Abstract
The association between the clinical use of nitroglycerin (NTG) and headache has led to the examination of NTG as a model trigger for migraine and related headache disorders, both in humans and laboratory animals. In this study in mice, we hypothesized that NTG could trigger behavioural and physiological responses that resemble a common manifestation of migraine in humans. We report that animals exhibit a dose-dependent and prolonged NTG-induced thermal and mechanical allodynia, starting 30–60 min after intraperitoneal injection of NTG at 5–10 mg/kg. NTG administration also induced Fos expression, an anatomical marker of neuronal activity in neurons of the trigeminal nucleus caudalis and cervical spinal cord dorsal horn, suggesting that enhanced nociceptive processing within the spinal cord contributes to the increased nociceptive behaviour. Moreover, sumatriptan, a drug with relative specificity for migraine, alleviated the NTG-induced allodynia. We also tested whether NTG reduces the threshold for cortical spreading depression (CSD), an event considered to be the physiological substrate of the migraine aura. We found that the threshold of CSD was unaffected by NTG, suggesting that NTG stimulates migraine mechanisms that are independent of the regulation of cortical excitability.
Keywords: Nitroglycerin, migraine, pain, hypersensitivity, sumatriptan
Introduction
Migraine is a common episodic, primary pain disorder affecting 6% of men and 17% of women in the USA (1). For many patients, diverse environmental triggers elicit the typical phases of the migraine attack: aura, head pain, nausea and hypersensitivity to sensory stimuli. During the headache phase, stimuli that are normally innocuous, such as ambient light, sound, applying make-up or wearing a watch can be unpleasant or even painful. In fact, two-thirds or more of those with migraine experience increased sensitivity to non-noxious thermal and mechanical stimulation of the skin in non-cranial regions of the body (2–4), by a mechanism that is likely to involve central sensitization (5).
The clinical use of nitroglycerin (NTG) reliably produces a dull headache within the first few minutes of administration, presumably from the direct activation of cranial afferents secondary to NTG-induced vasodilation of cranial blood vessels (6). However, 30 min to 4 h after administration of NTG, patients with a history of migraine also report the onset of delayed headache symptoms that are indistinguishable from their environmentally triggered migraines (7). Furthermore, experimentally administered NTG induces migrainous headaches that are accompanied by thermal allodynia (8), activate brainstem regions that are also active in a migraine attack (9) and can be blocked by migraine prophylactic agents (10,11). NTG can even trigger aura in some patients with an established history of migraine with aura (12). NTG has thus been used extensively to model migraine in human subjects (13). Interestingly, in other settings NTG reduces pain, such as its common use in myocardial syndrome, and topical NTG can reduce pain in patients with reflex sympathetic dystrophy or diabetic neuropathy (14,15).
The mechanism of NTG-induced hyperalgesia has been explored in rodents. Nitric oxide (NO) donors such as NTG (16) increase dorsal horn responses to afferent stimulation, sensitize a subpopulation of trigeminal afferents that innervate the dura, and have a role in inflammatory hyperalgesia (17–20). NTG induces hyperalgesia and Fos expression in the trigeminal nucleus caudalis (TNC) in rats (21–23). Most interestingly, the antimigraine drug eletriptan, a serotonin 1B, 1D and 1F receptor agonist, suppresses NTG-induced Fos induction in the TNC (24). NTG-induced hyperalgesia in animals may thus provide a behavioural model of the NTG-induced allodynia observed in humans with migraine.
In animal models, cortical spreading depression (CSD) manifests as a wave of concentric neuronal and glial depolarization (25,26), can induce activation of the TNC (27), can be inhibited by migraine prophylactic medications (28), and is more easily elicited in mice bearing mutations of the calcium channel subunit alpha 1A, which underlies familial hemiplegic migraine type I (29). Importantly, waves of cortical activity highly suggestive of CSD occur in awake patients during migraine (30–33). As these studies suggest that cortical excitability in CSD is a physiological correlate of a predisposition to migraine, we also tested the hypothesis that NTG can lower the threshold for induction of CSD.
We first determined the doses of NTG that are required to trigger hypersensitivity to mechanical and thermal stimuli in wild-type C57BL6 mice. We report that systemic NTG evokes a dose-dependent thermal and mechanical allodynia with a delayed time course similar to that of NTG-induced allodynia in humans. Furthermore, we report that NTG-induced allodynia in mice is relieved by the antimigraine drug, sumatriptan.
Methods
Experimental animals
All experiments were conducted on C57BL6 male mice weighing 20–30 g. Animals were housed in a 12-h light–dark cycle. Experiments were conducted between 09.00 and 14.00 h at a temperature of 22°C. All protocols were approved by the Institutional Animal Care and Use Committee at University of California San Francisco or University of California Los Angeles.
Nitroglycerin administration
A stock of 5.0 mg/ml NTG (American Regent, Inc., Shirley, NY, USA) dissolved in 30% alcohol, 30% propylene glycol, and water was freshly diluted each day in 0.9% saline in a polypropylene tube. Doses of 0.5, 1, 5 or 10 mg/kg were administered intraperitoneally. Control mice received an intraperitoneal (i.p.) injection of 0.9% saline. The doses of NTG that were sufficient to produce allodynia in mice are comparable to those used to produce allodynia in rats (10 mg/kg NTG i.p.; (17)), but are much higher than the total doses used in humans (40 μg/kg NTG by intravenous infusion) (34) or 0.9–1.0 mg sublingually (8). These large differences in dosing between human and rodent may be due to the much more efficient hepatic bioactivation of NTG into the pharmacologically active NO in humans (35).
