Abstract
Infections from Enterococcus faecalis and Enterococcus faecium are uncommon in the post-neurosurgical intervention setting., [1, 2, 3, 4] Intraventricular antibiotics are recommended when standard intravenous therapy fails. [5] Here we present a case of post-neurosurgical ventriculitis, meningitis, and cerebritis in an oncology patient caused by refractory Enterococcus faecalis successfully treated with intraventricular vancomycin.
Key Words: infection, oncology, intraventricular, vancomycin
Ventriculitis is a complication of meningitis in 30% of adult cases with the risk increasing to 45% in patients with an external ventricular drain (EVD). The most common pathogens identified to cause EVD or cerebral spinal fluid (CSF) shunt infections are gram-positive organisms such as Staphylococcus epidermidis and Staphylococcus aureus.1 However, Enterococcus is increasingly described in postneurosurgical intervention case reports.2,3 Management of most enterococcal infections involves single bacteriostatic agents. However, combination therapy with the addition of gentamicin is suggested for more severe infections such as meningitis.4,5 Although there is limited experience, patients not responding to intravenous therapy can then be considered for intraventricular (IVT) antibiotics such as vancomycin of 5 to 20 mg or gentamicin of 1 to 8 mg.6 Infectious Diseases Society of America guidelines recommend ceftriaxone plus vancomycin for empiric meningitis therapy when the organism is unknown. However, if the culture data reveal ampicillin-susceptible Enterococcus, then ampicillin plus an aminoglycoside remains the therapy of choice. There are limited studies that suggest a synergistic effect of the combination of ampicillin and ceftriaxone for the treatment of E. faecalis endocarditis.7–9 Unfortunately, no studies have been published confirming the efficacy of this regimen for the treatment of enterococcal meningitis, and therefore, ceftriaxone should not be continued.
CASE REPORT
A 44-year-old Kuwaiti woman with HER-2/Neu + score of +3 infiltrating ductal carcinoma of the right breast was transferred from Kuwait to the Cancer Treatment Centers of America in Philadelphia, Pennsylvania, on August 8, 2014. She arrived hyporesponsive, tachycardic, and hypotensive; with a temperature of 101.8°F; and mechanically ventilated. She had previously received multiple rounds of chemotherapy, radiation, and a craniotomy for resection of a tumor. Before her arrival, she developed increased intracranial pressure (ICP) and aspiration pneumonia leading to intubation. With presumed pneumonia and, possibly, meningitis, the intensive care unit medical team at Cancer Treatment Centers of America continued meropenem of 2 g every 8 hours because of an unknown penicillin allergy and provided medical support for septic shock. A magnetic resonance imaging of the brain revealed mild hydrocephalus and a large enhancing cavitary lesion in the right hemisphere. On August 12, a craniotomy was performed to place a ventricular drain and assist in monitoring ICPs due to her report of elevated ICP at the previous hospital. Epidural and intracavitary swabs were performed intraoperatively; these and CSF cultures revealed heavy growth of Enterococcus faecalis. The patient was initiated on therapeutic doses of vancomycin in addition to meropenem. The patient continued to be nonresponsive despite no use of analgesics or sedatives. With benefits outweighing the risks of a reaction to a potential penicillin allergy, the patient was changed from meropenem and vancomycin to ampicillin plus gentamicin for better Enterococcus coverage. However, all subsequent CSF cultures from August 13 through 27 also remained positive. Table 1 demonstrates the timeline of culture data as well as antibiotic treatment courses.
TABLE 1.
