Abstract
Ten percent of the population worldwide suffers from chronic kidney disease (CKD), characterized by fibrotic changes in the kidney. Fibrosis is associated with loss of epithelial parenchyma, and accumulation of myofibroblasts, fibrillary collagen and inflammatory cells. Two recent papers highlight the critical role of Twist and Snai1 transcription factors in kidney fibrosis.
The kidney has a tremendous capacity to regenerate. Exposure to toxins or hypoxia results in the death of renal tubule epithelial cells (TECs). This event causes an acute decline in kidney function, known as Acute Kidney Injury (AKI). The death of TECs triggers the proliferation and expansion of surviving cells. Surviving TECs then undergo differentiation, after which kidney function and histological features typically return to normal.
In contrast, chronic and repeated TEC injury leads to irreversible loss of function, termed Chronic Kidney Disease (CKD). Fibroblast accumulation is the most prominent feature of fibrosis (Reidy et al., 2014). The origin of activated myofibroblasts and collagen in renal tissue has long been debated (Kriz et al., 2011). Initial studies indicated that fibroblasts originated from injured epithelial cells via a mechanism called epithelial mesenchymal transition (EMT) (Iwano et al., 2002). Later studies however, demonstrated that the majority of activated myofibroblasts actually come from resident fibroblasts (Humphreys et al., 2010). While epithelial cells might not directly turn into myofibroblasts, they play a critical role in myofibroblast activation. Studies in mice have reproducibly shown that TEC-specific genetic manipulation of transcription factor pathways can protect mice from developing fibrosis (Bielesz et al., 2010, Chuang et al., 2013).
High expression of Wnt, Notch and Hedgehog pathway genes have been observed in injured kidneys, indicating that the injury response recapitulates aspects of development. The expression of these pathways is likely needed to enhance proliferation and lineage commitment of undifferentiated cells required to replace damaged cells. The expression of developmental genes in TECs might be associated with both beneficial and harmful effects. Though Wnt and Notch proteins can enhance proliferation and induce resistance to apoptosis, they also prohibit full differentiation of epithelial cells (Chuang et al., 2013). Their expression needs to be “turned off” for epithelial cells to undergo terminal differentiation. In CKD, large numbers of epithelial cells appear dedifferentiated and have mesenchymal-like characteristics; the term ‘epithelial to mesenchymal transition’, or EMT, has been used to describe these changes. To complicate matters, on the one hand, the notion that epithelial cells fully trans-differentiate and become activated myofibroblasts via the EMT process was originally proposed. But on the other hand, later studies indicated that very few TECs, if any, are unable to cross the basement membrane to actually become fibroblasts. To clarify this distinction, many scientists started to use the term “partial EMT” to describe this process. However, “partial EMT” would indicate that this is a unidirectional process in which TECs eventually become mesenchymal cells, which is not the case in kidney fibrosis. TECs in kidney fibrosis appear to be plastic; these cells are not locked into one differentiated state. While some TECs become more mesenchymal, others transition back to the epithelial phenotype or remain in a dedifferentiated state. Therefore, for the sake of clarity, we propose to refer to this process as “epithelial plasticity.”
A handful of transcription factors: Twist, Zeb1, Zeb2, Snai1, and Snai2 are known to play key role in EMT(Kalluri and Weinberg, 2009). Expression of any of these transcription factors in epithelial cells is able to induce complete mesenchymal reprogramming in vitro. To directly address the role of epithelial plasticity in renal fibrosis development, the Kalluri and Nieto groups genetically ablated Twist or Snai1 in TECs in mice (Grande et al., 2015, Lovisa et al., 2015). In the absence of Snai1 and Twist (thus inhibiting the EMT program), TECs were able to express certain markers of differentiated renal epithelial cells (e.g., aquaporins and solute transporters, among others). This was accompanied by a decrease in myofibroblast accumulation and inflammation. It is noteworthy that, in addition to these genetic models (ex vivo), they also inhibited Snai1 expression in vivo using anti-sense oligonucleotides targeting a Snai1 mRNA splicing site. Much like the genetic model, this inactivation could also ameliorate unilateral ureteral obstruction-induced fibrosis in mice, thereby potentially representing a novel therapeutic target site to treat kidney fibrosis.