Sumatriptan administration
Dilutions of sumatriptan were made from an injectable preparation of sumatriptan succinate at 12 mg/ml (GlaxoSmithKline Welcome, Chapel Hill, NC, USA). Five minutes after the administration of 10 mg/kg NTG, each animal was treated with i.p. sumatriptan (300 μg/kg, 600 μg/kg) or saline. Alternatively, an identical set of NTG-injected mice was treated with a single injection of intrathecal sumatriptan (0.06 μg in 5 μl) or saline at 5 min after NTG administration. Intrathecal (i.t.) injections were made in lightly restrained, awake mice using a 25-μl Hamilton syringe mounted onto a 30-G, 0.5-in needle inserted between the L4-5 lumbar interspace (36,37). As the effective i.t. dose of 0.06 μg is approximately 1/100 of the 300-μg/kg systemic dose, we believe that any observed effect is almost certainly via an action at a central target. Neither i.t. nor systemic sumatriptan at these doses produces any changes in acute nociceptive thresholds, nor do they cause any sensorimotor impairment that would interfere with these behavioural experiments (37).
Behavioural assays
Animals were habituated to the testing apparatus for 60 min on the day prior to and again immediately before determination of baseline nociceptive thresholds. Each group of animals underwent one of two different tests before and following NTG injection. We tested 10–12 animals at each dose of NTG.
Heat sensitivity
To determine thermal nociceptive thresholds, we used the Hargreaves assay, which focuses radiant light on the hind paw and measures the latency in seconds to withdrawal of the hind paw (PAW Thermal Stimulator, UC San Diego Department of Anesthesia, San Diego, CA, USA) (38). The heat intensity was calibrated so that a normal, untreated animal has an average latency of 8–10 s; to prevent tissue injury, a maximum cut-off value was set at 20 s. For each animal, the withdrawal latency is the average of three separate determinations, taken with at least 2 min between each trial. We used the Hargreaves test to measure thermal nociceptive behaviours immediately before and 30, 60, 90, 120 and 240 min after injection of NTG. The assays were conducted by a single experimenter blinded to the treatment groups.
Mechanical
Mechanical nociceptive thresholds were determined with von Frey (Semmes-Weinstein) monofilaments (North Coast Medical, Morgan Hill, CA, USA) using the up-and-down method (39). We determined the baseline for each hind paw before injection (baseline), 1, 2 and 4 h after injection.
Statistical analysis
We presented the average value of the nociceptive thresholds from each group of mice. Statistical significance was tested by anova analysis followed by Dunnett's test for multiple comparisons. Error bars represent s.e.m.
Immunohistochemistry
Each treatment group consisted of three mice. Two hours after i.p. injection of NTG (10 mg/kg) or saline, mice were anaesthetized with 2.5% Avertin and perfused intracardially with phosphate-buffered saline (PBS) and subsequently 4% paraformaldehyde. Whole brain and spinal cord were fixed for 15 h in 4% paraformaldehyde, and transferred to 30% sucrose in PBS for 24–30 h. Spinal cord and brain were frozen and transverse sections cut at 40 μm. Every third section was collected for staining. Free-floating sections were incubated in PBS and 0.3% Triton X-100 and 5% normal goat serum (NGS–T) for 1 h before incubation in primary anti-Fos (Oncogene Science, Cambridge, MA, USA) at a dilution of 1 : 50 000 in 1% NGS–T for 18 h at room temperature. Sections were washed in 1% NGS–T before incubation for 1 h in biotinylated goat antirabbit antibody (Vector Laboratories, Burlingame, CA, USA), washed in PBS–T and incubated in Extravidin peroxidase (Sigma-Aldrich Biotechnology, St Louis, MO, USA) for 1 h, followed by detection of the peroxidase with 3'diaminobenzidine (Sigma). Mounted and cover-slipped sections were counted for Fos-reactive nuclei by a single observer in a blinded manner. Cervical level was determined based upon the morphological appearance of the section under brightfield, and the areas of lamina I and II of the cervical spinal cord dorsal horn were identified by the area of lucency in darkfield. We counted 30 hemi-sections from each treatment group for each cervical level (6–12 per animal), and 50 hemi-sections that included TNC (10–20 per animal).
Cortical spreading depression thresholds
Fourteen male C57BL6 mice, weighing 25–35 g, were housed in temperature-controlled rooms on a 12-h light–dark cycle. All experiments were performed at the same time of day.
Anaesthesia was induced (5%) and maintained (0.8–1.5%) with isoflurane. Each animal was placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA, USA) and the parietal skull exposed. A glass microelectrode (2 μm diameter, 1 M KCl fill solution, 0.5 MΩ resistance) was advanced 500 μm into the cortex through a burr hole placed 0.5 mm anteromedial to lambda. A second burr hole was placed 0.5 mm from the temporal ridge midway between bregma and lambda, for CSD induction. A bipolar tungsten microelectrode (80 KΩ resistance, 300 μm tip diameters, tip spacing 200 μm) was inserted 500 μm into the parietal cortex through the second burr hole. The skull was coated with silicone oil to improve transparency. For CSD induction, a stimulus isolation unit (Grass SD9; Quincy, MA, USA) generated a bipolar stimulus train at 200 Hz, 200 μs pulse width, for 50 s, at increasing currents. Stimulus began at 12.5 μA and was increased every 100 s in fixed steps to a maximum of 1250 μA, until CSD was elicited.
