Abstract
Background
Morphological studies have revealed a close anatomical relationship between enteric nerve terminals and intramuscular ICC (ICC-IM) which supports a role for ICC-IM as intermediaries in enteric motor neurotransmission. Recently, a second type of interstitial cell previously described as ‘fibroblast-like’ but can now be identified by platelet-derived growth factor receptor-α expression, has also been implicated in enteric neurotransmission in rodents. The present study was performed to determine if enteric nerve fibers form close anatomical relationships with ICC and PDGFRα+ cells throughout the primate GI tract.
Methods
Immunohistochemical experiments and confocal microscopy were performed to examine the relationship between excitatory and inhibitory motor neurons, ICC and PDGFRα+ cells throughout the monkey GI tract.
Key Results
The pan neuronal marker. Protein gene product 9.5 (PGP9.5) was used to label all enteric neurons and substance-P (sub-P) and neuronal nitric oxide synthase (nNOS) to label excitatory and inhibitory neurons, respectively. Double labeling with Kit revealed that both classes of nerve fibers were closely apposed with ICC-IM in the stomach, small intestine and colon (taenia and inter-taenia regions), but not with ICC at the level of the myenteric plexus (ICC-MY). Varicose enteric nerve fibers were closely associated with ICC-IM for distances up to 250 μm. Both excitatory and inhibitory nerve fibers were also closely apposed to PDGFRα+ cells throughout the primate GI tract.
Conclusions & Inferences
The close anatomical relationship between enteric nerve fibers and ICC-IM and PDGFRα+ cells throughout the GI tract of the Cynomolgus monkey provides morphological evidence that these two classes of interstitial cells may provide a similar physiological function in primates as has been attributed in rodent animal models.
Keywords: enteric nervous system, excitatory and inhibitory motor neurons, interstitial cells of Cajal, PDGFRα+ cells
INTRODUCTION
The role of interstitial cells of Cajal (ICC) in the GI tract has been revealed by multi-faceted morphological, physiological, and genetic approaches that have been performed primarily on rodent animal models. Many of these studies exploited the finding that ICC express the receptor tyrosine kinase, Kit, and the Kit signaling pathway is required for development and maintenance of ICC.1–7 Manipulation of Kit has allowed investigations into the functional role of ICC that includes their critical role in pacemaker activity,2,4,5 and as mediators of enteric motor neurotransmission.3,6,7 Pathologists have also utilized Kit as a marker of ICC deficiency in GI tissues with motility disorders and in gain-of-function mutations that can lead to the development of gastrointestinal stromal tumors.8–13
A second type of interstitial cell, identified recently by the expression of platelet-derived growth factor receptor-α (PDGFRα), has also been described in GI muscles.14–18 Using electron microscopy, these cells were first described as ‘fibroblast-like’ and observed in close association with enteric nerve fibers.19–21 Expression of PDGFRα by these cells has allowed investigations into their morphological relationship with chemically identified enteric nerve fibers.14–16 PDGFRα+ cells also express guanylate cyclase, P2Y1 receptors, and SK3 K+ channels,16–18 demonstrating that they have major receptors and effectors that might mediate enteric inhibitory neurotransmission. Activation of inhibitory motor neurons produces a post-junctional response that is mediated by purines binding to P2Y1 receptors and is inhibited by apamin.22–26 Isolated PDGFRα+ cells have been studied using electrophysiological techniques, and it was shown that, unlike smooth muscle cells, PDGFRα+ cells produce robust outward currents in response to purines that were reduced by apamin and blocked by selective P2Y1R antagonists.17 It is therefore likely that PDGFRα+ cells generate inhibitory post junctional responses and like ICC contribute to enteric inhibitory neurotransmission in GI muscles.
Although rodents have been instrumental to our understanding of the functions of interstitial cells in the GI tract, a more complete morphological evaluation of the relationship between enteric neurons and ICC and PDGFRα+cells throughout the GI tract of larger animals and humans is lacking. In the case of human muscles this is mainly due to the paucity of tissues available for such studies and the processing methods utilized (i.e., wax sections that allow for two-dimensional bright-field immunohistochemistry vs frozen tissues or whole mounts that enable three-dimensional confocal imaging to be performed).27–30 We sought to investigate this question in GI tissues closely related to humans, Cynomolgus monkeys (Macaca fascicularis), and we examined the relationship between nerve fibers and interstitial cells throughout the GI tract. As in mice, we found a close anatomical relationship between nerve fibers and interstitial cells, providing morphological evidence that ICC/PDGFRα+ cells may play an analogous role in enteric motor neurotransmission in primates.
METHODS
Animals
Gastrointestinal tissues from 20 Cynomolgus monkeys (Macaca fascicularis) between the ages 2–8 years (both sexes) were obtained from Charles River Laboratories (CRL; Sparks, Nevada, USA). Monkeys were initially sedated with Ketamine (10 mg kg−1), then administered 0.7 ml Beuthanasia-D solution (Schering-Plough AH, Kenilworth, NJ, USA) (pentobarbital sodium and phenytoin sodium) followed by exsanguination. The animals were maintained and the experiments performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Tissues were transported from CRL to the University of Nevada in pre cooled Kreb’s Ringers solution (KRB) and fixed within 2 h after animals were sacrificed.
Morphological studies
For double-label immunohistochemical studies, tissues were pinned with the mucosa facing upward to the Sylgard elastomer (Dow Corning Corp., Midland, MI, USA) base of a dissecting dish containing fresh KRB. The mucosa was removed by sharp dissection and the remaining strips of tunica muscularis stretched to 110% of the resting length and width before being immersed in fixative paraformaldehyde (4% w/v) solution for 1 h at room temperature. Following fixation, tissues were washed overnight in phosphate buffered saline (PBS; 0.01 M, pH 7.2) and rewashed with fresh PBS the following day for 5 h, with a change of PBS every hour.
Because some of the organs within the monkey GI tract were too thick to be examined as whole mounts, they were processed as thick flat cryostat sections (flat mounts) which allowed three-dimensional, whole mount type, images to be obtained. Tissues were dehydrated in graded sucrose solutions (5, 10, 15, and 20% w/v in PBS, 15 min each for 5, 10, and 15% followed by overnight in 20%), embedded in a 50 : 50 mixture of 20% sucrose and Tissue Tek (Miles, Ill., USA), and rapidly frozen in 2-methylbutane pre-cooled in liquid nitrogen. Flat mount sections (100 μm), were then cut through the entire muscle layer using a Leica CM3050 cryostat. Tissue sections were placed in individual wells of a 24-well plate containing PBS. Sections were washed in PBS and subsequently incubated in bovine serum albumin (BSA; 1%) for ICC labeling or donkey serum (10%) for PDGFRα labeling to reduce non-specific antibody binding (1 h at room temperature). Tissues were then incubated for 48 h at 4 °C with a monoclonal antibody raised against Kit protein (hSCF-R, diluted 1 : 100 in 0.5% Triton-X 100) or a polyclonal antibody raised against PDGFRα (hPDGFRα, diluted 1 : 100 in 0.5% Triton-X 100). After washing the sections for 5–6 h they were subsequently placed in an appropriate secondary antibody (donkey anti-goat Alexa fluor 488 or 594, 1 : 1000 in PBS; 1 h, room temperature; Molecular Probes, Eugene, OR, USA). Tissues were then washed overnight and for several hours the next day before being incubated in a second primary antibody for 48 h at 4 °C (anti-PGP9.5, anti-sub-P or anti-nNOS) (see Table 1 for antibody details). Tissues were washed for a further 5–6 h and immunoreactivity was then detected by incubation in a second secondary antibody (donkey anti-rabbit Alexa fluor 594 or 488). Control tissues were prepared by either omitting primary or secondary antibodies from the incubation solutions.