Antibiotic Courses and Culture Data
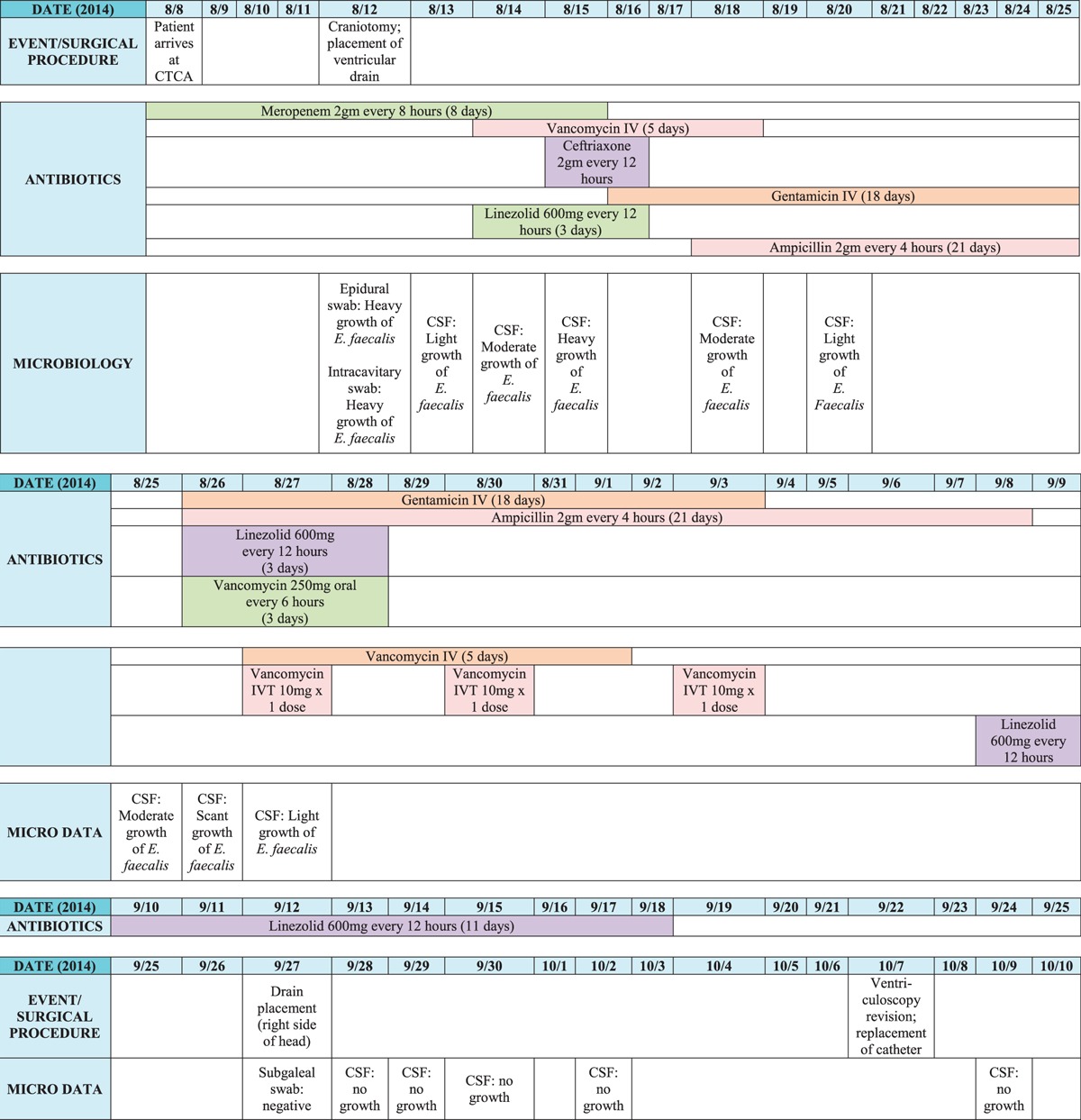
On August 18, the patient tried to open her eyes for the first time and soon began to regain her strength on the right side. She was then extubated on August 20 and began to verbally communicate during the following week. Despite improvement in her mental status, the cultures remained positive. The only option for further treating the CSF was vancomycin administered intraventricularly via the ventriculostomy drain. Minimal information is available in the literature to guide IVT therapy. However, it has been described that, in cases of ventriculitis, there may be a lack of significant inflammatory response. Therefore, drug penetration across the blood-brain barrier is compromised, and CSF concentrations may be reduced. Direct instillation of vancomycin into the CSF may be the optimal therapy to reach adequate concentrations.10 Recommended dosing is based on the estimated ventricular volume. In adults, recommended vancomycin dosing is 5 mg with slit ventricles, 10 mg with normal-sized ventricles, and 15 to 20 mg with enlarged ventricles.2 The frequency of administration is based on volume of CSF drainage: once-daily dosing for CSF drainage of greater than 100 mL/d, every other day if the drainage is 50 to 100 mL/d, and every third day if drainage is less than 50 mL/d.2 Some data suggest that monitoring concentrations of vancomycin in the CSF is also useful as elevated levels can be associated with neurotoxicity.2,10 The desired 24-hour trough concentration for vancomycin in the CSF is 10 to 20 μg/mL.10 This patient had normal-sized ventricles, and the CSF drainage frequently remained less than 50 mL/d. Therefore, vancomycin of 10 mg was administered intraventricularly every 3 days for a total of 3 doses. The antibiotic was compounded and administered based on the Intraventricular Antimicrobial Therapy for CSF Shunt Infections protocol created by the NYU Langone Medical Center (see Supplementary Figure 1, http://links.lww.com/IDCP/A18).11 Unfortunately, CSF vancomycin concentrations could not be monitored with the laboratory unable to process such samples. Therefore, the patient was monitored closely for neurological changes. The duration of therapy is based on bacteriologic and clinical response.10 Intraventricular vancomycin was administered to the patient on August 27 and 30 and September 3. As the patient's clinical status continued to improve, no subsequent doses were administered. The first CSF culture returned negative on September 28, including all subsequent cultures. Eventually, she was deemed safe enough to restart chemotherapy and any further treatments for her cancer. Six months later, she is still infection free and continues to undergo physical therapy with positive improvements.