The animal was placed on the imaging stage of a custom microscope and rested under anaesthesia for 1 h before imaging. The cortex was illuminated with a 940-nm light-emitting diode (Opto Technology, Wheeling, IL, USA). The use of infrared light allowed visualization of the CSD waveform through the intact skull. Reflected light was collected with a lens system consisting of two f/0.95 lenses connected front to front, focused on a high-sensitivity (0.00015 Lux) 8-bit charge-coupled device camera (Watec 902K; Tsuruoka, Japan). Field of view was 4.2 × 3.2 mm, pixel size was 6.6 μm. Images were acquired at 2 Hz for 10 min, with CSD induction beginning 25 s into the recording. Field potentials were acquired (bandpass 0–1 kHz), amplified (A-M Systems 3000; Carlsborg, WA, USA), digitized (at 1 kHz, PCI-6251; National Instruments, Austin, TX, USA) and recorded and synchronized with imaging data, by a custom LabView Virtual Instrument (National Instruments).
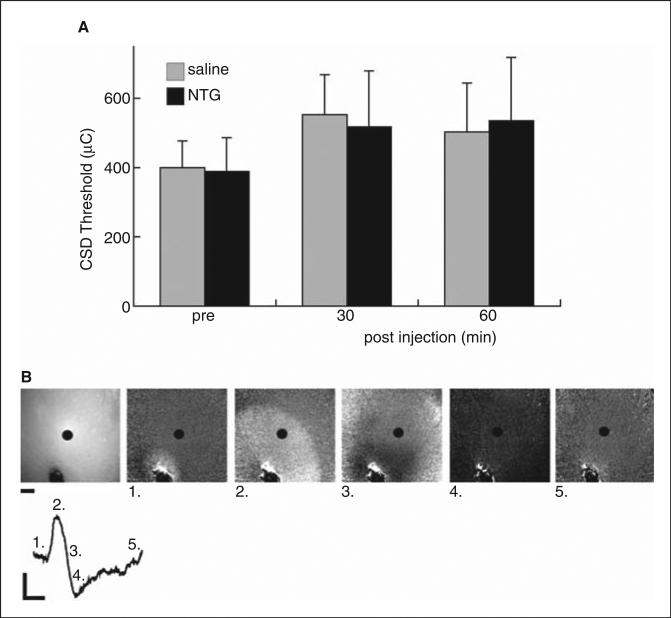
CSD threshold was determined in three sessions per animal. The first was a control session to determine baseline CSD threshold. Thirty minutes after the first session, the animals were treated with NTG (10 mg/kg i.p.; n = 7) or saline vehicle (n = 7). Thirty minutes and 1 h after treatment, repeat thresholding sessions were performed. CSD was demonstrated by a change in the propagated optical intrinsic signal and corresponding negative d.c. shift in field potential. Mean thresholds, as well as amplitude, duration and area under the curve for the d.c. shift were computed for the three time points and compared by anova (Microcal Origin 6.0; Northampton, MA, USA).
Results
NTG induces thermal hypersensitivity
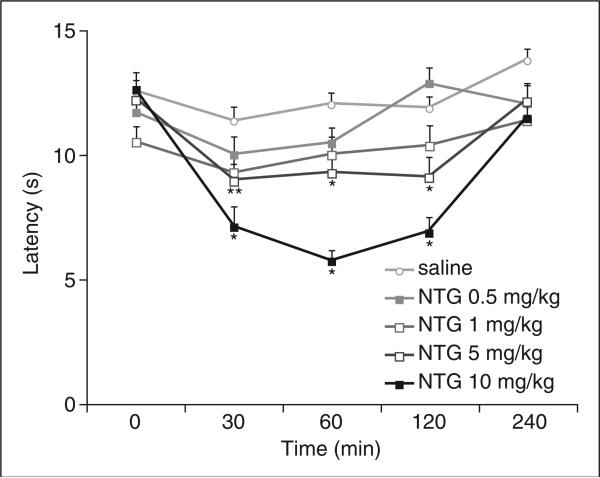
To test whether NTG can increase thermal sensitivity in mice, we measured the latency to hindpaw withdrawal from a focused beam of radiant light (Hargreaves test (38)) before and after administration of NTG. I.p. injection of NTG significantly reduced withdrawal latencies in a dose-dependent manner (Fig. 1). At a dose of 10 mg/kg, NTG-induced thermal hypersensitivity developed within 30 min, peaked at 60 min, and subsided by 4 h after injection (P < 0.01 for all time points). Administration of 5.0 mg/kg NTG also significantly reduced withdrawal latencies (P < 0.05 for all time points), but the lowest doses of NTG (0.5 or 1.0 mg/kg) did not significantly affect response latency (P > 0.05).
Figure 1.
Nitroglycerin (NTG) induces thermal hypersensitivity. Time course of thermal nociceptive thresholds, measured by hindpaw withdrawal latency to a radiant heat source (Hargreaves test), after various doses of NTG administration. Latency to respond is decreased in animals that received 5 or 10 mg/kg NTG compared with animals that received saline. n = 10–12 animals per group; *P < 1 × 10−2; **P < 5 × 10−2. Neither 1.0 nor 0.5 mg/kg NTG significantly affected the response to radiant heat (P > 5 × 10−2).