Table 1.
Details of antibodies used for immunohistochemistry
| Combined antibodies | Resource | Mono- or poly-clonal antibodies | Host | Dilution |
|---|---|---|---|---|
| Anti-hSCF-R or hPDGFRα/anti-PGP9.5 | R&D Systems Inc., Minneapolis, MN, USA/UltraClone Limited, Isle of Wight, England, UK | Poly-/Poly- | Goat/Rabbit | 1 : 100/1 : 2000 |
| Anti-hSCF-R or hPDGFRα/anti-sub-P | R&D Systems Inc., Minneapolis, MN, USA/Millipore. Billerica, MA, USA | Poly-/Poly- | Goat/Rabbit | 1 : 100/1 : 500 |
| Anti-hSCF-R or hPDGFRα/anti-nNOS | R&D Systems Inc., Minneapolis, MN, USA/Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA | Poly-/Poly- | Goat/Rabbit | 1 : 100/1 : 500 |
| Anti-histamine | ImmunoStar, Hudson, WI, USA | Poly | Rabbit | 1 : 500 |
Note: hSCF-R, human stem cell factor receptor; hPDGFRα, human platelet derived growth factor receptor α; PGP9.5, protein gene product 9.5; sub-P, substance P; nNOS, neuronal nitric oxide synthase.
Cross-sections (30 μm thick) were also cut from tissues that were dehydrated and frozen as described above. Sections were collected on Vectabond (Vector Laboratories, Burlingame, CA, USA) coated glass slides and processed through primary and secondary antibodies as described above, except primary antibody incubations, which were performed overnight rather than for 48 h.
Tissues sections were examined with a Zeiss LSM 510 Meta confocal microscope (Zeiss, Göttingen, Germany) with excitation wavelengths appropriate for Alexa fluor 488 and Alexa fluor 594. Images were typically taken with a 60 × objective with a z-step of 0.25 μm and a frame size up to 1024 × 1024 pixels, resulting in a pixel size of 0.14 μm. Since there were yellow pixels (co-labeled with green and red) in many images it is likely that these regions were separated by distances of 0.14 μm or less in the x and y axis and up to 0.25 μm in the z-axis. The term ‘close morphological relationship’ refers to the minimally resolved distance of 0.14 μm–1 μm. To determine the distance over which enteric nerve fibers were morphologically associated with ICC or PDGFRα+ cells, individual fibers were measured from the point of close apposition to the point of divergence within single scan planes (0.25 μm) using a 60 × objective. Often enteric nerves and ICC or PDGFRα+ cells were closely apposed across the entire field of view. Final images were constructed using Zeiss LSM 5 Image Examiner software and converted to Tiff files for final processing in Adobe Photoshop 7.0 (Adobe Co., Mountain View, CA, USA) and Corel Draw 7.0 (Corel Corp. Ontario, Canada).
Solutions
Tissue samples were collected in oxygenated KRB (4 °C) of the following composition (mM): NaCl 118.5; KCl 4.5; MgCl2 1.2; NaHCO3 23.8; KH2PO4 1.2; dextrose 11.0; CaCl2 2.4. The pH of the KRB was adjusted to pH 7.3–7.4 when saturated with 97% O2–3% CO2 at 4 °C.
RESULTS
Morphological relationship between enteric motor neurons and ICC or PDGFRα+ cells throughout the monkey GI tract
To examine the morphological association between chemically identified enteric nerve fibers and interstitial cells we performed double labeling with antibodies against protein gene product 9.5 (PGP9.5) substance-P (sub-P) and nNOS with Kit or PDGFRα. Within the muscle layers the varicose nature of PGP9.5, sub-P and nNOS fibers identified them as excitatory or inhibitory motor neurons.
Morphological relationship between enteric motor neurons and ICC
Stomach
Neuronal processes were closely associated with ICC-IM in the gastric fundus (Fig. 1A–C). Individual nerve fibers appeared to innervate multiple ICC-IM. Double labeling with antibodies against sub-P and nNOS and Kit showed that both excitatory (sub-P, Fig. 1D–F) and inhibitory enteric motor neurons (nNOS; Fig. 1G–I) formed a close morphological relationship with ICC-IM in the circular muscle layer (CM). ICC-IM were also observed in longitudinal muscle layer (LM), and in some cases these cells were also observed in close association with individual nerve fibers (Fig. 1A,D,G).
Figure 1.
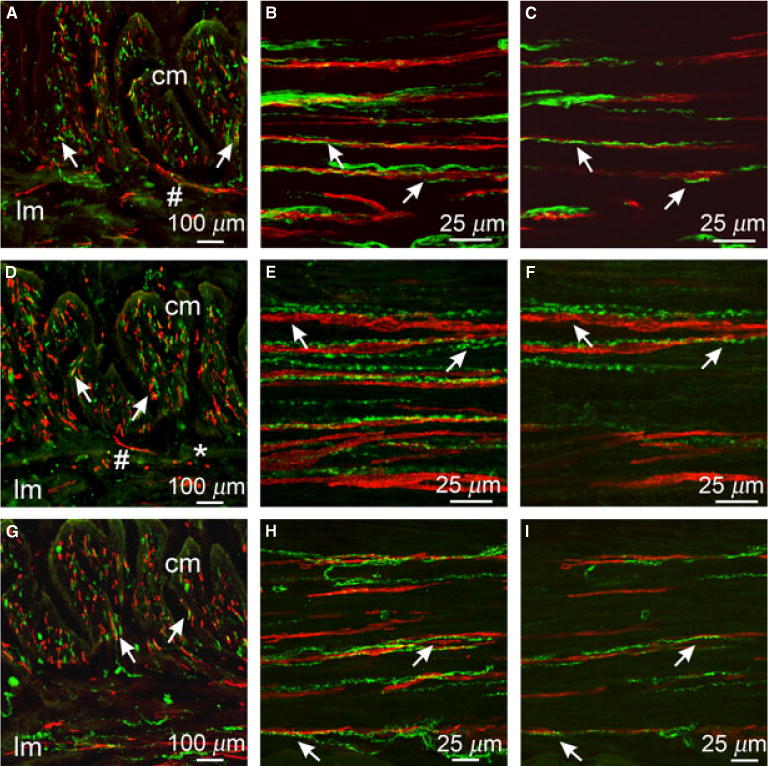
Relationship between enteric neurons and ICC in the gastric fundus. (A,D,G) Cryostat sections through the fundus tunica muscularis revealing CM and LM (cm & lm, respectively). (A) Double label of PGP9.5+ neurons (green) and Kit+ ICC (red) closely apposed to each other in both muscle layers (arrows). (D) Sub-P+ nerve fibers (green) and Kit+ ICC (red) in close apposition (arrows). (G) nNOS+ neurons (green) and Kit+ ICC (red) in close morphological association (arrows in CM). (B,C,E,F,H,I) Double labeled images of flat mounts through the CM of the fundus with Kit and PGP9.5 (B,C) sub-P (E,F) and nNOS (H,I) showing the close anatomical relationship between enteric neurons and ICC (arrows). (B,E,H) Multiple stacks through 10–30 μm. (C,F,I) single sections of 0.4 μm displaying morphological relationship between enteric neurons and ICC in the same optical plane. Single sections in C,F, and I were taken from the confocal stacks in B,E,H. Numerous small rounded Kit+ cells (*) with a morphology resembling mast cells were observed within the LM/myenteric plexus region. N.B. ICC were observed transversing from the CM into the LM (#) in cryostat sections (A, D). Scale bars are indicated on each panel.
