Abstract
BACKGROUND
Persistent severe left ventricular (LV) systolic dysfunction after myocardial infarction (MI) is associated with increased mortality and is a class I indication for implantation of a cardioverter-defibrillator.
OBJECTIVES
We developed models and assessed independent predictors of LV recovery to >35% and ≥50% after 90-day follow-up in patients presenting with acute MI and severe LV dysfunction..
METHODS
Our multicenter prospective observational study enrolled participants with ejection fraction (EF) of ≤35% at the time of MI (n = 231). Predictors for EF recovery to >35% and ≥50% were identified after multivariate modeling and validated in a separate cohort (n = 236).
RESULTS
In PREDICTS, 43% of patients had persistent EF ≤35%, 31% had an EF of 36% to 49%, and 26% had an EF ≥50%. The model that best predicted recovery of EF to >35%, included EF at presentation, length of stay, prior MI, lateral wall motion abnormality at presentation, and peak troponin. The model that best predicted recovery of EF to ≥50%, included EF at presentation, peak troponin, prior MI, and presentation with ventricular fibrillation or cardiac arrest. After predictors were transformed into point scores, the lowest point scores predicted a 9% and 4% probability of EF recovery to >35% and ≥50%, respectively, whereas profiles with the highest point scores predicted an 87% and 49% probability of EF recovery to >35% and ≥50%.
CONCLUSIONS
In patients with severe systolic dysfunction following acute MI with an EF ≤35%, 57% had EF recovery to >35%. A model using clinical variables present at the time of MI can help predict EF recovery.
Keywords: heart failure, remodeling, risk assessment, ventricular ejection fraction
Persistence of severe left ventricular (LV) dysfunction after acute myocardial infarction (MI) has important prognostic implications and is associated with increased morbidity and mortality from both congestive heart failure (HF) and sudden cardiac death. While implantable cardioverter-defibrillators (ICD) confer a survival benefit in patients with severe LV dysfunction, guidelines recommend implantation of an ICD after a 40-day waiting period (90 days if revascularization occurs) (1) for patients whose ejection fraction (EF) remains ≤35%. This waiting period is based on 2 studies showing no long-term mortality benefit from early implantation of an ICD (2,3). The proportion of patients and factors that predict which patients will continue to have an EF ≤35% 90 days after MI are unknown.
Creatine kinase (CK), troponin, Q waves, dyssynchrony, and wall motion abnormalities measured at the time of acute MI have all been shown to predict LV functional recovery (4–6). Cohorts in which these associations were made included heterogeneous acute MI patients, many of whom had EFs >35% (and often normal or near-normal EFs). Many of these studies occurred prior to the institution of modern HF therapies and rapid revascularization techniques, which may attenuate the inferences of these findings. Taken together, existing data provide limited utility to help us understand the unique risk profile of acute MI patients presenting with severe LV dysfunction. Therefore, it remains a clinical challenge to predict which acute MI patients with severe LV dysfunction will still meet the indications for an ICD at the end of 90 days. In the present study, we define the incidence, identify markers, and develop prediction models for LV recovery to >35% and ≥50% in patients with acute MI and EF ≤35% using data from the PREDiction of ICd Treatment Study (PREDICTS).
Methods
Study Samples
The model development study samples were drawn from PREDICTS, a 60-center international study conducted from July 2008 to May 2011 that followed participants previously randomized in VEST (Vest Prevention of Early Sudden Death Trial), a randomized, controlled clinical trial enrolling patients age 18 years or older, admitted with MI and LV systolic dysfunction (EF ≤35%) measured at least 8 hours after the MI or percutaneous coronary intervention (PCI). Upon discharge from the hospital, participants were randomized to a LifeVest® wearable defibrillator (ZOLL Medical Corporation, Chelmsford, Massachusetts) and optimal medical therapy (OMT) or OMT alone with the primary endpoint of 90-day sudden death mortality.
At the conclusion of VEST participation, 90 days after discharge from hospitalization for an index MI, participants were enrolled in PREDICTS. In the PREDICTS study, patients were implanted with an ICD based on clinical indications or a Reveal® XT (Medtronic, Minneapolis, Minnesota) if the EF recovered to >35% for arrhythmia monitoring. The purpose of PREDICTS was to develop a risk stratification algorithm that predicted future ICD shock or sudden death over 5 years in patients who were admitted for an acute MI with an EF ≤35%. Of these 364 participants, 231 had follow-up echocardiograms at 90 days before the study was prematurely terminated. Inclusion criteria for PREDICTS was the same as noted above for VEST. Exclusion criteria for VEST and PREDICTS included significant valve disease, planned coronary artery bypass graft (CABG) surgery within 2 months, existing ICD, contraindication to eventual ICD, terminal condition, chronic renal failure, chest circumference >56 inches or <26 inches, pregnancy, and discharge to a skilled nursing facility. PREDICTS was stopped early due to slower than expected enrollment and termination of funding (from the National Institutes of Health and Medtronic).
After the termination of PREDICTS, VEST continued and the VEST Registry was created to follow those enrolled in VEST for 1 year. The VEST Registry has the same inclusion/exclusion criteria. Distinct from PREDICTS, a 90-day echocardiogram in the VEST study was not mandatory, but rather occurred at the discretion of the treating physician. Of the 509 participants in the VEST Registry available at the time of this analysis, 236 had echocardiograms at or near 90 days. This cohort was used for model validation (Online Figure 1).
Echocardiograms
Baseline echocardiograms were obtained at study sites using standard echocardiographic views and the PREDICTS Standard Operating Procedure (based on the American Society of Echocardiography guidelines) (7), more than 8 hours after MI or acute PCI. Ejection fraction was calculated by Simpson’s Rule. PREDICTS sites underwent a certification process by the PREDICTS echocardiography core lab, during which the echocardiogram quality and EF calculation methods were verified. Sites were allowed to recruit only after they passed this certification process. The echocardiography core laboratory maintained quality assurance by randomly sampling 50% of the studies. Participants underwent follow-up echocardiograms 90 days after the initial MI systematically (PREDICTS) or as clinically indicated (VEST registry) as discussed earlier.
