Cardiovascular disease has emerged as one of the leading causes of morbidity and mortality in recent years. Despite considerable progress in managing the disease, we still face the daunting challenge of developing therapeutic interventions to effectively treat heart failure and improve heart function. Although conventional pharmacologic therapy has been quite successful in relieving symptoms, there is no cure for heart failure.1 However, several experimental studies have demonstrated that gene therapy could be an effective option to treat the failing myocardium.2–6 Many of these studies have focused on the calcium (Ca2+)-handling proteins of the sarcoplasmic reticulum in an effort to improving calcium cycling in the diseased heart.2,5–8
There have been promising animal studies, but the field has met with numerous hurdles in translating gene therapy to human patients. These include inefficient gene delivery systems, uncontrolled levels of target gene expression, limitations on the size of the therapeutic gene, severe host immune responses, and defining the time of administration. Designing a vector that not only delivers the gene of interest efficiently but does so without triggering any undesirable side effects, such as inflammatory responses, continues to be a major obstacle. Therefore recent efforts have been focused on developing viral vectors that are less immunogenic but at the same time can integrate into the host genome and continue to express the protein for a prolonged period. One such popular vector is the lentiviral vector, because it can easily infect nonreplicating, terminally differentiated cells and can also integrate into the genome without the need for cell division. In this issue, Niwano et al.9 report that they have used this vector very successfully to deliver the sarcoplasmic reticulum Ca2+ ATPase (SERCA2a) gene into the cardiac myocardium to rescue heart failure.
Several pathologic alterations that occur during heart failure have been targets for gene therapy, but restoring Ca2+ transport has received tremendous attention in the past few years. There has been particular emphasis on calcium-transport genes as candidates for gene therapy, including SERCA2a, phospholamban (PLB), ryanodine receptor (RyR2), and the sodium-calcium exchanger (NCX) (Figure 1). Among the major sarcoplasmic reticulum proteins that have been investigated, SER-CA has proven a very promising candidate because its expression and activity are decreased in a wide variety of pathologic conditions in heart failure.10–12 In addition, a decrease in SERCA pump level and sarcoplasmic reticulum Ca2+ transport is well correlated with negative force frequency seen in failing hearts.13–15 Results from studies using cell systems and rodent models have presented a clear proof of concept that increasing SERCA pump levels can improve cardiac muscle function.3,16,17
Figure 1. Altered Ca2+ transport in heart failure and targets for gene therapy.
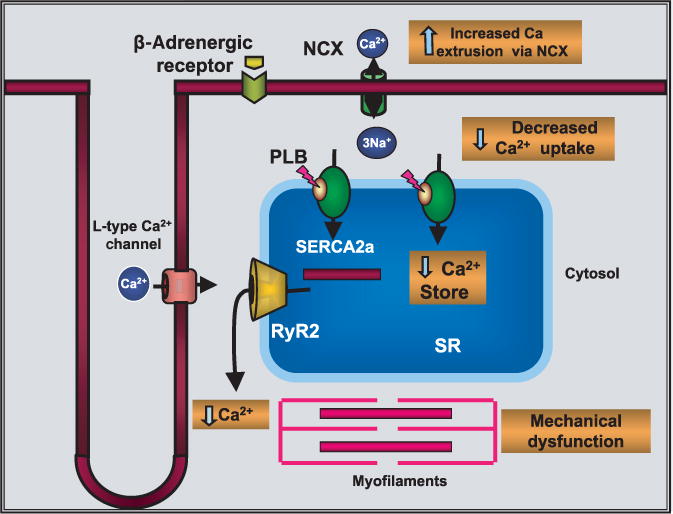
NCX, sodium-calcium exchanger; PLB, phospholamban; RyR2, ryanodine receptor; SR, sarcoplasmic reticulum.
Del Monte et al. first showed that over-expression of SERCA2a in failing human ventricular myocytes isolated from patients with end-stage heart failure can increase SERCA pump activity and enhance contraction and relaxation velocity.17 These studies led to the development of in vivo gene transfer using an elegant catheter-based technique to introduce SERCA2a into the myocardium.2–4,18–20 Adenoviral-mediated SERCA2a gene transfer in a rat model of pressure-overload hypertrophy (in which SERCA2a levels were decreased and severe contractile dysfunction was evident) restored both systolic and diastolic dysfunction to normal levels. Restoration of SERCA2a levels decreased left ventricular size and restored the slope of the end-diastolic pressure–dimension relationship to control levels.20 Interestingly, overexpression of SERCA2a in failing heart restored and normalized the levels of phosphocreatine and ATP and suggested that normalizing Ca2+ transport would improve energetics.3,20 Furthermore, adenoviral-mediated SERCA2a gene transfer into the infarcted myocardium significantly decreased ventricular arrhythmias, reduced infarct size, and improved wall thickening in the anterior wall.8 An increase in Ca2+ transport and a decrease in diastolic Ca2+ and better handling of intracellular ions during the rush of reperfusion should result in improved survival of the cardiomyocytes. Improving Ca2+ transport by SERCA2a gene transfer is therefore beneficial for maintaining cardiac inotropy and for preventing the pathologic effects of Ca2+ overload. Many of the earlier studies used adenoviral gene transfer to deliver target genes.3,5,7,8,19,20 But a major disadvantage of adenoviral vectors lies in the activation of the host immune system and potential destruction of cardiac myocytes when applied in vivo.