A similar close proximity was found between ICC and enteric nerve processes in the antrum. Within the region of the myenteric plexus, between the CM and LM, a dense neuropil of PGP9.5+ nerve fibers was observed between ganglia (Fig. 2A,B). These fibers were both sub-P+ (Fig. 2E,F) and nNOS+ (Fig. 2I,J). In the region of the myenteric plexus this definition on chemical coding was more ambiguous as interneurons also expressed these proteins. ICC-MY surrounded myenteric ganglia on both the CM and LM aspects but neither class of motor neuron formed close morphological associations with ICC-MY. Within the CM individual enteric nerve fibers were closely associated with ICC-IM for distances up to 150 μm (measured in a single optical plane; Fig. 2D,H,L). ICC-IM associated with neuron processes often displayed lateral projections that formed contacts with adjacent ICC or nerve fibers. Numerous rounded Kit+ mast cells were noted at the serosal surface and were occasionally also observed within the CM and LM (Fig. 2).
Figure 2.
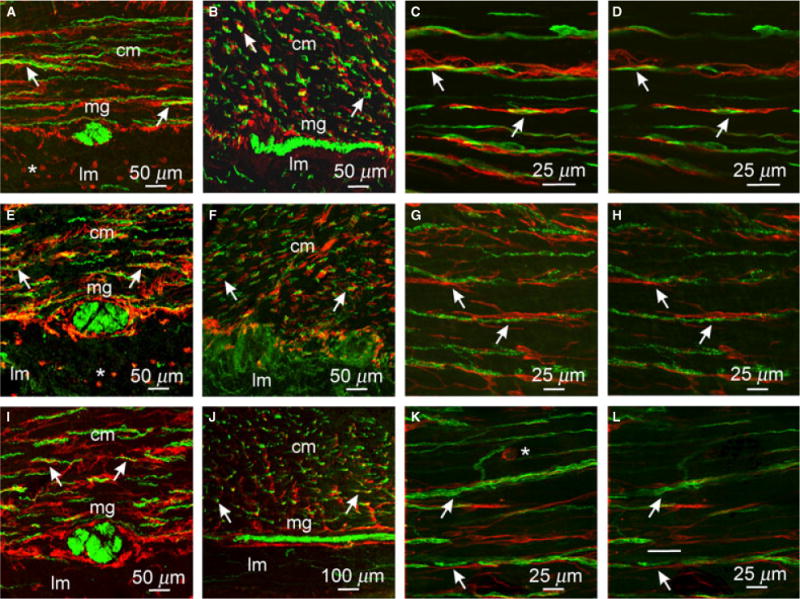
Close morphological relationship between enteric neurons and ICC in the gastric antrum. (A–D) Cryostat sections (A,B) and flat mount sections (C,D) of PGP9.5+ neurons (green) and Kit+ ICC (red). PGP9.5+ nerve fibers were observed in close association with ICC (arrows) in cryostat sections cut parallel (A) or transverse (B) to the CM and in flat mounts (C,D) within the CM (cm) but not the LM (lm). (E–H) Double labeling with sub-P and Kit in the antrum revealed a close anatomical relationship between excitatory nerve fibers and ICC. Cryostat sections cut parallel (E) and transverse (F) to the CM and flat mounts (G,H) cut parallel to CM fibers showed close apposition between sub-P containing neurons and ICC-IM (arrows). (I–L) nNOS neurons and ICC were also in close apposition to one another. (I, J) cryostat sections (K,L) flat mounts of nNOS (green) and Kit+ ICC (red) closely apposed to one another in the CM (cm; arrows). Both nerve fibers and ICC were sparse in the LM. (D,H,L) Single sections (0.4 μm) taken from the reconstructed stacks in C,G, and K, respectively. ICC-MY also surrounded myenteric ganglia (mg) but did not penetrate ganglionic sheaths. Numerous small rounded mast cells (*) were observed in the LM and occasionally within the CM. Scale bars are as indicated in each panel.
Small intestine
ICC-MY were observed at the level of the myenteric plexus, and ICC were also found in the CM, near the submucosal surface, at the level of the deep muscular plexus (ICC-DMP). ICC-IM were also dispersed within the muscle bundles, and were less dense than ICC-MY or ICC-DMP, as previously described in other species.31,32 Double labeling with PGP9.5 and Kit revealed that enteric neuronal processes were closely associated with ICC-DMP (Fig. 3B,C). Double labeling with Kit and sub-P (Fig. 3F,G) or nNOS (Fig. 3J,K) antibodies showed that both excitatory and inhibitory enteric neurons were closely apposed to ICC-DMP. Although PGP9.5+ enteric nerve fibers (Fig. 3A,D) containing sub-P+ (Fig. 3E) and nNOS+(Fig. 3I) were abundant in the region of the myenteric plexus, these fibers were not closely associated with ICC-MY. Rounded Kit+ mast cells were observed throughout the monkey intestine and were found within the mucosa, submucosa and muscle layers (Fig. 3H,L).
Figure 3.
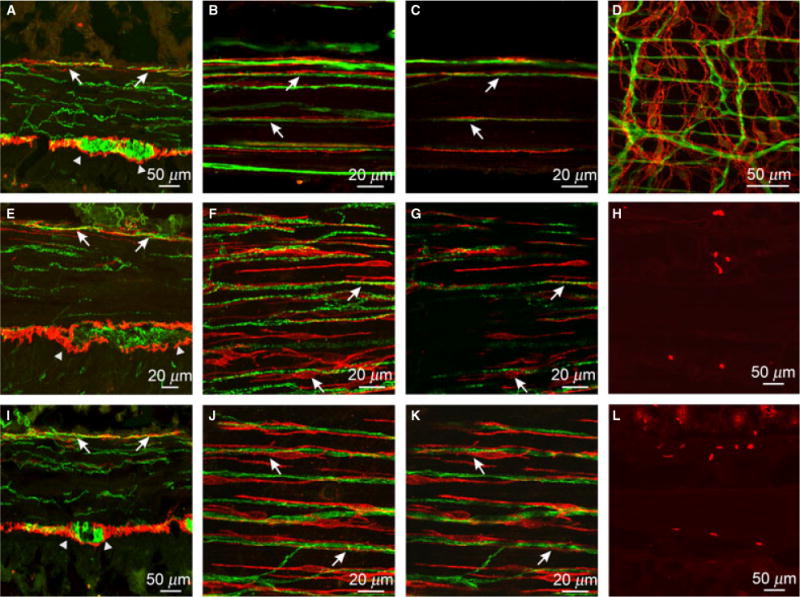
Morphological relationship between enteric neurons and ICC in the small intestine. (A) Cryostat cross-section through the intestine wall labeled with PGP9.5 and Kit. PGP9.5+ nerve fibers (green) were evenly distributed throughout the CM but Kit+ ICC (red) were observed in close apposition to PGP9.5+ nerve fibers predominantly at the level of the deep muscular plexus (DMP, arrows). (B,C) Flat mounts showing the DMP. PGP9.5+ nerves fibers were in close apposition to ICC (arrows). (D) Flat mount of the myenteric plexus region (also seen in A, arrowheads) showing little or no morphological relationship between PGP9.5+ nerves fibers and ICC-MY in this region of the intestinal wall. (E–G) Relationship between sub-P containing nerve fibers and Kit+ ICC in the intestine. (I–K) Relationship between nNOS containing nerve fibers and Kit+ ICC. Both sub-P and nNOS containing neurons were readily observed throughout the CM but few fibers were observed in the LM. (F,G) and (J,K) show flat mounts of sub-P and nNOS nerve fibers and Kit+ ICC at the level of the DMP, respectively. Excitatory and inhibitory neurons were apposed to ICC in this region of the intestine (arrows). (C,G,K) Single sections of reconstructed stacks in (B,F,J), respectively. (H,L) Cryostat sections labeled with an anti-histamine antibody to identify mast cells in the intestine. Mast cells were located in the mucosa, lamina propria, tunica muscularis and serosa. Scale bars are as indicated on each panel.