Risk Factors of Persistent LV Dysfunction
Patient demographics, clinical characteristics (including prior cardiovascular disease and pre-hospitalization medications), characteristics of the MI hospitalization (e.g., electrocardiographic parameters, biomarkers, length of hospital stay, and primary treatment of the MI), baseline echocardiographic parameters, and discharge medications were evaluated as potential predictors of persistent LV dysfunction.
Prolonged hospital stay was defined as a hospital stay >4 days, based on previously published studies demonstrating an association between hospital stays >4 days at the time of acute MI presentation and subsequent poor outcomes (8). Discharge medications were categorized as the following: beta-blockers (carvedilol specifically), angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), ACEI or ARBs, aldosterone receptor blockers, statins, aspirin, and diuretics.
Statistical Analysis
For model development, baseline characteristics of study participants were compared according to their EF at 90 days, categorized as ≤35%, 36% to 49%, and ≥50%, using Wilcoxon rank sum test and chi-square tests as appropriate, followed by pairwise tests between categories as well as tests for trend. We used student’s t-tests to assess the association of baseline characteristics with change in EF.
We developed 2 logistic regression models: one to predict recovery of EF defined as 90-day values of >35% and one for the prediction of 90-day EF ≥50%. First we identified baseline characteristics associated with each recovery measure in single-predictor models at a significance level of p < 0.1. We determined which of the continuous predictors identified in the first step had nonlinear associations with each outcome variable in unadjusted models and used flexible 3-knot restricted cubic spline transformations to achieve a better fit. For each possible candidate logistic model, with 4 to 7 of the identified predictors, we estimated the c-statistic (to measure of discrimination) using 10 repetitions of 10-fold cross-validation to avoid optimism and overfitting. We estimated the Hosmer-Lemeshow goodness of fit statistic using the cross-validated predictions. Among the models with the highest cross-validated c-statistics, we selected the best performing model based on the following criteria: 1) competitive c-statistic; 2) Hosmer-Lemeshow p > 0.1; and 3) simplicity. When ranking the models, if there were other models within 0.03 of the model with the highest c-statistic, we selected the model with the best calibration as measured by the Hosmer-Lemeshow statistics. If more than 1 model was identified with equally high measures of discrimination and calibration, we chose the model that contained the most easily obtainable clinical variables. We then derived point scores based on the selected models for each outcome by categorizing continuous predictors, refitting the models, and rounding the logistic regression coefficients. We estimated the c-statistic and Hosmer-Lemeshow goodness of fit statistic for the point scores using 10 repetitions of 10-fold cross-validation.
For model validation, baseline characteristics of participants used in the derivation cohort were compared with those in the validation set and baseline characteristics of participants in the validation set without echocardiograms at 90 days were compared to those with echocardiograms at or near 90 days, using Wilcoxon rank sum and chi-square tests as appropriate. We then applied the models derived for the prediction of EF recovery to the data for registry participants’ data. Predicted risk scores for sustained LV dysfunction were calculated using point scores derived from the PREDICTS derivation cohort and applied to the VEST registry cohort to estimate discriminative ability.
Results
Baseline Characteristics
Per the baseline characteristics of the 231 PREDICTS participants (Table 1), 40% were routinely taking aspirin prior to the index admission and 25% had a history of MI. Only 13% of participants had a prior history of congestive HF. The EF prior to the acute MI was not known for all patients and thus is not included in this analysis.
Table 1.
Baseline Characteristics at Time of Index MI
| Characteristics at Presentation |
Total (n = 231) |
≤35 (n = 100) |
90-day EF (%) 36–49 (n = 72) |
≥50 (n = 59) |
p Value |
|---|---|---|---|---|---|
| Age, yrs | 60.4 ± 11.8 | 60 ± 12 | 59 ± 11 | 62 ± 12 | 0.5029 |
| Male | 165 | 76 (46.1) | 53 (32.1) | 36 (21.8)** | 0.0455 |
| BMI, kg/m2 | 28.6 ± 4.9 | 29.1 (4.8) | 28.2 (4.9) | 28.3 (5.0) | 0.3116 |
| Diabetes Mellitus |
80 | 34 (42.5) | 27 (33.8) | 19 (32.2) | 0.8164 |
| History of congestive HF |
31 | 17 (54.8) | 9 (29.0)* | 5 (16.1) | 0.1326 |
| History of MI | 66 | 37 (56.1) | 18 (28.8)* | 10 (15.2) | 0.0074 |
| History of PCI | 84 | 39 (46.4) | 27 (32.1) | 18 (21.4) | 0.2808 |
| History of CABG |
26 | 14 (53.8) | 10 (38.5) | 2 (8.7) ** | 0.0317 |
| History of CVA | 12 | 7 (58.3) | 3 (25.0) | 2 (16.7) | 0.3413 |
| History of AF | 21 | 12 (57.1) | 4 (19.1) | 5 (23.8) | 0.2140 |
| History of ASA | 90 | 46 (51.1) | 26 (28.9) | 18 (20.0) | 0.1303 |
| Tobacco use | |||||
| Never | 86 | 36 (41.9) | 26 (30.2) | 24 (28.6) | 0.8269 |
| Former | 80 | 36 (45.0) | 25 (31.2) | 19 (23.7) | |
| Current | 65 | 28 (43.1) | 21 (32.3) | 16 (24.6) | |
Values are mean ± SD or n (%).
p < 0.05 for EF ≤35% vs. EF >35%
p < 0.05 for EF <50% vs. ≥50%
AF = atrial fibrillation; ASA = aspirin; BMI = body mass index; CABG = coronary artery bypass graft; CVA = cerebrovascular accident; EF = ejection fraction; HF = heart failure; MI = myocardial infarction; PCI = percutaneous coronary intervention.