NTG induces mechanical hypersensitivity
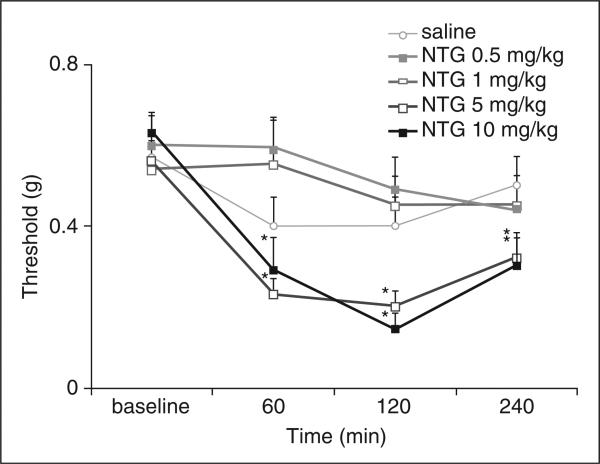
To evaluate NTG-induced changes in mechanical nociceptive threshold, we examined the hindpaw withdrawal in response to calibrated von Frey (Semmes-Weinstein) monofilaments (Fig. 2). Administration of 0.5 and 1.0 mg/kg NTG intraperitoneally did not produce a significant difference in mechanical pain threshold compared with i.p. injection of saline. However, 5.0 and 10.0 mg/kg NTG produced a small but significant reduction in the mechanical nociceptive threshold 60 min after NTG injection (0.23 and 0.28 g following 5.0 or 10.0 mg/kg NTG, P < 0.05). Mechanical hypersensitivity persisted even at 4 h after injection of 5.0 or 10 mg/kg NTG (0.32 and 0.29 g, P < 0.05).
Figure 2.
Nitroglycerin (NTG) induces dose-dependent mechanical hypersensitivity. NTG triggers changes in hindpaw withdrawal thresholds to mechanical stimulation with von Frey monofilaments. Administration of 5 or 10 mg/kg NTG significantly reduces mechanical pain thresholds at 60, 120 and 240 min compared with animals treated with saline (n = 10–12 animals per group; *P = 3 × 10−2; **P = 9 × 10−3). Mechanical thresholds in animals treated with lower doses (0.5, 1.0 and 5.0 mg/kg NTG) are not statistically different from animals treated with saline.
NTG and blood pressure
Because the vasodilator effects of NTG could cause significant changes in blood pressure, which could influence nociceptive behaviours (40), we tested the effects of NTG on blood pressure. Intermittent monitoring of blood pressure after i.p. injection of 5.0 or 10.0 mg/kg NTG in awake C57BL6 mice revealed no significant changes in blood pressure or heart rate at any of the experimental time points (supplemental Fig. S1).
Sumatriptan reduces NTG-induced thermal hypersensitivity
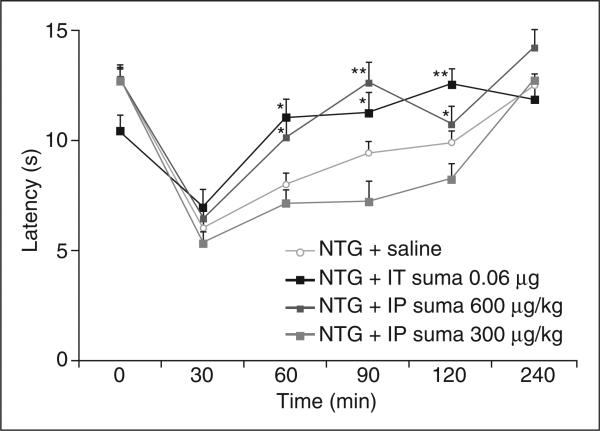
To determine further the parallels between NTG-induced allodynia in humans and mice, we next determined whether the antimigraine drug, sumatriptan, could reverse NTG-induced thermal hypersensitivity (Fig. 3). At 5 min after the administration of NTG, animals were treated with sumatriptan or saline. As noted above, withdrawal latencies were reduced in all animals 30 min after NTG injection (Fig. 1). However, in the sumatriptan-treated mice, withdrawal latencies returned to baseline at 60 min after NTG injection (10.1 ± 0.77 s for i.p. administration at 600 μg/kg and 11.02 ± 0.83 s for i.t. administration). In contrast, we found that neither saline (either systemic or i.t.) nor the lower dose of systemic sumatriptan (300 μg/kg) altered thermal thresholds during the 4 h after NTG injection (Fig. 3).
Figure 3.
Sumatriptan reverses nitroglycerin (NTG)-induced thermal hypersensitivity. Intrathecal injection of 0.06 μg sumatriptan or intraperitoneal (i.p.) injection of 600 μg/kg sumatriptan alleviates NTG-induced thermal hypersensitivity (10 mg/kg i.p. NTG) (anova analysis **P < 5 × 10−3; *P < 5 × 10−2). The difference between i.p. injection of 600 μg/kg sumatriptan and saline is not significant at other time points.