Proximal colon
Within the CM of the proximal colon, PGP9.5+ nerve fibers, ran parallel to the smooth muscle cells and tracked ICC-IM for at least 130 μm (Fig. 4B,C). Sub-P antibodies showed varicose excitatory motor nerve fibers closely associated with ICC-IM for at least 130 μm and were often closely aligned with more than a single ICC-IM (Fig. 4G,H). Likewise, double labeling with nNOS and Kit revealed that inhibitory motor nerve processes were closely associated with ICC-IM (Fig. 4L,M). A dense network of PGP9.5+ enteric neurons was located at the level of the myenteric plexus, but double labeling with Kit suggested no close associations with ICC-MY. Double labeling with sub-P or nNOS and Kit also showed that these different neuronal populations were not closely associated with ICC-MY (not shown).
Figure 4.
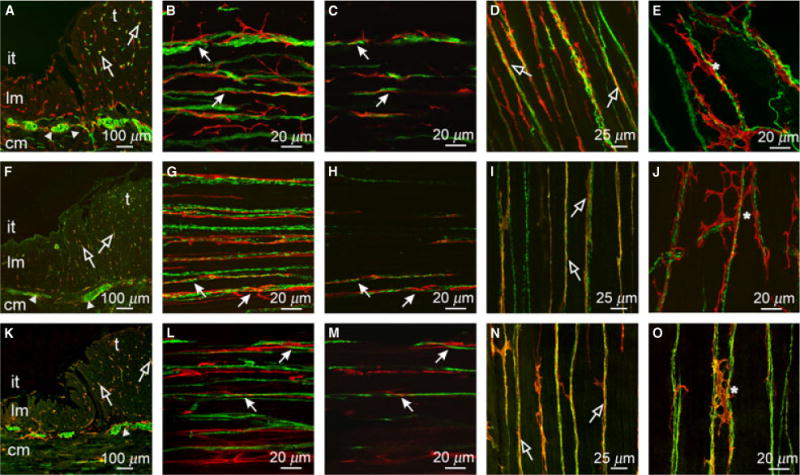
Relationship between enteric neurons and ICC within the taenia (t) and inter-taenia (it) regions of the proximal colon. (A,F,K) Cryostat cross-sections of PGP9.5+, sub-P and nNOS containing neurons (green) with Kit+ ICC (red), respectively. Close appositions are seen in the CM and taenia region of the colon (open arrows). (B,C) CM (cm in A,F,K) double labeled with PGP9.5 (green) and Kit (red). (G,H) CM double labeled with sub-P and Kit. (L,M) CM double labeled with nNOS and Kit. (C,H,M) Single sections of reconstructed stacks taken from (B,G,L). ICC ran parallel to and in close apposition with excitatory and inhibitory neurons. (D,I,N) & (E,J,O) Taenia LM and serosal surface of taenia double labeled with PGP9.5 (D,E), sub-P (I,J) and nNOS (N,O) and Kit, respectively. Enteric nerve fibers formed close anatomical associations with ICC throughout the taenia. ICC were observed to form an anastomosing network (*) along and between nerve fibers adjacent to the serosa of the taenia. Scale bars are as indicated in each panel.
Taenia
Double labeling experiments with PGP9.5, sub-P or nNOS and Kit revealed the anatomical relationship between specific classes of nerve fibers and ICC within this LM band. Flat and profile sections showed that similar to the CM of the colon, individual PGP9.5, sub-P and nNOS nerve fibers were closely apposed to a single ICC-IM within the taenia for distances greater than 230 μm (Fig. 4D,I,N). ICC located along the sub-serosal surface (ICC-SS; Fig. 4E,J,O) were also anatomically associated with enteric nerve fibers. ICC-SS also had lateral processes that interconnected with one another and adjacent nerve fibers that may assist in the transfer of neuronal information between ICC and smooth muscle cells. Varicose nerve fibers from both classes of neurons were seen to come into close apposition with more than one ICC, suggesting the possibility that single nerve fibers innervate multiple ICC (Fig. 4). In the colon the inter-taenia LM did not contain many ICC-IM. Likewise the number of nerve fibers innervating this layer was also significantly less than in taenia muscle bands. The occasional nerve fibers that were observed in this region of the colon were not closely apposed to ICC-IM.
Morphological relationship between enteric motor neurons and PDGFRα+ cells throughout the monkey GI tract
We performed double labeling experiments using PGP9.5, sub-P and nNOS and an antibody against PDGFRα to characterize the extent of the associations between motor neurons and PDGFRα+ cells in the primate GI tract.
Stomach
In both fundus and antrum PGP9.5+ nerve fibers were closely apposed to PDGFRα+ cells in CM (Figs 5A,B and 6B,C). Individual PGP9.5+ nerve processes tracked PDGFRα+ cells for distances greater than 230 μm. Excitatory (sub-P; Figs 5C,D and 6E,F) and inhibitory (nNOS; Figs 5E,F and 6L,M) neurons were closely associated with PDGFRα+ cells in both fundus and antrum. PDGFRα+ cells appeared to make contact with multiple nerve processes (Fig. 6C). At the level of the myenteric plexus, a second population of PDGFRα+ cells surrounded ganglia, but these cells did not appear to be closely associated with enteric nerve fibers. In LM, PDGFRα+ cells were present in similar density as in CM. However, there are few motor nerve projections in antral LM, so there were also fewer associations between nerve fibers and PDGFRα+ cells in these muscles (Fig. 6).
Figure 5.
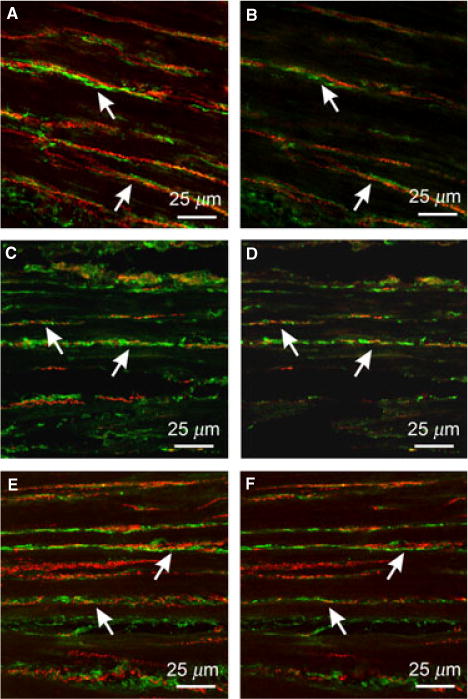
Relationship between enteric neurons and PDGFRα+ cells in the gastric fundus. (A,B) Double labeling with PGP9.5 and PDGFRα antibodies reveal close apposition between enteric neurons (red) and PDGFRα+ cells (green) in CM and LM. PGP9.5+ fibers ran parallel to PDGFRα+ cells for at least 130 μm. Double labeling with sub-P (C,D) or nNOS (E,F) reveal that both excitatory and inhibitory nerve fibers run parallel to PDGFRα+ cells for at least 150 μm. Scale bars are as indicated in each panel.
Figure 6.