Characteristics of the index MI hospitalization are shown in Table 2. The mean EF was 28 ± 6.6%. Most participants (84%) had wall motion abnormalities noted at the time of presentation, with 78% and 73% of participants having apical and anterior wall motion abnormalities, respectively. The majority of the patients presented with ST-elevation MI (81%) and another 7% had elevated troponin with a new or presumed new left bundle branch block. Nearly 20% had cardiac arrest or ventricular fibrillation (VF) arrest at the time of presentation for their MI and an additional 7% had sustained ventricular tachycardia or ventricular tachycardia requiring cardioversion. PCI was performed in 84%, 13% of whom were first treated with lytic therapy. Forty percent of the patients required ventilator support and/or circulatory support with an intra-aortic balloon pump.
TABLE 2.
Clinical Characteristics at Time of Index MI
| Characteristics at Presentation |
Total (n = 231) |
≤35 (n = 100) |
90-day EF 36–49 (n = 72) |
≥50 (n = 59) |
p Value |
|---|---|---|---|---|---|
| EF at index MI | 28.1 ± 6.6 | 25 ± 6.9 | 30 ± 5.6 | 31 ± 5.0 | < 0.0001 |
| EF ≤25 % | 88 | 60 (68.2) | 17 (19.3) | 11 (12.5) | < 0.0001 |
| EF 26%–30 % | 64 | 24 (37.5) | 24 (37.5) | 16 (25.0) | |
| EF >30 % | 79 | 16 (20.3) | 31 (39.2) | 32 (40.5) | |
| Mitral Regurgitation |
0.2124 | ||||
| None-Mild | 176 | 76 (43.2) | 53 (30.1) | 47 (26.7) | |
| Mild-Moderate | 25 | 10 (40.0) | 10 (20.0) | 5 (20.0) | |
| Moderate- Severe | 6 | 5 (83.3) | 1 (16.7) | 0 | |
| WMA | |||||
| Any | 204 | 89 (43.6) | 64 (31.4) | 51 (25.0) | 0.6727 |
| Anterior | 177 | 80 (45.2) | 52 (29.4) | 45 (25.4) | 0.8124 |
| Inferior/posterior | 111 | 54 (48.7) | 33 (29.7) | 24 (21.7) | 0.1355 |
| Septal | 166 | 76 (45.8) | 50 (30.1) | 40 (24.1) | 0.3669 |
| Lateral | 93 | 49 (52.7) | 26 (28.0) | 18 (19.4) | 0.293 |
| Apical | 181 | 81 (44.8) | 53 (29.4) | 47 (26.0) | 0.8590 |
| STEMI | 186 | 79 (42.5) | 62 (33.3) | 45 (24.2) | 0.6883 |
| New LBBB | 18 | 10 (55.6) | 5 (27.8) | 3 (16.7) | 0.2745 |
| New Q waves | 59 | 26 (44.1) | 19 (32.2) | 14 (23.7) | 0.7498 |
| AF on presentation | 27 | 15 (55.6) | 7 (25.9) | 5 (18.5) | 0.2306 |
| Acute HF | 35 | 14 (40.0) | 11 (31.4) | 10 (28.6) | 0.6158 |
| Cardiogenic shock | 40 | 20 (50.0) | 12 (30.0) | 8 (20.0) | 0.3030 |
| Revascularization | |||||
| None | 37 | 16 (43.2) | 14 (37.8) | 7 (18.9) | 0.4302 |
| Lytics | 1 | 1 (100) | 0 | 0 | |
| PCI | 166 | 72 (43.3) | 48 (29.1) | 46 (27.7) | |
| Both | 27 | 11 (40.7) | 10 (37.0) | 6 (22.2) | |
| Intubation | 34 | 16 (47.1) | 7 (20.6) | 11 (32.4) | 0.6679 |
| Balloon pump | 53 | 28 (52.8) | 14 (26.4) | 11 (20.8) | 0.1853 |
| Arrest or VF | 43 | 16 (37.2) | 13 (30.2) | 14 (32.6)** | 0.2289 |
| VT | 18 | 10 (55.6) | 5 (27.8) | 3 (16.7) | 0.2745 |
| LOS, days | 6.1 ± 4.6 | 7.1 ± 5.4 | 5.5 ± 3.8 * | 5.3 ± 3.5 | 0.0089 |
| Prolonged HS (>4 days) |
118 | 62 (52.5) | 31 (26.3) | 25 (21.2) | 0.0163 |
| Laboratory analysis |
|||||
| Maximum Cr, mg/dl |
1.2 ± 0.5 | 1.2 ± 0.5 | 1.2 ± 0.4 | 1.2 ± 0.7 | 0.4409 |
| Baseline glucose, mg/dl |
207 ± 467 | 158 ± 203 | 324 ± 782 | 145 ± 118 | 0.4094 |
| Fasting glucose, mg/dl |
185 ± 373 | 152 ± 234 | 265 ± 584 | 140 ± 140 | 0.2015 |
| LDL-C, mg/dl | 109 ± 40 | 114 ± 40.4 | 102 ± 41 | 107 ± 39 | 0.3159 |
| HDL-C, mg/dl | 39.7 ±12.2 | 40 ± 12 | 39 ± 14 | 40 ±11 | 0.9987 |
| Triglyceride, mg/dl |
129 ± 64 | 132 ±75 | 120 ± 48 | 135 ± 65 | 0.5728 |
| Troponin max, x ULN |
1,592 ± 3,180 |
2,347 ± 4,408 |
1,238 ± 1,560 |
743 ± 1,455 ** |
0.0067 |
| BNP max, pg/ml | 1,054 ± 1,735 |
987 ± 1,506 |
936 ± 1,208 |
1,381 ± 2,681 |
0.1363 |
| Medications on discharge |
|||||
| ACEI or ARB | 175 | 81 (46.3) | 55 (31.4) | 39 (22.3)** | 0.0349 |
| Aspirin | 202 | 89 (44.1) | 62 (30.7) | 51 (25.2) | 0.6308 |
| Beta-blocker | 207 | 94 (45.4) | 65 (31.4) | 48 (23.2) | 0.0127 |
| Statin | 196 | 86 (43.9) | 57 (29.1) | 53 (27.0) | 0.4816 |
| Furosemide | 75 | 38 (50.7) | 24 (32.0) | 13 (17.3) | 0.0372 |
| Spironolactone | 57 | 34 (59.6) | 17 (29.8)* | 6 (10.5) ** | 0.0008 |
| Any diuretic | 110 | 57 (51.8) | 36 (32.7) | 17 (15.5)** | 0.0006 |
Values are mean ± SD or n (%).