Sumatriptan reduces NTG-induced mechanical hypersensitivity
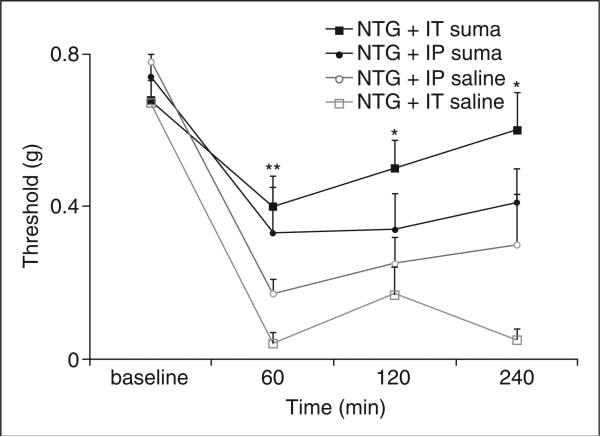
We determined whether sumatriptan could also alleviate NTG-induced mechanical allodynia in animals injected with sumatriptan or saline at 5 min after the injection of NTG 10 mg/kg (Fig. 4). As noted above (Fig. 2), mechanical nociceptive thresholds in the control group treated with NTG followed by saline at 5 min were significantly reduced at 60 min after NTG injection, and persisted throughout the 4-h study period. However, animals injected with NTG followed by a direct central nervous system (CNS) injection of sumatriptan (0.06 μg i.t.) had significantly higher mechanical nociceptive thresholds than control animals injected with saline (0.41 ± 0.08 g vs. 0.05 ± 0.05 g). Four hours after NTG injection, animals that received sumatriptan (0.06 μg i.t.) had recovered normal mechanical nociceptive thresholds, whereas NTG-induced hypersensitivity persisted in animals treated with systemic sumatriptan (600 μg/kg i.p.).
Figure 4.
Sumatriptan reduces nitroglycerin (NTG)-induced mechanical hypersensitivity. Intrathecal (i.t.) 0.06 μg sumatriptan reduces NTG-induced mechanical hypersensitivity [10 mg/kg intraperitoneal (i.p.) NTG] (**P < 5 × 10−4; *P = 6 × 10−3 compared with animals that received an i.t. saline injection). Sumatriptan (600 μg/kg, i.p.) did not significantly change the mechanical pain threshold after NTG compared with i.p. saline injection.
NTG activates trigeminal nucleus and spinal cord Fos expression
The pathophysiology of migraine is thought to involve the activation of upper cervical dorsal horn and TNC, which receive sensory input from craniofacial deep tissues (41). We hypothesized that the NTG-induced hypersensitivity we observed in the hindpaw could also be observed throughout the spinal cord dorsal horn, including the cervical spinal cord dorsal horn and TNC. To measure NTG-induced neuronal activation, we used immunohistochemistry to detect the expression of the Fos early gene product, at 2 h after injection of 10 mg/kg NTG or saline. Figure 5 illustrates the distribution of Fos-immunoreactive neurons in the upper cervical spinal cord and TNC. Compared with saline injection, NTG injection approximately doubled the average number of Fos-immunoreactive neurons in the primary nociceptive regions of laminae III–V of the cervical dorsal horn, an area associated primarily with processing non-nociceptive information (Fig. 5). NTG did not significantly increase Fos expression in laminae I–II or VII–X. In the TNC, NTG more than doubled the number of Fos-expressing neurons compared with saline.
Figure 5.
Nitroglycerin (NTG)-induced Fos expression in the spinal cord and trigeminal nucleus. (A) Quantification of Fos-immunoreactive nuclei in the cervical spinal cord lamina I–II, III–V, VI–X, and trigeminal nucleus caudalis 2 h after systemic 10 mg/kg NTG or saline. n = 3 animals per group, *P < 5 × 10−3. Representative examples of Fos immunoreactivity in the trigeminal nucleus caudalis at 2 h after the administration of (B) saline or (C) NTG 10 mg/kg, shown here with a 20× objective.
NTG effects on CSD threshold
To test whether NTG administration influenced CSD, we measured the threshold stimulus required to induce CSD before and after administration of NTG. No significant differences were noted in CSD thresholds for NTG (10 mg/kg) vs. vehicle-treated animals at any of the time points tested (Fig. 6). We also measured field potentials during CSD and again found no significant difference in amplitude, duration or area under the curve of the extracellular negative d.c. shift characteristic of CSD, in NTG- vs. vehicle-treated animals (data not shown).
Figure 6.
Determination of the cortical spreading depression (CSD) threshold with nitroglycerin (NTG) treatment. Mean CSD threshold 30 min prior to, and 30 and 60 min subsequent to treatment with saline vehicle or NTG 10 mg/kg. No significant difference was noted in CSD threshold in vehicle (n = 7) vs. NTG-treated (n = 7) animals. (b) Panels: Leftmost panel shows appearance of intact skull and underlying cortex with 940-nm illumination. Stimulation pipette at bottom centre of image; field potential electrode at top right corner. Scale bar: 500 μm. 1–5: Ratiometric images showing progression of CSD. Line trace: Optical intrinsic signal CSD waveform at 940 nm, from region of interest at centre of each image. Time point of each image is labelled. Vertical scale bar: 2% reflectance change; horizontal scale bar: 1 min.