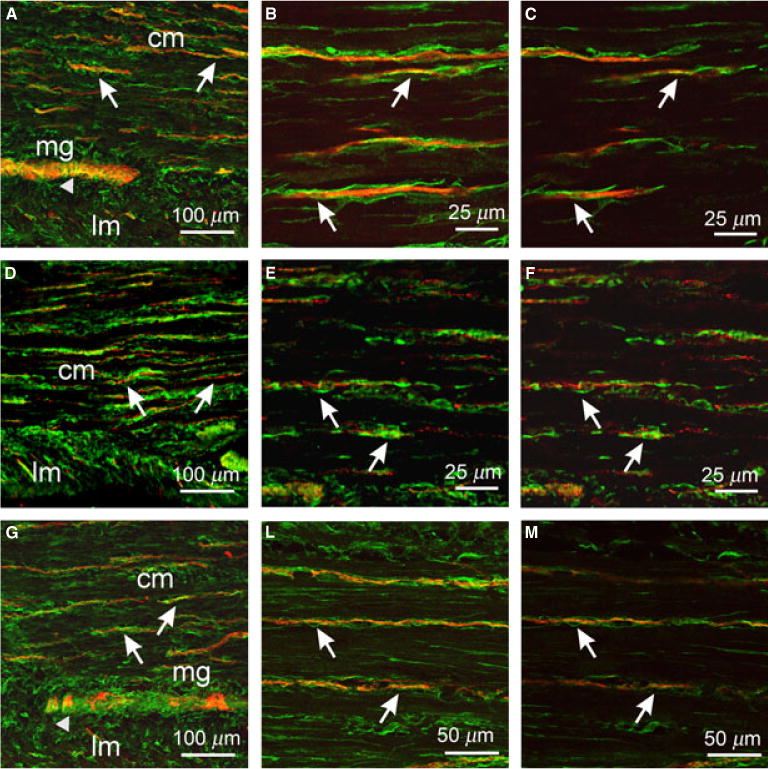
Anatomical relationships between enteric neurons and PDGFRα+ cells in the gastric antrum. (A,D,G) Cryostat sections through the antral wall labeled with PGP9.5 (A, red), sub-P (D, red) and nNOS (G, red) and PDGFRα (green) antibodies. (B,C) Flat mounts of antrum labeled with PGP9.5 and PDGFRα antibodies showing close apposition of PGP9.5+ nerve fibers and PDGFRα+ cells (arrows). Flat mounts labeled with sub-P (E,F) and nNOS (H,I) along with PDGFRα. Sub-P and nNOS nerve fibers (arrows) were closely apposed to PDGFRα+ cells for at least 100 μm and 230 μm, respectively. (C,F,I) Single sections of reconstructed stacks taken from (B,E,H). Scale bars are as indicated in each panel.
Small intestine
Double labeling with antibodies against PGP9.5 and PDGFRα revealed close contacts between nerve fibers and PDGFRα+ cells in jejunal CM (Fig. 7B,C), and both excitatory (sub-P; Fig. 7E,F) and inhibitory (nNOS; Fig. 7H,I) nerve fibers were associated with PDGFRα+ cells. The relationship between neural processes and PDGFRα+ cells in the intestine was different than observed with ICC. PDGFRα+ cells were more widely distributed throughout the CM and their contacts with enteric motor neurons were therefore more evenly distributed than neural contacts with ICC, which were concentrated at the level of the DMP. The reason for the different distribution between these two classes of interstitial cells remains to be resolved. Although the main axis of PDGFRα+ cells ran parallel with circular muscle fibers, these interstitial cells displayed lateral processes that made contacts with adjacent PDGFRα+ cells, forming a 3-dimensional network of cells (Fig. 7B,C,E,F,H,I). PDGFRα+ cells within the CM made contacts with several nerve fibers (Fig. 7-B,C,E,F,H,I). PDGFRα+ cells within the myenteric region surrounded myenteric ganglia but were not closely associated with enteric neurons in this region. Numerous PDGFRα+ cells were observed in the LM, but a low level of close contacts were formed with enteric motor neurons because there were few neural processes in the LM (Fig. 7A,D,G).
Figure 7.
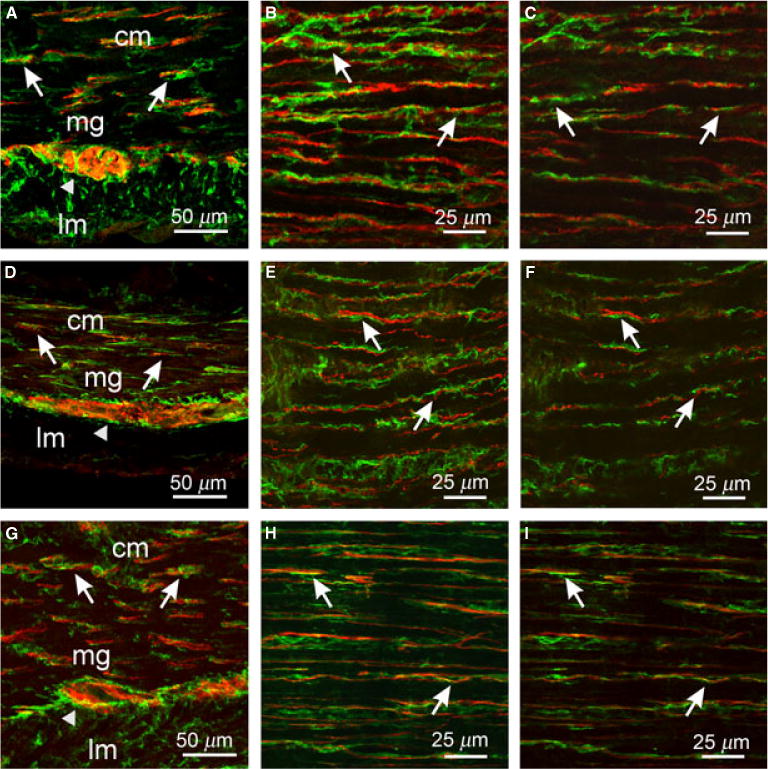
Enteric neurons and PDGFRα+ cells in the small intestine. (A–C) PGP9.5+ neurons and PDGFRα+ cells in cryostat sections (A) and flat mounts (B,C). (D–F) Sub-P+ neurons and PDGFRα+ cells in cryostat sections (D) and flat mounts (E,F). (G–I) nNOS+ neurons and PDGFRα+ cells in sections (G) and flat mounts (H,I). Both classes of enteric neurons (red) were closely apposed to PDGFRα+ cells (green, arrows). Note the even distribution of PDGFRα+ cells and close association with nerve fibers throughout the CM compared to the dense distribution of ICC at the DMP and scant distribution within the CM (see Fig. 4). PDGFRα+ cells also surrounded myenteric ganglia (arrowheads) but did not infiltrate ganglionic sheaths. (C,F,I) Single sections of reconstructed stacks taken from (B,E,H) show both cell types are closely apposed in one optical section. Scale bars are as indicated in each panel.
Colon
PDGFRα+ cells were closely associated with enteric motor neurons in the CM between taenia and under the taenia coli (Fig. 8B,C). As in other regions, both excitatory (sub-P; Fig. 8F,G) and inhibitory (nNOS; Fig. 8J,K) motor neurons were in close contact with PDGFRα+ cells. As in the stomach and small intestine, the PDGFRα+ cells appeared to envelope nerve fibers, and their processes formed contacts with adjacent nerve cell processes, as shown within single 0.25 μm image planes. This observation provides morphological evidence for a close three-dimensional relationship between enteric nerve fibers and PDGFRα+ cells. A dense network of PDGFRα+ cells was observed in the taenia coli, and these cells formed close associations with neural processes (Fig. 8L). As in other regions of the GI tract, a dense population of PDGFRα+ cells was found at the level of the myenteric plexus, and these cells were found at the CM and LM aspects of ganglia. However, there were no obvious structural associations between PDGFRα+ cells and enteric nerve fibers within the myenteric region (Fig. 8D,H).
Figure 8.