p < 0.05 for EF <35% vs. EF ≥ 35%
p < 0.05 for EF<50% vs. ≥50%
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BNP = B-type natriuretic peptide; Cr = creatinine; HDL-C = high-density lipoprotein cholesterol; HS = hospital stay; LBBB = left bundle branch block; LDL-C = low-density lipoprotein cholesterol; LOS = length of stay; STEMI = ST-elevation myocardial infarction; ULN = fold increase above upper limit of normal; VF = ventricular fibrillation; VT = ventricular tachycardia; WMA = wall motion abnormalities; other abbreviations as in Table 1.
Follow-Up Characteristics
The mean time from discharge to followup echocardiogram was 81.3 ± 32.9 days. The mean EF increased by 12.2 ± 11.9% to a mean of 40.2 ± 11.5% at follow-up. Of the participants, 57% had an EF of greater than 35% and 26% had EF recovery to 50% or greater (Table 2). Only 18.6% had a worse EF at follow-up than at baseline. Univariate analysis demonstrated the following predictors of persistent severe systolic dysfunction: lower baseline EF, elevated baseline (nonfasting) glucose levels, prolonged hospital stay, a prior history of MI, troponin elevation, and a lateral wall motion abnormality (Online Table 1). A history of congestive HF was also associated with persistent EF <35% (Table 2). Analysis of the interaction between multiple wall motion abnormalities demonstrated that there were small multiplicative interactions between anterior or septal and apical wall motion abnormalities. No interaction was found between anterior, septal, or apical and lateral or inferior wall motion abnormalities.
In univariate analysis, EF at the time of MI was directly correlated with EF recovery to ≥50% (Online Table 2). Males had a lower chance of EF recovery to ≥50% (odds ratio [OR]: 0.37; p = 0.006). Notably, those who had VF or cardiac arrest at the time of presentation had higher odds of EF recovery to ≥50% (OR: 2.41; p = 0.03). Increasing level of peak troponin was associated with lower odds of EF recovery to ≥50%. A history of CABG or MI had a negative association with the recovery of EF to ≥50% (Table 1).
Most patients were discharged on guideline-directed medical therapy specific for post-MI (Table 3). Receipt of either beta-blockers or spironolactone was associated with persistent LV dysfunction (p = 0.013 and p ≤ 0.001, respectively). Receipt of a prescription of either furosemide or ACEI or ARBs was associated with a trend toward less EF recovery. Consistent with the concern that confounding by indication explained this apparent association; length of hospital stay was significantly associated with receipt of ACEI, ARBs, diuretics, beta-blockers, and aldosterone inhibitors, and the receipt of diuretics was significantly associated with acute HF on presentation, history of HF, or lower EF at the time of presentation (Online Table 3).
Table 3.
Clinical Characteristics at Time of Index MI: Derivation and Validation Cohorts
| Characteristics at Presentation |
PREDICTS (Derivation) |
VEST Registry Overall Cohort |
VEST Registry without 90- day Echocardiogra m |
VEST Registry with 90-day Echocardiograpm (Validation) |
p Value* |
|---|---|---|---|---|---|
| (n = 231) | (n = 509) | (n = 273) | (n = 236) | ||
| EF at index MI | 28.1 ± 6.6 | 28.2 ± 5.9 | 28.2 ± 6.0 | 28.2 ± 5.7 | (0.54,0.87) |
| EF <25 % | 88 (38.1) | 188 (36.9) | 95 (34.8) | 93 (39.4) | |
| EF 26%–30 % | 64 (27.7) | 171 (33.6) | 98 (35.9) | 73 (30.9) | |
| EF >30 % | 79 (34.2) | 150 (29.5) | 80 (29.3) | 70 (29.7) | |
| Mitral regurgitation | (0.94,0.85) | ||||
| None-Mild | 176 (76.2) | 398 (84.1) | 212 (84.1) | 186 (84.2) | |
| Mild-Moderate | 25 (10.8) | 60 (12.7) | 31 (12.3) | 29 (13.1) | |
| Moderate- Severe | 6 (2.6) | 15 (3.2) | 9 (3.6) | 6 (2.7) | |
| WMA | |||||
| Any | 204 (88.3) | 395 (90.4) | 198 (88.0) | 197 (92.9) | (0.20,0.08) |