Discussion
In the present studies in the mouse, we monitored allodynia, susceptibility to CSD and a marker of neuronal activation produced by systemic injection of NTG. NTG induced thermal and mechanical hypersensitivity that persisted for up to 4 h after its administration. We found that NTG also induced significant Fos expression in neurons of the TNC and upper cervical dorsal horn, but did not reduce the threshold for CSD. Finally, we demonstrated that i.t. sumatriptan significantly reversed the behavioural hypersensitivity produced by NTG in non-cranial regions of the body.
The behavioural assessment of allodynia in mice
It is difficult, if not impossible, to determine behaviourally the subjective quality of headache in mice. However, the observation that migraine attacks in humans often initiate a generalized central sensitization affecting the entire body (2,3) suggests that reduced nociceptive thresholds, even in non-cranial regions of the body, could constitute a behavioural correlate of the progression of a migraine attack (5). Thus, we hypothesized that NTG could also be used in mice to model the allodynia that occurs in migraine.
We found that 10.0 and 5.0 mg/kg NTG, but not lower doses, induced significant and prolonged hypersensitivity to mechanical and thermal stimuli in mice. Using Fos expression as a marker of neuronal activity, we showed that systemic 10 mg/kg NTG also increased activation of neurons in the C1 and C2 dorsal horn and in the TNC. The increase in NTG-evoked Fos expression in the TNC and cervical dorsal horn is a correlate of neuronal activity that may underlie the observed thermal and mechanical hypersensitivity.
NTG-induced mechanical and thermal allodynia can be attenuated by sumatriptan
Importantly, we have shown that administration of either 600 μg/kg i.p. or 0.06 μg sumatriptan i.t. alleviated the NTG-induced thermal and mechanical hypersensitivity. In these experiments, i.t. sumatriptan was more effective than systemic sumatriptan at reducing NTG-evoked mechanical and thermal hypersensitivity, suggesting that NTG exerts its effects on CNS targets (17), as does sumatriptan (37,42–44).
NTG does not affect CSD thresholds
Susceptibility to CSD has been used as a measure of cortical excitability in migraine models, and the fact that female mice have reduced thresholds for CSD compared with male mice interestingly parallels the increased prevalence of migraine in women (45). Here we found that NTG did not change the threshold required to induce CSD in C57BL6 mice. Furthermore, we found no effect of NTG on the electrophysiological correlates of CSD. Our experiments, however, do not entirely rule out an effect of NTG on CSD. We tested CSD threshold at times and doses comparable to those that produced the greatest effect on behaviour (i.e. at 30 and 60 min after injection of 10 mg/kg NTG). It is possible that NTG could alter CSD thresholds at times earlier than we tested, given its rapid onset of action.
There is evidence that other NO donors can modulate CSD. For example, NO contributes to the arteriolar dilation that takes place during CSD (46), and Petzold and colleagues have reported that nitric oxide synthase (NOS) inhibitors, applied topically to rat brain, reduced the CSD threshold, probably via NO-mediated modulation of n-methyl d-aspartate glutamate receptors and P/Q voltage gated calcium channels (47). In these experiments, NO donors prevented the effects of NOS inhibitors when applied concurrently; whether NO donors alone affected CSD thresholds was not investigated, but these data suggest that NO donors in fact work to increase the CSD threshold.
Although mutations that potentially contribute to migraine have been identified, an established method to address their contribution to pain in mice has been lacking (29,48). Nitroglycerin sensitivity may be a useful tool to examine mice bearing mutations associated with migraine. Thus, we anticipate that a measure of sensitivity to NTG will complement a broad panel of studies, to examine other elements in the migraine constellation.
Supplementary Material
Acknowledgements
This work was funded by Howard Hughes Medical Institute, NIH NHLBI HL059596 (Y-H.F., L.J.P.), Sandler Neurogenetics Fund (L.J.P.), NS 14627 (A.I.B.), NS 48499 (A.I.B.), NIH NS 47113 (A.H.A.) and an A. P. Giannini Foundation Postdoctoral Fellowship (E.A.B.).