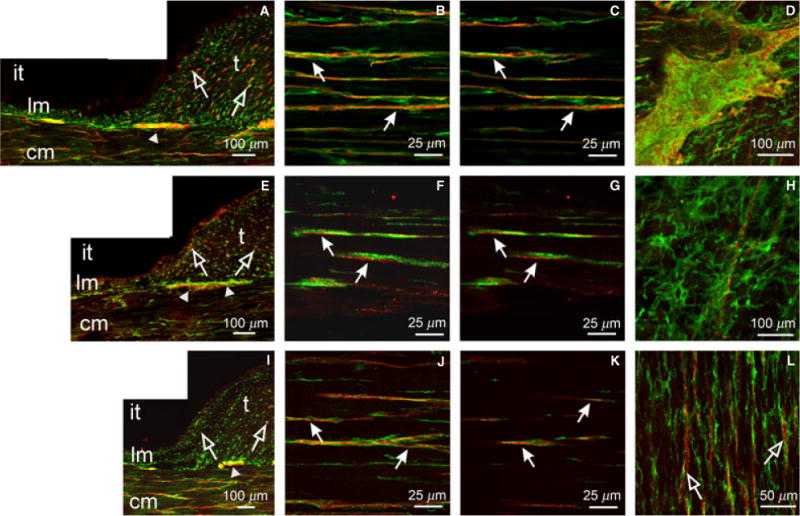
Relationship between enteric neurons and PDGFRα+ cells in the colon. (A–D) PGP9.5+ neurons (red) and PDGFRα+ cells (green) in the taenia (t) and inter-taenia (it) regions of the proximal colon. (A) Cryostat cross-section in the region of the taenia and non-taenia revealing LM (lm) and CM (cm). Close relationships between enteric nerve fibers and PDGFRα+ cells exist (open arrows). (B,C) Confocal reconstructions of PGP9.5+ fibers and PDGFRα+ cells in the CM showing the close apposition (closed arrows). (D) Myenteric ganglia revealing PDGFRα+ cells lie on the surface and between myenteric ganglia. (E–H) Sub-P+ neurons and PDGFRα+ cells and (I–L) nNOS+ neurons and PDGFRα+ cells in the proximal colon. Both neuron types (red) were closely apposed to PDGFRα+ cells (green; arrows) in the CM (F,G for sub-P and J,K for nNOS). (H) A reconstruction through the myenteric region showing a single sub-P+ nerve fiber not encased within a ganglionic sheath and with no apparent structural relationship with PDGFRα+ cells. (L) nNOS nerve fibers also observed in close apposition to PDGFRα+ cells in the taenia muscle layer.
DISCUSSION
We examined the morphological relationship between excitatory motor neurons and two distinct populations of interstitial cells, ICC and PDGFRα+ cells, throughout the tunica muscularis of Cynomolgus monkeys. The structural arrangement between enteric nerve fibers and PDGFRα+ cells in the primate GI tract is remarkably similar to that recently reported for guinea-pig and mouse.14,16,17,33 These cells are closely apposed to individual excitatory and inhibitory nerve fibers for distances greater than 230 μm, providing morphological evidence for a possible role of these cells in motor neurotransmission in the primate GI tract.
Our data are consistent with the hypothesis that ICC and PDGFRα+ cells may be involved in transducing neural signals. Both these types of cells form gap junctions with smooth muscle cells,20,34,35 so activation of ionic conductances in interstitial cells can be conducted to the smooth muscle syncytium. Thus, the neuroeffector junction in GI muscles appears to be composed of enteric nerve terminals and at least three cell types. From studies of rodents, it appears that the post-junctional responses in GI muscles are integrated responses of the smooth muscle/ICC/PDGFRα+ cell (SIP) syncytium. Our data show that the morphology of the primate GI tract is very similar to other mammals, and therefore it is possible that interstitial cells also have an important role in mediating enteric motor inputs in primate species.
Close associations occurred predominantly between PGP9.5 (pan-neuronal marker), sub-P (excitatory) and nNOS (inhibitory) nerve fibers and intramuscular ICC (ICC-IM) in the gastric fundus and antrum. In the small intestine, excitatory and inhibitory motor neurons were closely associated with ICC at the level of the deep muscular plexus (ICC-DMP). In the colon enteric nerve fibers formed close contacts with ICC-IM in the CM but also in the taenia where innervation of the LM in the colon was concentrated. Few neural processes were found in the LM in regions between taenia. Similar proximity existed between enteric nerve fibers and PDGFRα+ cells in many regions of the primate GI tract, however, we observed no concentration of PDGFRα+ cells within the deep muscular plexus in the small intestine, as observed with ICC-DMP. PDGFRα+ cells were more widely distributed and thus closely associated with enteric neurons throughout the CM. Although dense populations of ICC-MY and PDGFRα+ cells were observed in the myenteric regions of each organ, neither type of interstitial cell appeared to be closely associated with nerve cell processes in this region. Based on these morphological observations, it seems ICC-IM and PDGFRα+ cells may be highly innervated in the primate GI tract.
The hypothesis that ICC act as an intermediary cell between nerve terminals and smooth muscle cells was originally proposed by Cajal.36 Numerous electron microscopy studies have provided ultrastructural evidence that varicose enteric nerve terminals form close structural contacts (<20 nm) with ICC.34,37,38 More recently molecular and ultrastructural evidence have also suggested that synaptic specializations exist between enteric neurons and ICC,39 and the structural relationship between enteric motor nerve varicosities and ICC might have similarities to synaptic structures in the CNS40 or at skeletal neuromuscular junctions.41 Electron microscopy (EM) is excellent at providing a close look at the contacts between cells, however, the extent of cell-to-cell contacts within tissues is laborious to deduce from EM. Finding specific labels for ICC and PDGFRα+ cells has allowed analysis of morphological association between enteric neurons and interstitial cells at the light microscopy level.
Many of the morphological observations that have provided evidence that ICC act as cellular mediators in enteric motor neuron inputs have come from studies on rodent animal models.42 Structural studies for a role of ICC in enteric neurotransmission have been supported by functional studies performed on mutant animals. Mutants such as the Sl/Sld or W/WV mutant mouse, or the Ws/Ws mutant rat, have reduced Kit expression and reduced numbers of ICC in different regions of their GI tract. In the absence of ICC-IM in the stomach of the Sl/Sld or W/WV mutant mouse there was reduced cholinergic and nitrergic motor neuron input to the muscularis.3,6,7,43 Others have argued against a role of ICC in enteric motor neurotransmission and suggested that defects in neural responses in W mutants are a consequence of smooth muscle dysfunction.44–46 However, responses to exogenous excitatory and inhibitory transmitters appear to be intact in these mutants and similar to that of wild-type controls, suggesting that the absence or reduction in post junctional responses may be more related to defects in ICC.3,6,7
There have been only a limited number of morphological studies examining the relationship between enteric neurons and ICC in larger animal species including primates.31,42,47,48 Furthermore, many of these studies have been performed using immunohistochemistry on thin paraffin or cryostat sections that often obscure the complex three-dimensional relationships between nerve terminals and interstitial cells.27–30 In the present study, immunohistochemical analysis of whole mounts and flat mounts was performed and combined with confocal imaging to provide improved visualization of the structural relationships between enteric neurons and ICC in the thicker tunica muscularis of primates. This study provides a comprehensive examination of the tissue wide associations between enteric motor neurons and interstitial cells within the major organs of the GI tract. Until functional studies are performed on ICC isolated from primate tissues, or imaging studies directed toward ICC in motor function, the exact role of these cells in enteric motor neurotransmission remains to be determined.