DISCUSSION
Enterococcus is a significant nosocomial pathogen, which commonly causes urinary tract, intra-abdominal, and bacteremia infections. However, enterococcal meningitis continues to be rare and described in the literature only as case reports. A review article identified 94 cases of enterococcal meningitis from 1966 to 1992. Thirty-two of the 94 cases were included in the final analysis, and of these 32 cases, 6 adults (38%) had central nervous system damage due to trauma, surgery, or the placement of an epidural catheter. The majority of the patients (67%) received ampicillin in combination with an aminoglycoside. Five patients received intrathecal (IT) antibiotics with an aminoglycoside or vancomycin. Of the 24 patients who received combination therapy, 96% (23) had a full recovery.5 A second retrospective review identified Enterococcus as the causative organism for 7.6% (20/261) of all nosocomial meningitis cases during the study period (1994–2003). All Enterococcus meningitis cases were reported after neurosurgical procedures. Intrathecal vancomycin was added to intravenous antibiotic therapy in 5 patients; however, no difference was observed in outcomes of these patients compared with those not treated with IT antibiotics.3 As these reviews demonstrate, neurosurgical procedures and surgical devices are common risk factors for the development of enterococcal meningitis. Additional risk factors include patients with comorbidities requiring steroids or other immunosuppressive therapy (63%, n = 10) and underlying infections with Enterococcus from other sites (31%, n = 5).5 It should be noted that vancomycin is not approved for IT use.
Per Infectious Disease Society of America guidelines, combination therapy with the addition of gentamicin is suggested for enterococcal meningitis, with vancomycin recommended as an alternative agent in the event of a penicillin allergy.4,5 Patients not responding to intravenous therapy can be considered for vancomycin of 10 or 20 mg administered intraventricularly. Direct instillation of antibiotics in the CSF certainly may result in serious complications if instructions are not closely followed. Improper technique with needle or catheter placement can result in hemorrhage, CSF leakage, new infections, and seizures. All antibiotic doses must be prepared using only preservative-free diluents to prevent reactions such as arachnoiditis. Despite these challenges, IVT antibiotics still remain novel treatment options in severe cases of meningitis nonresponsive to conventional therapy.
Vancomycin can be administered directly into the CSF using 2 different techniques: lumbar IT or IVT injection. Intraventricular injection is the preferred technique, as administration of antibiotics into the lumbar sac produces unreliable cerebral CSF concentrations and does not allow for measurement of drug concentrations in the cerebral fluid compartments. If access to the CSF via an indwelling catheter is not available, then the lumber IT route of administration should be used.10
Intraventricular vancomycin can be administered using 3 different methods: direct injection into an EVD, injection into an IVT shunt, or into a subcutaneous Ommaya reservoir. The clearance of antibiotics instilled into the cerebral ventricles is dependent on the drainage facilitated by these devices. This may contribute to the previously mentioned recommendation on frequency of dosing recommended based on volume of CSF drainage.2
If a shunt infection is suspected, removal of the infected shunt components, external drainage, and antimicrobial therapy has been proposed as the optimal treatment strategy.6 Organisms such as Enterococcus have been associated with formation of biofilms on indwelling devices that contributes to the challenge of eradicating nosocomial infections.12 One study demonstrated that the cure rate for ventriculitis significantly increased from 30% in patients treated with antimicrobial therapy alone without shunt removal to 90% in 1-stage shunt replacement to 100% cure rate in a 2-stage shunt replacement (removal of infected CSF shunt, replacement with a new shunt, and a second surgery after ventriculitis were treated successfully).2 Although this patient did not have a shunt, the EVD was replaced when cultures continued to remain positive.