Inflammatory responses induced by adenoviral particles can be very strong and fatal to the recipient, as reported in initial human trials. New classes of vectors that provide an alternative to the adenovirus include recombinant adeno-associated virus (AAV) and lentiviral vectors. The recombinant AAV vectors can also infect nondividing cells; they are less immunogenic and do not contain viral genes, but they can accommodate only up to 4.8 kilobases of DNA, somewhat limiting their use for larger genes. On the other hand, lentiviral vectors are becoming increasingly popular because they can easily infect nonreplicating, terminally differentiated cells and can incorporate into the genome without the need for cell division. Lentiviral vectors have also been increasingly popular for long-term stable gene expression in a variety of cells, including vascular smooth muscle cells and endothelial cells.21
In the new study reported in this issue, Niwano et al. infused a lentiviral vector containing the SERCA2 gene into the rat heart by a hypothermic intracoronary delivery method 2 weeks after myocardial infarction (MI).9 They have shown for the first time that the SERCA2 gene can be targeted to the myocardium using lentiviral vectors and they have documented improved cardiac function in a rat model of ischemic cardiomyopathy. In addition, their study demonstrates that the therapy prevented geometrical left ventricular remodeling after MI and also improved the survival rate.
These data are significant in several respects. One is that they identify an efficient vector that is superior in many ways to the existing options. The lentiviral system used in this study is a third-generation vector with several safety features that are necessary for minimal production of replication-competent viruses. The data also demonstrate that SERCA2 administration is significantly effective even 2 weeks after an MI episode, thereby offering a potentially translatable therapy. Third, the study shows that 6 months after transduction SERCA2 gene transfer significantly prevented left ventricular dilation and improved systolic and diastolic function, resulting in reduction of mortality. These results are very promising in that the lentiviral vectors could be tailored to situations in which stable long-term transgene expression is needed, without the adverse effects of adenoviral vectors and multiple viral administrations. Such long-term interventions could present effective treatment options for both vascular and cardiac disease.
Exciting findings such as those from Niwano et al.9 take us one step closer to achieving what has been pursued for more than two decades. This study has identified a better tool for delivering the gene and tested the possibility of delayed gene delivery following MI. However, potential complications, including electrical heterogeneity resulting from uneven expression of the target gene, will need to be rigorously tested and optimized. Future research must identify tissue-specific gene delivery and expression systems to ensure targeted integration of the gene as well as its controlled expression. As technology continues to improve, gene therapy is no longer at an experimental stage to treat heart disease, because the translation of these results into tangible clinical therapy is already in progress. At least two clinical trials using SERCA2 gene transfer are in preparation: a phase I, randomized double-blinded, placebo-controlled study using AAV1-SERCA2a (Mydicar; Celladon Corporation, La Jolla, CA) in patients with congestive heart failure, and a phase I study using AAV6-SERCA2a to evaluate efficacy and safety in ischemic patients undergoing left ventricular assist placement.2 With further development of improved delivery methods and advanced viral vectors, SERCA gene therapy may not be far from reality.
Acknowledgments
We thank Robert M. Frederickson, Editor of Molecular Therapy, for his input and valuable comments. MP is supported by RO1 HL 08855 and R01 HL-64140-06.