A second interstitial cell, previously referred to in ultrastructural studies as ‘fibroblast-like cells’ (FLCs) also forms close associations with enteric nerve varicosities.20,21 Antibodies against PDGFRα label this class of interstitial cell specifically.14–17 Studies on mouse and guinea-pig have shown that PDGFRα+ cells are a distinct population of interstitial cells that are Kit−. These cells are distributed throughout GI organs, often closely associated with enteric neurons, and express receptors that mediate nitrergic inhibitory motor neurotransmission.14,16,17,33 PDGFRα+ cells form gap junctions with smooth muscle cells that would allow for electrical communication between these cells.20 PDGFRα+ cells express SK3 channels, which are apamin sensitive and are thought to contribute to the purinergic post-junctional inhibitory responses in the GI tract.15 Isolated PDGFRα cells respond to ß-NAD and ATP, two purines that have been proposed to be involved in mediating inhibitory post-junctional responses in the colon.17 Therefore, structural and functional evidence is accumulating that this second class of interstitial cell also acts as an intermediary cell in enteric motor neurotransmission. Interestingly PDGFRα+ cells are also closely apposed to excitatory motor nerve terminals, but to date little is known about the potential role of these cells in excitatory motor neurotransmission or whether these cells express receptors that could transduce excitatory inputs from motor neurons.
Noticeably there were also the differences in the distribution of enteric neurons and interstitial cells between the CM and LM in some organs of the primate GI tract. In the gastric antrum and small intestine there was a paucity of nerve fibers and ICC-IM in the LM vs the adjacent CM. Similar morphological observations have been observed in the murine antrum.49 Likewise in the proximal colon there was a marked difference in neuron density and ICC within the LM. The taenia was densely innervated and contained numerous ICC compared to the LM in inter-taenia regions. These data would suggest that the major innervation of the LM in the colon occurred in the three bands of taenia muscle. Although morphological reports have described differences in the density of innervation of the CM and LM in rodent stomachs,49 studies describing differences in functional responses between muscle layers in primates are not yet available. PDGFRα+ cells were densely distributed in the LM throughout the primate GI tract and in regions where few nerves fibers were evident suggesting that these cells have additional functions other than a role in enteric neurotransmission and raises the possibility that different classes of these cells exist within the primate tunica muscularis. A dense population of PDGFRα+ cells is also resident within the lamina propria and mucosa of rodents which do not appear associated with nerve fibers.14,50 Therefore the diverse distribution of PDGFRα+ cells would support different physiological roles for these cells in GI function.
In summary, at least two discrete populations of interstitial cells, ICC and PDGFRα+ cells form close associations with excitatory and inhibitory motor neurons in the tunica muscularis of all organs of the primate GI tract. The relationship between enteric nerve terminals and ICC/PDGFRα+ cells was similar as previously documented in rodents, in which functional studies on animals lacking interstitial cells demonstrates that these cells participate in mediating neural responses. From the similarity in morphology between rodents and primates, it is likely that both ICC and PDGFRα+ cells of the primate GI tract are also mediators of enteric motor neurotransmission.
Acknowledgments
The authors are grateful to Nancy Horowitz for the collection of monkey tissues. The authors are also grateful to Charles Rivers, Sparks, NV for supplying monkey tissue samples.
FUNDING
This work was supported by NIH DK57236 S.M.W. and NIH P01 DK41315 to K.M.S. and S.M.W. and an equipment grant from the NCRR for the Zeiss LSM510 confocal microscope (1 S10 RR16871).
Footnotes
CONFLICT OF INTEREST
The authors have no competing interests to declare.
AUTHOR CONTRIBUTION
SMW designed the research study; PJAB and YB performed the research in the study; PJAB analyzed the data; PJAB and SMW wrote the manuscript; SMW and KMS reviewed the paper.
References
- 1.Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–75. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- 2.Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480(Pt 1):91–7. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20:1393–403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bern-stein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–9. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 5.Ordög T, Ward SM, Sanders KM. Interstitial cells of cajal generate electrical slow waves in the murine stomach. J Physiol. 1999;518(Pt 1):257–69. doi: 10.1111/j.1469-7793.1999.0257r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci. 1996;93:12008–13. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckett EA, Horiguchi K, Khoyi M, Sanders KM, Ward SM. Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sl(d) mice. J Physiol. 2002;543:871–87. doi: 10.1113/jphysiol.2002.021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakahara M, Isozaki K, Hirota S, et al. A novel gain-of-function mutation of c-kit gene in gastrointestinal stromal tumors. Gastroenterology. 1998;115:1090–5. doi: 10.1016/s0016-5085(98)70079-4. [DOI] [PubMed] [Google Scholar]
- 9.Vanderwinden JM, Rumessen JJ. Interstitial cells of Cajal in human gut and gastrointestinal disease. Microsc Res Tech. 1999;47:344–60. doi: 10.1002/(SICI)1097-0029(19991201)47:5<344::AID-JEMT6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 10.Burns AJ. Disorders of interstitial cells of Cajal. J Pediatr Gastroenterol Nutr. 2007;45(Suppl 2):S103–6. doi: 10.1097/MPG.0b013e31812e65e0. [DOI] [PubMed] [Google Scholar]
- 11.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008;20(Suppl 1):54–63. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 12.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 13.Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M. CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol. 1998;11:728–34. [PubMed] [Google Scholar]
- 14.Iino S, Horiguchi K, Horiguchi S, Nojyo Y. c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor alpha in the murine gastrointestinal musculature. Histochem Cell Biol. 2009;131:691–702. doi: 10.1007/s00418-009-0580-6. [DOI] [PubMed] [Google Scholar]
- 15.Iino S, Nojyo Y. Immunohistochemical demonstration of c-Kit-negative fibroblast-like cells in murine gastro intestinal musculature. Arch Histol Cytol. 2009;72:107–15. doi: 10.1679/aohc.72.107. [DOI] [PubMed] [Google Scholar]
- 16.Cobine CA, Hennig GW, Kurahashi M, Sanders KM, Ward SM, Keef KD. Relationship between interstitial cells of Cajal, fibroblast-like cells and inhibitory motor nerves in the internal anal sphincter. Cell Tissue Res. 2011;344:17–30. doi: 10.1007/s00441-011-1138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurahashi M, Zheng H, Dwyer L, Ward SM, Don Koh S, Sanders KM. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol. 2011;589:697–710. doi: 10.1113/jphysiol.2010.201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurahashi M, Nakano Y, Hennig GW, Ward SM, Sanders KM. Platelet derived growth factor receptor α-positive cells in the tunica muscularis of human colon. J Cell Mol Med. 2012;16:1397–404. doi: 10.1111/j.1582-4934.2011.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komuro T, Tokui K, Zhou DS. Identification of the interstitial cells of Cajal. Histol Histopathol. 1996;11:769–86. [PubMed] [Google Scholar]