Footnotes
Supporting information
Additional Supporting Information may be found in the online version of this article:
Supplemental Data Cardiovascular monitoring after NTG injection does not reveal haemodynamically significant changes.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–9. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- 2.Selby G, Lance JW. Observations on 500 cases of migraine and allied vascular headache. J Neurol Neurosurg Psychiatry. 1960;23:23–32. doi: 10.1136/jnnp.23.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol. 2000;47:614–24. [PubMed] [Google Scholar]
- 4.Bigal ME, Ashina S, Burstein R, Reed ML, Buse D, Serrano D, Lipton RB. Prevalence and characteristics of allodynia in headache sufferers: a population study. Neurology. 2008;70:1525–33. doi: 10.1212/01.wnl.0000310645.31020.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000;123:1703–9. doi: 10.1093/brain/123.8.1703. [DOI] [PubMed] [Google Scholar]
- 6.Iversen HK, Olesen J. Headache induced by a nitric oxide donor (nitroglycerin) responds to sumatriptan. A human model for development of migraine drugs. Cephalalgia. 1996;16:412–18. doi: 10.1046/j.1468-2982.1996.1606412.x. [DOI] [PubMed] [Google Scholar]
- 7.Christiansen I, Thomsen LL, Daugaard D, Ulrich V, Olesen J. Glyceryl trinitrate induces attacks of migraine without aura in sufferers of migraine with aura. Cephalalgia. 1999;19:660–7. doi: 10.1046/j.1468-2982.1999.019007660.x. discussion 26. [DOI] [PubMed] [Google Scholar]
- 8.de Tommaso M, Libro G, Guido M, Difruscolo O, Losito L, Sardaro M, Cerbo R. Nitroglycerin induces migraine headache and central sensitization phenomena in patients with migraine without aura: a study of laser evoked potentials. Neurosci Lett. 2004;363:272–5. doi: 10.1016/j.neulet.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 9.Bahra A, Matharu MS, Buchel C, Frackowiak RS, Goadsby PJ. Brainstem activation specific to migraine headache. Lancet. 2001;357:1016–17. doi: 10.1016/s0140-6736(00)04250-1. [DOI] [PubMed] [Google Scholar]
- 10.Tvedskov JF, Thomsen LL, Iversen HK, Gibson A, Wiliams P, Olesen J. The prophylactic effect of valproate on glyceryltrinitrate induced migraine. Cephalalgia. 2004;24:576–85. doi: 10.1111/j.1468-2982.2003.00720.x. [DOI] [PubMed] [Google Scholar]
- 11.Tvedskov JF, Thomsen LL, Iversen HK, Williams P, Gibson A, Jenkins K, et al. The effect of propranolol on glyceryltrinitrate-induced headache and arterial response. Cephalalgia. 2004;24:1076–87. doi: 10.1111/j.1468-2982.2004.00796.x. [DOI] [PubMed] [Google Scholar]
- 12.Afridi SK, Kaube H, Goadsby PJ. Glyceryl trinitrate triggers premonitory symptoms in migraineurs. Pain. 2004;110:675–80. doi: 10.1016/j.pain.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Magis D, Bendtsen L, Goadsby PJ, May A, Sanchez del Rio M, Sandor PS, et al. Evaluation and proposal for optimization of neurophysiological tests in migraine: part 2—neuroimaging and the nitroglycerin test. Cephalalgia. 2007;27:1339–59. doi: 10.1111/j.1468-2982.2007.01435.x. [DOI] [PubMed] [Google Scholar]
- 14.Agrawal RP, Choudhary R, Sharma P, Sharma S, Beniwal R, Kaswan K, Kochar DK. Glyceryl trinitrate spray in the management of painful diabetic neuropathy: a randomized double blind placebo controlled cross-over study. Diabetes Res Clin Pract. 2007;77:161–7. doi: 10.1016/j.diabres.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Manahan AP, Burkman KA, Malesker MA, Benecke GW. Clinical observation on the use of topical nitroglycerin in the management of severe shoulder-hand syndrome. Nebr Med J. 1993;78:87–9. [PubMed] [Google Scholar]
- 16.Harrison DG, Bates JN. The nitrovasodilators. New ideas about old drugs. Circulation. 1993;87:1461–7. doi: 10.1161/01.cir.87.5.1461. [DOI] [PubMed] [Google Scholar]
- 17.Meller ST, Gebhart GF. Nitric oxide (NO) and nociceptive processing in the spinal cord. Pain. 1993;52:127–36. doi: 10.1016/0304-3959(93)90124-8. [DOI] [PubMed] [Google Scholar]
- 18.Lambert GA, Donaldson C, Boers PM, Zagami AS. Activation of trigeminovascular neurons by glyceryl trinitrate. Brain Res. 2000;887:203–10. doi: 10.1016/s0006-8993(00)02919-x. [DOI] [PubMed] [Google Scholar]
- 19.Levy D, Strassman AM. Modulation of dural nociceptor mechanosensitivity by the nitric oxide-cyclic GMP signaling cascade. J Neurophysiol. 2004;92:766–72. doi: 10.1152/jn.00058.2004. [DOI] [PubMed] [Google Scholar]
- 20.Tassorelli C, Greco R, Wang D, Sandrini G, Nappi G. Prostaglandins, glutamate and nitric oxide synthase mediate nitroglycerin-induced hyperalgesia in the formalin test. Eur J Pharmacol. 2006;534:103–7. doi: 10.1016/j.ejphar.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 21.Tassorelli C, Greco R, Wang D, Sandrini M, Sandrini G, Nappi G. Nitroglycerin induces hyperalgesia in rats —a time-course study. Eur J Pharmacol. 2003;464:159–62. doi: 10.1016/s0014-2999(03)01421-3. [DOI] [PubMed] [Google Scholar]
- 22.Tassorelli C, Blandini F, Greco R, Nappi G. Nitroglycerin enhances cGMP expression in specific neuronal and cerebrovascular structures of the rat brain. J Chem Neuroanat. 2004;27:23–32. doi: 10.1016/j.jchemneu.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache. 2007;47:1026–36. doi: 10.1111/j.1526-4610.2007.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tassorelli C, Joseph SA. Systemic nitroglycerin induces Fos immunoreactivity in brainstem and forebrain structures of the rat. Brain Res. 1995;682:167–81. doi: 10.1016/0006-8993(95)00348-t. [DOI] [PubMed] [Google Scholar]