The Nieto group showed that TEC-specific overexpression of Snai1 was not only necessary, but also sufficient to induce fibrosis. Mice with genetic overexpression of Snai1 exhibited epithelial plasticity, myofibroblast accumulation, and inflammatory cell accumulation. While these studies provide strong evidence for the role of epithelial plasticity in the development of fibrosis, it remains unclear how epithelial dedifferentiation causes inflammatory cell and myofibroblast accumulation. In future studies, it will be important to determine the direct or paracrine signals that mediate epithelial cell-induced myofibroblast accumulation. Such interactions have proved to be vitally important in studies of tumor progression. Indeed, a recent publication by the Kalluri group indicates that activated fibroblasts play a critical role in restricting cancer spread. This type of epithelial-stromal interaction should also be studied in renal fibrosis (Ozdemir et al., 2014).
It will also be important to elucidate the mechanism that holds TECs in a “partial EMT” state. In other organs, this is a critical step that actually prevents cancer formation. When cells become mesenchymal, they dedifferentiate into a stem cell-like state that is associated with highly proliferative and migratory capacities which are indeed, important cancer characteristics. In order to fully understand the TEC “pause” mechanism, it will be necessary to determine the upstream factors that regulate Snai1 and Twist expression. TGF-β and Notch are very strong regulators of Snai1 and Twist in vitro, but it is not clear whether or not they are as effective in vivo.
In summary, these two new and exciting articles fit well into the existing paradigm of the role epithelial plasticity and EMT play in kidney fibrosis. They indicate that in cases of CKD, most likely caused by sustained renal injury, the terminal differentiation of epithelial cells is blocked by increased levels of Snai1 and Twist. These transcription factors most likely work in concert with β-catenin, Notch, and Smad3, although this has not been fully proven. Because the expression of these pathways is associated with resistance to apoptosis, cells that activate these pathways might preferentially expand in CKD. Releasing the terminal differentiation block by inhibiting Twist and Snai1 leads to amelioration of fibrosis. As such, these signaling pathways may well represent novel therapeutic targets in renal disease.
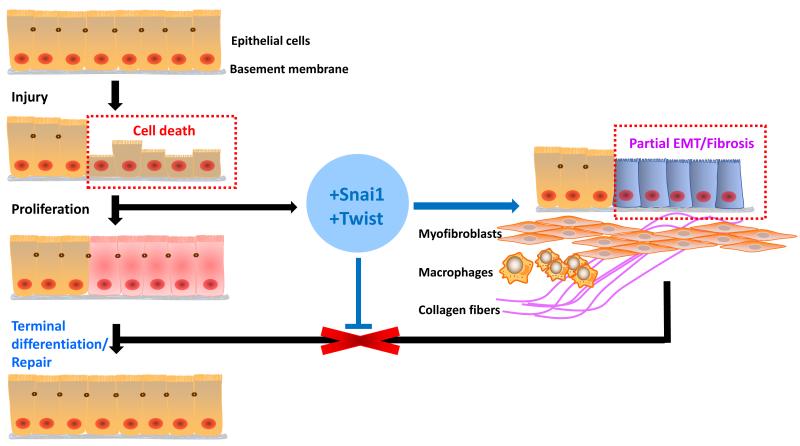
Figure 1. Twist and Snai1 expression contributes to the development of kidney fibrosis.
Chronic kidney disease is characterized by increased TEC death. Cell death triggers a regenerative process, including epithelial cell proliferation. Expression of Snai1 and Twist prohibits TEC differentiation and results in myofibroblast and inflammatory cell accumulation and fibrosis development. Blocking Snai1 and Twist expression allows the differentiation of epithelial cells and ameliorates fibrosis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bielesz B, Sirin Y, Si H, Niranjan T, Gruenwald A, Ahn S, Susztak K. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest. 2010;120:4040–4054. doi: 10.1172/JCI43025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chuang PY, Menon MC, He JC. Molecular targets for treatment of kidney fibrosis. J Mol Med (Berl) 2013;91:549–559. doi: 10.1007/s00109-012-0983-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grande MT, Sanchez-Laorden B, Lopez-Blau C, De Frutos CA, Boutet A, Arevalo M, Nieto MA. Snail1-induced partial epithelial-to mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med. 2015 doi: 10.1038/nm.3901. [DOI] [PubMed] [Google Scholar]
- 4.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. The American journal of pathology. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Invest. 2011;121:468–474. doi: 10.1172/JCI44595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovisa S, LeBleu VS, Tampe B, Sugimoto H, Vadnagara K, Carstens JL, Kalluri R. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med. 2015 doi: 10.1038/nm.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Kalluri R. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. 2014;124:2333–2340. doi: 10.1172/JCI72271. [DOI] [PMC free article] [PubMed] [Google Scholar]