The toxicities and adverse effects of vancomycin IVT are controversial, and information is available only through case reports. Some patients have experienced reduced consciousness, prolonged headache, CSF eosinophilia, ototoxicity, and arachnoiditis. Unfortunately, there is a lack of adequate data to correlate such adverse effects with specific vancomycin CSF concentrations. Some effects also may have been due to pH of the injecting solution. Therefore, patients should be monitored clinically in addition to CSF vancomycin trough levels.10 Fortunately, in this patient, despite the inability to monitor such levels, they did not experience any adverse effects or signs of toxicity.
Although literature on optimal treatment of postsurgical meningitis due to Enterococcus still remains elusive, cases such as this may pave the path for further research and stronger national guideline recommendations for IVT therapy.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Dr Jeffrey Hoag, Director of the ICU and pulmonologist, and Dr Eric St. Clair, Director of Neurosurgery, for their support, expertise, and invaluable insights in treating this patient.
Footnotes
The authors have no funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal'ss Web site (www.infectdis.com).
REFERENCES
- 1. Ziai WC, Lewin JJ., III Update in the diagnosis and management of central nervous system infections. Neurol Clin. 2008; 26: 427– 468. [DOI] [PubMed] [Google Scholar]
- 2. Bhimraj A. Healthcare-acquired meningitis and ventriculitis. In: Garcia-Monco JC, ed. CNS Infections: A Clinical Approach. London, England: Springer; 2014: 29– 44. [Google Scholar]
- 3. Guardado R, Asensi V, Torres JM, et al. Post-surgical enterococcal meningitis: clinical and epidemiological study of 20 cases. Scand J Infect Dis. 2006; 38: 584– 588. [DOI] [PubMed] [Google Scholar]
- 4. Moellering RC., Jr Emergence of Enterococcus as a significant pathogen. Clin Infect Dis. 1992; 14(6): 1173– 1176. [DOI] [PubMed] [Google Scholar]
- 5. Stevenson KB, Murray EW, Sarubbi FA. Enterococcal meningitis: report of four cases and review. Clin Infect Dis. 1994; 18(2): 233– 239. [DOI] [PubMed] [Google Scholar]
- 6. Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004; 39: 1267– 1284. [DOI] [PubMed] [Google Scholar]
- 7. Euba G, Lora-Tamayo J, Murillo O, et al. Pilot study of ampicillin-ceftriaxone combination for treatment of orthopedic infections due to Enterococcus faecalis. Antimicrob Agents Chemother. 2009; 53(10): 4305– 4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gavaldá J, Onrubia PL, Gómez MT, et al. Efficacy of ampicillin combined with ceftriaxone and gentamicin in the treatment of experimental endocarditis due to Enterococcus faecalis with no high-level resistance to aminoglycosides. J Antimicrob Chemother. 2003; 52(3): 514– 517. [DOI] [PubMed] [Google Scholar]
- 9. Pasticci MB, Mencacci A, Moretti A, et al. In vitro antimicrobial activity of ampicillin-ceftriaxone and ampicillin-ertapenem combinations against clinical isolates of Enterococcus faecalis with high levels of aminoglycoside resistance. Open Microbiol J. 2008; 2: 79– 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leur MS, Hatton J. Vancomycin administration into the cerebrospinal fluid: a review. Ann Pharmacother. 1993; 27: 912– 919. [DOI] [PubMed] [Google Scholar]
- 11.Dr. Jeffrey Wisoff and Antimicrobial Stewardship Program. Intraventricular Antimicrobial Therapy for CSF Infections. NYU Langone Medical Center; 2014. [Google Scholar]
- 12. Tendolkar PM, Baghdayan AS, Gilmore MS, et al. Enterococcal surface protein, Esp, enhances biofilm formation by Enterococcus faecalis. Infect Immun. 2004; 72: 6032– 6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