- 25.Gorji A. Spreading depression: a review of the clinical relevance. Brain Res Brain Res Rev. 2001;38:33–60. doi: 10.1016/s0165-0173(01)00081-9. [DOI] [PubMed] [Google Scholar]
- 26.Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev. 2001;81:1065–96. doi: 10.1152/physrev.2001.81.3.1065. [DOI] [PubMed] [Google Scholar]
- 27.Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002;8:136–42. doi: 10.1038/nm0202-136. [DOI] [PubMed] [Google Scholar]
- 28.Ayata C, Jin H, Kudo C, Dalkara T, Moskowitz MA. Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol. 2006;59:652–61. doi: 10.1002/ana.20778. [DOI] [PubMed] [Google Scholar]
- 29.van den Maagdenberg AM, Pietrobon D, Pizzorusso T, Kaja S, Broos LA, Cesetti T, et al. A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron. 2004;41:701–10. doi: 10.1016/s0896-6273(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 30.Bowyer SM, Aurora KS, Moran JE, Tepley N, Welch KM. Magnetoencephalographic fields from patients with spontaneous and induced migraine aura. Ann Neurol. 2001;50:582–7. doi: 10.1002/ana.1293. [DOI] [PubMed] [Google Scholar]
- 31.Cao Y, Welch KM, Aurora S, Vikingstad EM. Functional MRI-BOLD of visually triggered headache in patients with migraine. Arch Neurol. 1999;56:548–54. doi: 10.1001/archneur.56.5.548. [DOI] [PubMed] [Google Scholar]
- 32.Hadjikhani N, Sanchez Del Rio M, Wu O, Schwartz D, Bakker D, Fischl B, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci USA. 2001;98:4687–92. doi: 10.1073/pnas.071582498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olesen J, Larsen B, Lauritzen M. Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann Neurol. 1981;9:344–52. doi: 10.1002/ana.410090406. [DOI] [PubMed] [Google Scholar]
- 34.Thomsen LL, Brennum J, Iversen HK, Olesen J. Effect of a nitric oxide donor (glyceryl trinitrate) on nociceptive thresholds in man. Cephalalgia. 1996;16:169–74. doi: 10.1046/j.1468-2982.1996.1603169.x. [DOI] [PubMed] [Google Scholar]
- 35.Sokolowska M, Bednarski M, Kwiecien I, Filipek B, Wlodek L. Bioactivation of nitroglycerin to nitric oxide (NO) and S-nitrosothiols in the rat liver and evaluation of the coexisting hypotensive effect. Fundam Clin Pharmacol. 2004;18:449–56. doi: 10.1111/j.1472-8206.2004.00265.x. [DOI] [PubMed] [Google Scholar]
- 36.Fairbanks CA. Spinal delivery of analgesics in experimental models of pain and analgesia. Adv Drug Deliv Rev. 2003;55:1007–41. doi: 10.1016/s0169-409x(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 37.Nikai T, Basbaum AI, Ahn AH. Profound reduction of somatic and visceral pain in mice by intrathecal administration of the anti-migraine drug, sumatriptan. Pain. 2008;139:533–40. doi: 10.1016/j.pain.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 39.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 40.Taylor BK, Roderick RE, St Lezin E, Basbaum AI. Hypoalgesia and hyperalgesia with inherited hypertension in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;280:R345–54. doi: 10.1152/ajpregu.2001.280.2.R345. [DOI] [PubMed] [Google Scholar]
- 41.Hu JW, Sun KQ, Vernon H, Sessle BJ. Craniofacial inputs to upper cervical dorsal horn: implications for somatosensory information processing. Brain Res. 2005;1044:93–106. doi: 10.1016/j.brainres.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Kaube H, Hoskin KL, Goadsby PJ. Inhibition by sumatriptan of central trigeminal neurones only after blood–brain barrier disruption. Br J Pharmacol. 1993;109:788–92. doi: 10.1111/j.1476-5381.1993.tb13643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shepheard SL, Williamson DJ, Williams J, Hill RG, Hargreaves RJ. Comparison of the effects of sumatriptan and the NK1 antagonist CP-99,994 on plasma extravasation in Dura mater and c-fos mRNA expression in trigeminal nucleus caudalis of rats. Neuropharmacology. 1995;34:255–61. doi: 10.1016/0028-3908(94)00153-j. [DOI] [PubMed] [Google Scholar]
- 44.Storer RJ, Goadsby PJ. Microiontophoretic application of serotonin (5HT)1B/1D agonists inhibits trigeminal cell firing in the cat. Brain. 1997;120:2171–7. doi: 10.1093/brain/120.12.2171. [DOI] [PubMed] [Google Scholar]
- 45.Brennan KC, Romero Reyes M, Lopez Valdes HE, Arnold AP, Charles AC. Reduced threshold for cortical spreading depression in female mice. Ann Neurol. 2007;61:603–6. doi: 10.1002/ana.21138. [DOI] [PubMed] [Google Scholar]
- 46.Colonna DM, Meng W, Deal DD, Busija DW. Nitric oxide promotes arteriolar dilation during cortical spreading depression in rabbits. Stroke. 1994;25:2463–70. doi: 10.1161/01.str.25.12.2463. [DOI] [PubMed] [Google Scholar]
- 47.Petzold GC, Haack S, von Bohlen Und Halbach O, Priller J, Lehmann TN, Heinemann U, et al. Nitric oxide modulates spreading depolarization threshold in the human and rodent cortex. Stroke. 2008;39:1292–9. doi: 10.1161/STROKEAHA.107.500710. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z, Winborn CS, Marquez de Prado B, Russo AF. Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J Neurosci. 2007;27:2693–703. doi: 10.1523/JNEUROSCI.4542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.