| Anterior | 177 (76.6) | 341 (74.0) | 166 (69.2) | 175 (79.2) | (0.60,0.01) |
| Inferior/Posterior | 111 (48.1) | 247 (53.6) | 125 (52.1) | 122 (55.2) | (0.37,0.50) |
| Septal | 166 (71.9) | 318 (69.0) | 161 (67.1) | 157 (71.0) | (0.23,0.36) |
| Lateral | 93 (40.3) | 205 (44.5) | 103 (42.9) | 102 (46.2) | (0.46,0.49) |
| Apical | 181 (78.4) | 338 (73.3) | 173 (72.1) | 165 (74.7) | (0.03,0.53) |
| STEMI | 186 (80.5) | 357 (70.3) | 188 (68.9) | 169 (71.9) | (0.03,0.45) |
| New LBBB | 18 (7.8) | 30 (5.9) | 17 (6.3) | 13 (5.5) | (0.32,0.72) |
| New Q waves | 59 (25.5) | 108 (21.3) | 57 (21.0) | 51 (21.6) | (0.32,0.86) |
| AF on presentation | 27 (11.7) | 53 (10.5) | 28 (10.3) | 25 (10.6) | (0.71,0.92) |
| Acute HF | 35 (15.2) | 69 (13.6) | 36 (13.3) | 33 (14.0) | (0.72,0.82) |
| Cardiogenic shock | 40 (17.3) | 62 (12.2) | 25 (9.2) | 37 (15.7) | (0.63,0.03) |
| Revascularization | (0.57,0.06) | ||||
| None | 37 (16.0) | 98 (19.3) | 52 (19.2) | 46 (19.5) | |
| Lytics | 1 (0.4) | 7 (1.4) | 7 (2.6) | 0 (0.0) | |
| PCI | 166 (71.9) | 357 (70.4) | 192 (70.9) | 165 (69.9) | |
| Both | 27 (11.7) | 45 (8.9) | 20 (7.4) | 25 (10.6) | |
| Intubation | 34 (14.7) | 54 (10.7) | 28 (10.3) | 26 (11.0) | (0.23,0.08) |
| Balloon pump | 53 (22.9) | 73 (14.4) | 36 (13.3) | 37 (15.7) | (0.05,0.44) |
| Arrest or VF | 43 (18.6) | 51 (10.1) | 20 (7.4) | 31 (13.1) | (0.11,0.03) |
| VT | 18 (7.8) | 27 (5.3) | 12 (4.4) | 15 (6.4) | (0.55,0.33) |
| LOS, days | 6.1 ± 4.6 | 6.2 ± 4.9) | 6.3 ± 5.1 | 6.2 ± 4.7 | (0.84,0.88) |
| Prolonged HS (>4 days) | 119 (51.5) | 268 (52.9) | 146 (53.9) | 122 (51.7) | (0.89,0.62) |
| Laboratory analysis | 34 (14.7) | 54 (10.7) | 28 (10.3) | 26 (11.0) | (0.23,0.08) |
| Maximum Cr, mg/dl | 1.2 ± 0.5 | 1.3 ± 0.6 | 1.3 ± 0.7 | 1.2 ± 0.76 | (0.90,0.42) |
| Baseline glucose, mg/dl | 207 ± 466 | 170 ± 311 | 174 ± 388 | 164 ± 194 | (0.24,0.81) |
| Fasting glucose, mg/dl | 185 ± 373 | 165 ± 341 | 177 ± 452 | 152 ± 147 | (0.05,0.37) |
| LDL-C, mg/dl | 109 ± 40 | 95 ± 44 | 92 ± 45 | 98 ± 42 | (0.04,0.20) |
| HDL-C, mg/dl | 39.7 ± 12.2 | 42 ± 17 | 42 ±19 | 42 ± 16 | (0.13,0.75) |
| Triglyceride, mg/dl | 129 ± 64 | 128 ± 98 | 125 ± 84 | 132 ± 111 | (0.21,0.99) |
| Troponin max, x ULN | 1,592 ± 3,180 | 1,069 ± 3,124 | 1,061 ± 2,655 | 1,076 ± 3,583 | (0.01,0.73) |
| BNP max, pg/ml | 1,054 ± 1735 | 3,180 ± 5,812 | 3,231 ± 5,609 | 3,119 ± 6,073 | (0.05,0.48) |
| Medications on discharge | |||||
| ACEI or ARB | 175 (75.8) | 383 (75.3) | 206 (75.5) | 177 (75.0) | (0.89,0.91) |
| Aspirin | 202 (87.4) | 447 (87.8) | 233 (85.4) | 214 (90.7) | (0.26,0.07) |
| Beta-blocker | 207 (89.6) | 441 (86.6) | 231 (84.6) | 210 (89.0) | (0.83,0.15) |
| Statin | 196 (84.8) | 435 (85.5) | 239 (87.6) | 196 (83.1) | (0.60,0.15) |
| Furosemide | 75 (32.5) | 146 (28.7) | 77 (28.2) | 69 (29.2) | (0.62,0.80) |
| Spironolactone | 57 (24.7) | 129 (25.3) | 74 (27.1) | 55 (23.3) | (0.73,0.33) |
| Any diuretic | 110 (47.6) | 231 (45.4) | 124 (45.4) | 107 (45.3) | (--,0.99) |
Values are mean ± SD or n (%).
The first p value is shows denotes the significance of the difference between the Predicts Derivation cohort and those in the VEST Registry without 90 day echocardiograms, the second p value demonstrates the significance of the difference between the cohort in the VEST registry that had a 90 day echocardiograms and those without a 90-day echo cardiograms.
Predictors of Systolic Recovery
The model with the highest discrimination and calibration for EF recovery to >35% included EF at the time of MI, prolonged hospital stay, history of MI, lateral wall motion abnormalities, and elevated troponin level. The overall c-statistic for this model was 0.72, increasing to 0.75 after transformation to a point score scale. The calibration of the model, as estimated by the Hosmer-Lemeshow goodness of fit, was 0.34 and improved to 0.99 after transformation into point score scale.