References
- 1.Cohn JN. Overview of the treatment of heart failure. Am J Cardiol. 1997;80:2L–6L. doi: 10.1016/s0002-9149(97)00843-6. [DOI] [PubMed] [Google Scholar]
- 2.Ly H, Kawase Y, Yoneyama R, Hajjar RJ. Gene therapy in the treatment of heart failure. Physiology (Bethesda) 2007;22:81–96. doi: 10.1152/physiol.00037.2006. [DOI] [PubMed] [Google Scholar]
- 3.del Monte F, Hajjar RJ, Harding SE. Overwhelming evidence of the beneficial effects of SERCA gene transfer in heart failure. Circ Res. 2001;88:E66–E67. doi: 10.1161/hh1101.092004. [DOI] [PubMed] [Google Scholar]
- 4.Lebeche D, Kaprelian R, del Monte F, Tomaselli G, Gwathmey JK, Schwartz A, et al. In vivo cardiac gene transfer of Kv4.3 abrogates the hypertrophic response in rats after aortic stenosis. Circulation. 2004;110:3435–3443. doi: 10.1161/01.CIR.0000148176.33730.3F. [DOI] [PubMed] [Google Scholar]
- 5.Hajjar RJ, Schmidt U, Matsui T, Guerrero JL, Lee KH, Gwathmey JK, et al. Modulation of ventricular function through gene transfer in vivo. Proc Natl Acad Sci USA. 1998;95:5251–5256. doi: 10.1073/pnas.95.9.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajjar RJ, Samulski RJ. Heart failure: a silver bullet to treat heart failure. Gene Ther. 2006;13:997. doi: 10.1038/sj.gt.3302747. [DOI] [PubMed] [Google Scholar]
- 7.del Monte F, Kizana E, Tabchy A, Hajjar RJ. Targeted gene transfer in heart failure: implications for novel gene identification. Curr Opin Mol Ther. 2004;6:381–394. [PubMed] [Google Scholar]
- 8.del Monte F, Lebeche D, Guerrero JL, Tsuji T, Doye AA, Gwathmey JK, et al. Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targeting myocardial calcium cycling. Proc Natl Acad Sci USA. 2004;101:5622–5627. doi: 10.1073/pnas.0305778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niwano K, Arai M, Koitabashi N, Watanabe A, Ikeda Y, Miyoshi H, et al. Lentiviral vector–mediated SERCA2 gene transfer protects against heart failure and left ventricular remodeling after myocardial infarction in rats. Mol Ther. 2008;16:1026–1032. doi: 10.1038/mt.2008.61. [DOI] [PubMed] [Google Scholar]
- 10.Arai M, Alpert NR, MacLennan DH, Barton P, Periasamy M. Alterations in sarcoplasmic reticulum gene expression in human heart failure: a possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res. 1993;72:463–469. doi: 10.1161/01.res.72.2.463. [DOI] [PubMed] [Google Scholar]
- 11.Pieske B, Maier LS, Bers DM, Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ Res. 1999;85:38–46. doi: 10.1161/01.res.85.1.38. [DOI] [PubMed] [Google Scholar]
- 12.Hasenfuss G. Alterations of calcium-regulatory proteins in heart failure. Cardiovasc Res. 1998;37:279–289. doi: 10.1016/s0008-6363(97)00277-0. [DOI] [PubMed] [Google Scholar]
- 13.Hasenfuss G, Holubarsch C, Hermann HP, Astheimer K, Pieske B, Just H. Influence of the force-frequency relationship on haemodynamics and left ventricular function in patients with non-failing hearts and in patients with dilated cardiomyopathy. Eur Heart J. 1994;15:164–170. doi: 10.1093/oxfordjournals.eurheartj.a060471. [DOI] [PubMed] [Google Scholar]
- 14.Hasenfuss G, Reinecke H, Studer R, Meyer M, Pieske B, Holtz J, et al. Relation between myocardial function and expression of sarcoplasmic reticulum Ca(2+)-ATPase in failing and nonfailing human myocardium. Circ Res. 1994;75:434–442. doi: 10.1161/01.res.75.3.434. [DOI] [PubMed] [Google Scholar]
- 15.Hasenfuss G, Reinecke H, Studer R, Pieske B, Meyer M, Drexler H, et al. Calcium cycling proteins and force-frequency relationship in heart failure. Basic Res Cardiol. 1996;91(Suppl 2):17–22. doi: 10.1007/BF00795357. [DOI] [PubMed] [Google Scholar]
- 16.Loukianov E, Ji Y, Grupp IL, Kirkpatrick DL, Baker DL, Loukianova T, et al. Enhanced myocardial contractility and increased Ca2+ transport function in transgenic hearts expressing the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+-ATPase. Circ Res. 1998;83:889–897. doi: 10.1161/01.res.83.9.889. [DOI] [PubMed] [Google Scholar]
- 17.del Monte F, Harding SE, Schmidt U, Matsui T, Kang ZB, Dec GW, et al. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation. 1999;100:2308–2311. doi: 10.1161/01.cir.100.23.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beeri R, Guerrero JL, Supple G, Sullivan S, Levine RA, Hajjar RJ. New efficient catheter-based system for myocardial gene delivery. Circulation. 2002;106:1756–1759. doi: 10.1161/01.cir.0000035240.92015.e4. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto MI, del Monte F, Schmidt U, DiSalvo TS, Kang ZB, Matsui T, et al. Adenoviral gene transfer of SERCA2a improves left ventricular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci USA. 2000;97:793–798. doi: 10.1073/pnas.97.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.del Monte F, Williams E, Lebeche D, Schmidt U, Rosenzweig A, Gwathmey JK, et al. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase in a rat model of heart failure. Circulation. 2001;104:1424–1429. doi: 10.1161/hc3601.095574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dishart KL, Denby L, George SJ, Nicklin SA, Yendluri S, Tuerk MJ, et al. Third-generation lentivirus vectors efficiently transduce and phenotypically modify vascular cells: implications for gene therapy. J Mol Cell Cardiol. 2003;35:739–748. doi: 10.1016/s0022-2828(03)00136-6. [DOI] [PubMed] [Google Scholar]