- 20.Horiguchi K, Komuro T. Ultrastructural observations of fibroblast-like cells forming gap junctions in the W/ W(nu) mouse small intestine. J Auton Nerv Syst. 2000;80:142–7. doi: 10.1016/s0165-1838(00)00089-8. [DOI] [PubMed] [Google Scholar]
- 21.Mitsui R, Komuro T. Direct and indirect innervation of smooth muscle cells of rat stomach, with special reference to the interstitial cells of Cajal. Cell Tissue Res. 2002;309:219–27. doi: 10.1007/s00441-002-0592-1. [DOI] [PubMed] [Google Scholar]
- 22.Grasa L, Gil V, Gallego D, Martín MT, Jiménez M. P2Y(1) receptors mediate inhibitory neuromuscular transmission in the rat colon. Br J Pharmacol. 2009;158:1641–52. doi: 10.1111/j.1476-5381.2009.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang SJ, Durnin L, Dwyer L, et al. β-nicotinamide adenine dinucleotide is an enteric inhibitory neurotransmitter in human and nonhuman primate colons. Gastroenterology. 2011;140:608–17, e606. doi: 10.1053/j.gastro.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang SJ, Blair PJ, Durnin L, Mutafova-Yambolieva V, Sanders KM, Ward SM. P2Y1 purinoreceptors are fundamental to inhibitory motor control of murine colonic excitability and transit. J Physiol. 2012;590:1957–72. doi: 10.1113/jphysiol.2011.224634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mutafova-Yambolieva VN, Hwang SJ, Hao X, et al. Beta-nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci USA. 2007;104:16359–64. doi: 10.1073/pnas.0705510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallego D, Gil V, Martinez-Cutillas M, Mañe N, Martin MT, Jimenez M. Purinergic neuromuscular transmission is absent in the colon of P2Y1 knocked out mice. J Physiol. 2012;590:1943–56. doi: 10.1113/jphysiol.2011.224345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagger R, Gharaie S, Finlayson C, Kumar D. Regional and transmural density of interstitial cells of Cajal in human colon and rectum. Am J Physiol. 1998;275:G1309–16. doi: 10.1152/ajpgi.1998.275.6.G1309. [DOI] [PubMed] [Google Scholar]
- 28.Bernardini N, Segnani C, Ippolito C, et al. Immunohistochemical analysis of myenteric ganglia and interstitial cells of Cajal in Ulcerative Colitis. J Cell Mol Med. 2011;16:318–27. doi: 10.1111/j.1582-4934.2011.01298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yun HY, Sung R, Kim YC, et al. Regional distribution of Interstitial Cells of Cajal (ICC) in human stomach. Korean J Physiol Pharmacol. 2010;14:317–24. doi: 10.4196/kjpp.2010.14.5.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanderwinden JM, Liu H, Menu R, Conreur JL, De Laet MH, Vander-haeghen JJ. The pathology of infantile hypertrophic pyloric stenosis after healing. J Pediatr Surg. 1996;31:1530–4. doi: 10.1016/s0022-3468(96)90171-2. [DOI] [PubMed] [Google Scholar]
- 31.Torihashi S, Horisawa M, Watanabe Y. c-Kit immunoreactive interstitial cells in the human gastrointestinal tract. J Auton Nerv Syst. 1999;75:38–50. doi: 10.1016/s0165-1838(98)00174-x. [DOI] [PubMed] [Google Scholar]
- 32.Wang XY, Paterson C, Huizinga JD. Cholinergic and nitrergic innervation of ICC-DMP and ICC-IM in the human small intestine. Neurogastroenterol Motil. 2003;15:531–43. doi: 10.1046/j.1365-2982.2003.00429.x. [DOI] [PubMed] [Google Scholar]
- 33.Iino S, Horiguchi K, Nojyo Y. Interstitial cells of Cajal are innervated by nitrergic nerves and express nitric oxide-sensitive guanylate cyclase in the guinea-pig gastrointestinal tract. Neuroscience. 2008;152:437–48. doi: 10.1016/j.neuroscience.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 34.Daniel EE, Posey-Daniel V. Neuro-muscular structures in opossum esophagus: role of interstitial cells of Cajal. Am J Physiol. 1984;246:G305–15. doi: 10.1152/ajpgi.1984.246.3.G305. [DOI] [PubMed] [Google Scholar]
- 35.Horiguchi K, Sanders KM, Ward SM. Enteric motor neurons form synaptic-like junctions with interstitial cells of Cajal in the canine gastric antrum. Cell Tissue Res. 2003;311:299–313. doi: 10.1007/s00441-002-0657-1. [DOI] [PubMed] [Google Scholar]
- 36.Cajal SR. Histologie du système nerveux de l’homme et des vertébrés. Vol. 2. Paris: Maloine; 1911. pp. 891–942. [Google Scholar]
- 37.Thuneberg L. Interstitial cells of Cajal: intestinal pacemaker cells? Adv Anat Embryol Cell Biol. 1982;71:1–130. [PubMed] [Google Scholar]
- 38.Faussone Pellegrini MS, Cortesini C, Romagnoli P. [Ultrastructure of the tunica muscularis of the cardial portion of the human esophagus and stomach, with special reference to the so-called Cajal’s interstitial cells] Arch Ital Anat Embriol. 1977;82:157–77. [PubMed] [Google Scholar]
- 39.Beckett EA, Takeda Y, Yanase H, Sanders KM, Ward SM. Synaptic specializations exist between enteric motor nerves and interstitial cells of Cajal in the murine stomach. J Comp Neurol. 2005;493:193–206. doi: 10.1002/cne.20746. [DOI] [PubMed] [Google Scholar]
- 40.Kennedy MB. Signal-processing machines at the postsynaptic density. Science. 2000;290:750–4. doi: 10.1126/science.290.5492.750. [DOI] [PubMed] [Google Scholar]
- 41.Boaro SN, Soares JC, König B. Comparative structural analysis of neuromuscular junctions in mice at different ages. Ann Anat. 1998;180:173–9. doi: 10.1016/S0940-9602(98)80020-4. [DOI] [PubMed] [Google Scholar]
- 42.Sanders KM, Hwang SJ, Ward SM. Neuroeffector apparatus in gastroin testinal smooth muscle organs. J Physiol. 2010;588:4621–39. doi: 10.1113/jphysiol.2010.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki H, Ward SM, Bayguinov YR, Edwards FR, Hirst GD. Involvement of intramuscular interstitial cells in nitrergic inhibition in the mouse gastric antrum. J Physiol. 2003;546:751–63. doi: 10.1113/jphysiol.2002.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sivarao DV, Mashimo HL, Thatte HS, Goyal RK. Lower esophageal sphincter is achalasic in nNOS(−/−) and hypotensive in W/W(v) mutant mice. Gastroenterology. 2001;121:34–42. doi: 10.1053/gast.2001.25541. [DOI] [PubMed] [Google Scholar]
- 45.Goyal RK, Chaudhury A. Mounting evidence against the role of ICC in neurotransmission to smooth muscle in the gut. Am J Physiol Gastrointest Liver Physiol. 2010;298:G10–3. doi: 10.1152/ajpgi.00426.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Carmichael SA, Wang XY, Huizinga JD, Paterson WG. Neuro-transmission in lower esophageal sphincter of W/Wv mutant mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G14–24. doi: 10.1152/ajpgi.00266.2009. [DOI] [PubMed] [Google Scholar]
- 47.Cobine CA, Hennig GW, Bayguinov YR, Hatton WJ, Ward SM, Keef KD. Interstitial cells of Cajal in the cynomolgus monkey rectoanal region and their relationship to sympathetic and nitrergic nerves. Am J Physiol Gastrointest Liver Physiol. 2010;298:G643–56. doi: 10.1152/ajpgi.00260.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horisawa M, Watanabe Y, Torihashi S. Distribution of c-Kit immunopositive cells in normal human colon and in Hirschsprung’s disease. J Pediatr Surg. 1998;33:1209–14. doi: 10.1016/s0022-3468(98)90152-x. [DOI] [PubMed] [Google Scholar]
- 49.Song G, David G, Hirst S, Sanders KM, Ward SM. Regional variation in ICC distribution, pacemaking activity and neural responses in the longitudinal muscle of the murine stomach. J Physiol. 2005;564:523–40. doi: 10.1113/jphysiol.2004.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karlsson L, Lindahl P, Heath JK, Betsholtz C. Abnormal gastrointestinal development in PDGF-A and PDGFR-(alpha) deficient mice implicates a novel mesenchymal structure with putative instructive properties in villus morphogenesis. Development. 2000;127:3457–66. doi: 10.1242/dev.127.16.3457. [DOI] [PubMed] [Google Scholar]