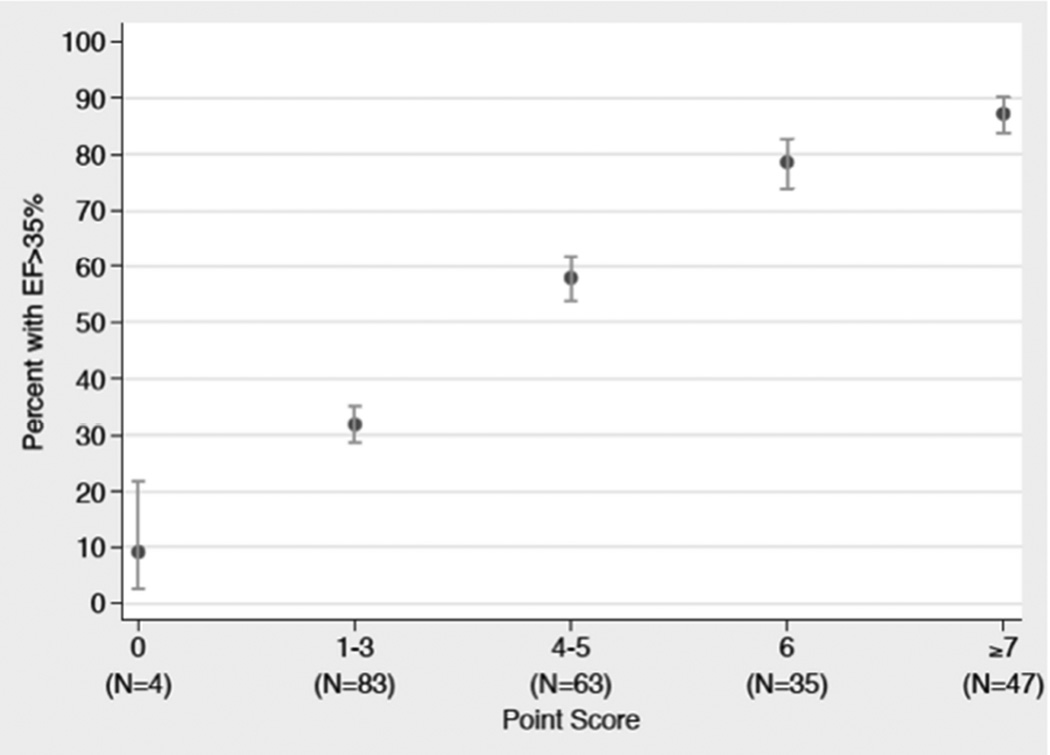
Ejection fraction on admission showed a strong and independent association with recovery to EF >35%. Compared to those with an admission EF of ≤25%, participants with EF of 26% to 30% and EF of 31% to 35% had increased chance of recovery to EF >35% (OR: 2.77; 95% confidence interval [CI]: 1.34 to 5.70; p < 0.01; and OR: 6.88; 95% CI: 3.26 to 14.5; p < 0.01, respectively). Predictors of hospital discharge within 4 days – lack of lateral wall motion on echocardiogram and no prior history of MI – all had a trend towards a higher odds ratio of EF recovery to >35% (OR: 1.58; 95% CI; 0.86 to2.89; p = 0.14; OR: 1.46; 95% CI: 0.79 to 2.72; p = 0.23; and OR: 1.52; 95% CI: 0.78 to 2.96; p = 0.22, respectively). A troponin peak of ≤50- and 51- to 500-fold above the upper limit of normal (ULN) had a trend towards higher odds of EF recovery to EF >35% compared to maximum troponin level >500-fold above the ULN (OR: 1.74; 95% CI: 0.82 to 3.69; p = 0.15; and OR: 1.81; 95% CI: 0.91 to 3.62; p = 0.09, respectively) (Online Table 1, top 5 models). After transforming model predictors into point scores, predictor profiles with the lowest score of 0 had a 9% (95% CI: 2.5% to 21.7%) probability of EF recovery to >35%, whereas predictor profiles with a score of 7 had an 87% (95% CI: 83.8% to 90.1%) probability of EF recovery to >35% (Table 4 and Figure 1; Online Table 4 for probability table).
Table 4.
EF Recovery to >35% 90 Days After MI
| Characteristic | Points | |
|---|---|---|
| EF at MI presentation | 31%–35 % |
4 |
| 26%– 30% |
2 | |
| Length of stay | ≤4 days | 1 |
| No history of MI | 1 | |
| No lateral WMA | 1 | |
| Troponin max fold increase |
< 500 | 1 |
| Total Possible Points | 8 | |
Figure 1. Frequency of EF Recovery ≥35%.
The observed left ventricular functional recovery to an ejection fraction (EF) >35% 90 days after the index myocardial infarction improved as point score increased (n = number of participants in the derivation set).
