10.1118/1.4754592. [DOI] [PubMed] [Google Scholar]
- 11.Keall P.J., Cattell H., Pokhrel D., Dieterich S., Wong K.H., Murphy M.J. Geometric accuracy of a real-time target tracking system with dynamic multileaf collimator tracking system. Int J Radiat Oncol Biol Phys. 2006;65:1579–1584. doi: 10.1016/j.ijrobp.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 12.Fast M.F., Nill S., Bedford J.L., Oelfke U. Dynamic tumor tracking using the elekta agility mlc. Med Phys. 2014;41:111719. doi: 10.1118/1.4899175. [DOI] [PubMed] [Google Scholar]
- 13.Falk M., af Rosenschöld P.M., Keall P., Cattell H., Cho B.C., Poulsen P. Real-time dynamic mlc tracking for inversely optimized arc radiotherapy. Radiother Oncol. 2010;94:218–223. doi: 10.1016/j.radonc.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menten M.J., Guckenberger M., Herrmann C., Krauß A., Nill S., Oelfke U. Comparison of a multileaf collimator tracking system and a robotic treatment couch tracking system for organ motion compensation during radiotherapy. Med Phys. 2012;39:7032–7041. doi: 10.1118/1.4761868. [DOI] [PubMed] [Google Scholar]
- 15.Hansen C.R., Bertelsen A., Riis H.L., Christiansen R.L., Hansen O., Sykes J.B., Thwaites D.I., Brink C. Plan quality and delivery accuracy of flattening filter free beam for sbrt lung treatments. Acta Oncol. 2015:1–6. doi: 10.3109/0284186X.2014.956184. [DOI] [PubMed] [Google Scholar]
- 16.Ng J.A., Booth J.T., Poulsen P.R., Fledelius W., Worm E.S., Eade T. Kilovoltage intrafraction monitoring for prostate intensity modulated arc therapy: first clinical results. Int J Radiat Oncol Biol Phys. 2012;84:e655–e661. doi: 10.1016/j.ijrobp.2012.07.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suh Y., Dieterich S., Cho B., Keall P.J. An analysis of thoracic and abdominal tumour motion for stereotactic body radiotherapy patients. Phys Med Biol. 2008;53:3623. doi: 10.1088/0031-9155/53/13/016. [DOI] [PubMed] [Google Scholar]
- 18.Langen K.M., Willoughby T.R., Meeks S.L., Santhanam A., Cunningham A., Levine L. Observations on real-time prostate gland motion using electromagnetic tracking. Int J Radiat Oncol Biol Phys. 2008;71:1084–1090. doi: 10.1016/j.ijrobp.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 19.Ezzell G.A., Burmeister J.W., Dogan N., LoSasso T.J., Mechalakos J.G., Mihailidis D. Imrt commissioning: multiple institution planning and dosimetry comparisons, a report from aapm task group 119. Med Phys. 2009;36:5359–5373. doi: 10.1118/1.3238104. [DOI] [PubMed] [Google Scholar]
- 20.Depuydt T., Poels K., Verellen D., Engels B., Collen C., Haverbeke C. Initial assessment of tumor tracking with a gimbaled linac system in clinical circumstances: a patient simulation study. Radiother Oncol. 2013;106:236–240. doi: 10.1016/j.radonc.2012.12.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mean (mean ± 1 SD) percentage of points that failed the 3%/3 mm, 2%/2 mm and 1%/1 mm γ-tests with and without adaptation for each of the motion traces (all adaptation type results combined). * indicate Wilcoxon sign rank p-values < 0.05 for the adaptation to no motion correction pairs.
Mean (mean ± 1 SD) percentage of points that failed the 3%/3 mm, 2%/2 mm and 1%/1 mm γ-tests with and without adaptation for each of the adaptation systems (all motion trace results combined). * indicate Wilcoxon sign rank p-values < 0.05 for the adaptation to no motion correction pairs.