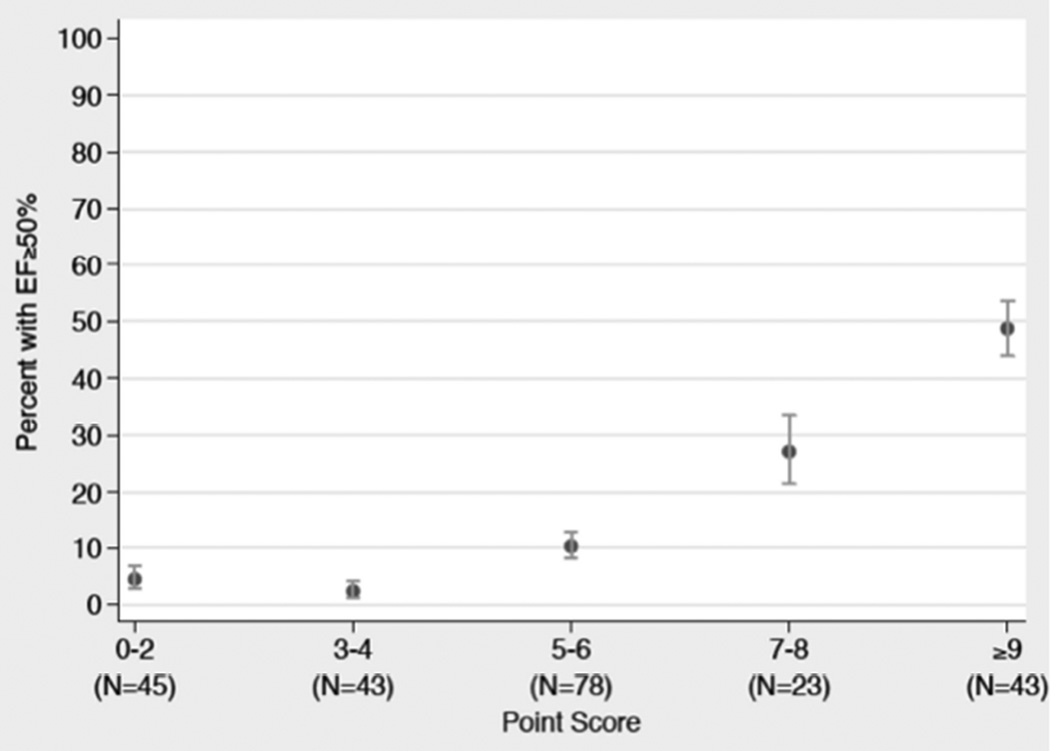
For EF recovery to ≥50%, the model with the highest discrimination and calibration included EF at the time of MI, history of MI, troponin elevation, and VF and/or cardiac arrest at presentation. The overall c-statistic for this model was 0.79 with a calibration of 0.34, as estimated by the Hosmer-Lemeshow goodness of fit calibration statistic. Neither the discrimination nor calibration changed after transformation into a point score scale. Ejection fraction on admission, VF or cardiac arrest on presentation, and troponin elevation all showed strong and independent associations with recovery to EF ≥50%. Compared to those with an admission EF of ≤25%, participants with EF of 26% to 30% or EF of 31% to 35% had an increased chance of recovery to EF ≥50% (OR: 3.08; 95% CI: 0.93 to 10.24; p = 0.07; and OR: 7.61; 95% CI: 2.48 to 23.33; p < 0.01, respectively). VF or cardiac arrest on presentation was associated with 5.53-fold higher odds of EF recovery to EF ≥50% (95% CI: 2.04 to 14.99; p < 0.01). A troponin peak of ≤50- or 51- to 500-fold above the ULN increased odds of EF recovery to ≥50% (OR: 12.02; 95% CI: 3.53 to 40.9; p < 0.01; and OR: 9.02; 95% CI: 2.82 to 28.83; p < 0.01, respectively) compared to a troponin peak of >500-fold above the ULN. The lack of prior history of MI approached significance for predicting EF recovery to ≥50% (odds ratio of 2.40; 95% CI: 0.85 to 6.78; p = 0.10) (Online Appendix, top 5 models). After transforming model predictors into point scores, predictor profiles with the lowest score of 0 to 2 had a 4% (95% CI: 3% to 7%) probability of EF recovery to ≥50%, whereas predictor profiles with a score of 9 to 11 had a 49% (95% CI: 44 to 54%) probability of EF recovery to ≥50% (Table 5 and Figure 2; Online Table 3 for probability table).
Table 5.
EF Recovery to ≥50% 90 Days After MI
| Characteristic | Points | |
|---|---|---|
| EF at MI presentation | 31%–35% | 4 |
| 26%–30 % | 1 | |
| No history of MI | 1 | |
| Troponin max fold increase | < 500 | 4 |
| Present with VF or arrest | 3 | |
| Total Possible Points | 11 | |
Figure 2. Frequency of EF Recovery ≥50%.
The observed left ventricular functional recovery to an EF ≥50% 90 days after the index myocardial infarction also improved as point score rose (n = number of participants in the derivation set). Abbreviations as in Figure 1.
Validation in the vest registry cohort
Characteristics of VEST registry participants, as well as differences between VEST registry and PREDICTs participants, are depicted in Tables 3 and 6. Echocardiograms were performed 20 days later in VEST registry compared to the PREDICTS patients (101 ± 36.9 vs. 81.3 ± 32.9 days; p < 0.01). VEST registry participants had a lower mean EF on follow-up (37.2% vs. 40.2%), and more patients in the VEST registry had a decrease in EF at follow-up (30.1% vs. 18.6%). VEST registry participants were less likely to have a prior history of PCI (22.9 vs. 36.1%; p = 0.02) and there was a trend toward lower prevalence of prior MI, HF, or prior CABG. Registry participants were less likely to have apical wall motion abnormalities (74.7 vs. 78.4%; p = 0.03). VEST registry participants also had higher B-type natriuretic peptide (BNP) values (3,231 vs. 1,054; p = 0.05), but lower peak troponin (1,061- vs. 1,592-fold increase above the ULN; p < 0.01) and lower low-density lipoprotein cholesterol on presentation (98 mg/dl vs. 109 mg/dl; p = 0.04).
Table 6.
Baseline Characteristics at Time of Index MI: Derivation and Validation Cohorts
| Characteristics at Presentation |
PREDICTS (Derivation) |
VEST Registry Overall Cohort |
VEST Registry without 90-day Echocardiogram |
VEST Registry with 90-day Echocardiogram (Validation) |
p Value* |
|---|---|---|---|---|---|
| (n = 231) | (n = 509) | (n = 273) | (n = 236) | ||
| Age, yrs | 60.4 ± 11.8 | 61 ± 11.7 | 61.7 ±11.9 | 60.4 ±11.5 | (0.81, 0.32) |
| Male | 165 (71.4) | 385 (75.8) | 213 (78.3) | 172 (72.9) | (0.73,0.15) |
| BMI, kg/m2 | 28.6 ± 4.9 | 28.2± 5.8 | 28.0 ± 6.2 | 28.5 ± 5.4 | (0.63, 0.24) |
| Diabetes mellitus | 80 (34.6) | 159 (31.2) | 87 (31.8) | 72 (30.5) | (0.34,0.74) |
| History of congestive HF |
31 (13.4) | 77 (15.1) | 50 (18.3) | 27 (11.4) | (0.52,0.03) |
| History of MI | 66 (28.6) | 129 (25.3) | 76 (27.8) | 53 (22.5) | (0.13,0.16) |
| History of PCI | 84 (36.4) | 128(25.2) | 74 (27.1) | 54 (22.9) | (0.02,0.27) |
| History of CABG | 26 (11.3) | 48 (9.4) | 30 (11.0) | 18 (7.6) | (0.18,0.20) |
| History of CVA | 12 (5.2) | 46 (9.0) | 29 (10.6) | 17 (7.2) | (0.37,0.18) |
| History of AF | 21 (9.1) | 45 (8.8) | 27 (9.9) | 18 (7.6) | (0.57,0.37) |
| History of ASA | 90 (39.0) | 210 (42.7) | 111 (42.4) | 99 (43.0) | (0.25,0.88) |
| Tobacco use | (0.10,0.89) | ||||
| Never | 86 (37.3) | 140 (27.5) | 73 (26.7) | 67 (28.4) | |
| Former | 80 (34.6) | 190 (37.4) | 103 (37.9) | 87 (36.9) | |
| Current | 65 (28.1) | 179 (35.2) | 97 (35.7) | 82 (34.8) | |
Values are mean ± SD or n (%).
The first p value is shows denotes the significance of the difference between the Predicts Derivation cohort and those in the VEST Registry without 90 day echocardiograms, the second p value demonstrates the significance of the difference between the cohort in the VEST registry that had a 90-day echocardiograms and those without a 90 day echocardiograms
Abbreviations as in Table 1.
When applied to the VEST registry patients, the prediction models remained significantly predictive, though they performed less well. The c-statistic for the model that predicts partial recovery to EF of ≥35% was 0.66 with a Hosmer-Lemeshow goodness of fit p value of 0.25. The model predicting EF recovery to ≥50% remained robust with a c-statistic of 0.72 with excellent calibration (Hosmer-Lemeshow goodness of fit p value of 0.85).
Discussion
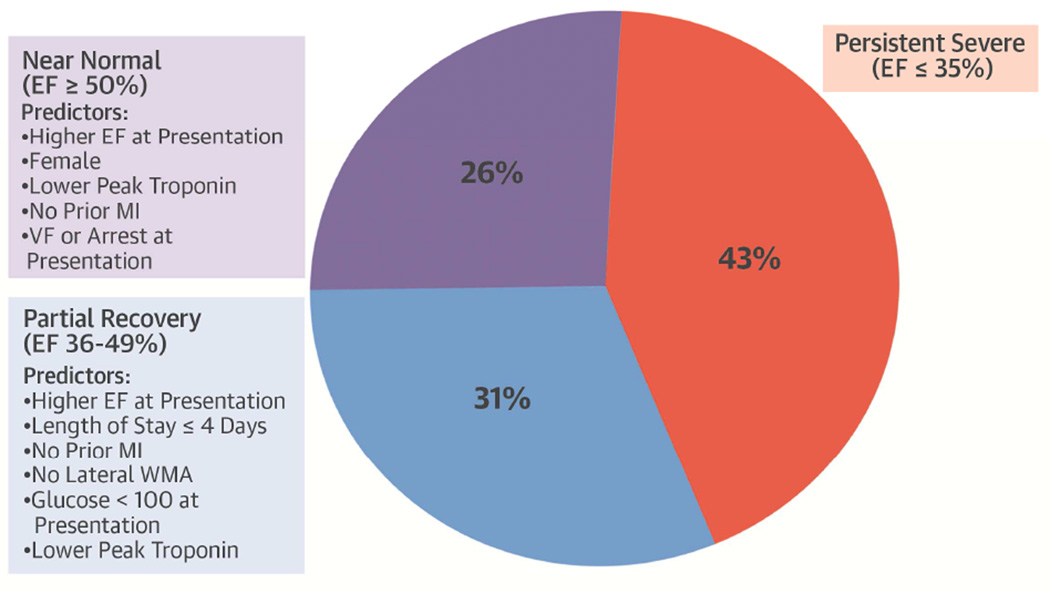
The incidence of EF recovery in patients presenting with severe LV dysfunction at the time of acute MI has not been well described. In this study of patients with EF ≤35% at the time of MI, 57% of patients had recovered to an EF >35% by 90 days, and 26% had an EF that returned to normal or near normal (≥50%). Systolic function at the time of MI was an independent predictor of EF recovery to >35%. A history of MI, prolonged hospital stay, serum troponin level, and presence of lateral wall motion abnormalities demonstrated large associations with EF recovery to >35% that approached statistical significance (Central Illustration). A model incorporating these variables had fair discrimination and good calibration for predicting EF recovery to >35%.
Central Illustration. Left Ventricular Dysfunction after Acute MI: Ejection Fraction 90 Days Acute Myocardial with Severe Systolic Dysfunction (EF ≤35%).
Severe left ventricular (LV) systolic dysfunction after myocardial infarction (MI) is associated with increased mortality. To better determine which patients with an ejection fraction (EF) ≤35% at time of acute MI may be more likely to improve systolic function, models assessing variables that predict LV recovery to an EF >35% and ≥50% 90 days after the event were developed. Although more patients continue to experience severe dysfunction, several variables predict partial or near normal recovery in these patients. VF = ventricular fibrillation.
Independent predictors of EF recovery to ≥50% included systolic function at the time of MI, troponin elevation, and VF and/or cardiac arrest at presentation. A history of MI approached significance for EF recovery to ≥50%. A model incorporating these variables had good discrimination and good calibration for predicting EF recovery to ≥50%.
In a large study involving more than 10,000 registry participants with EF ≤35% at the time of MI, Pokorney et al. demonstrated that only 8% of patients received an ICD within 1 year. Those who received an ICD had 36% lower risk of death within 2 years of their MI compared to those who did not receive an ICD, after adjusting for age, sex, prior MI, prior stroke, and other covariates (9). A crucial limitation of this study was that measures of EF used to define ICD eligibility were available only at the time of hospitalization for MI. We found that 43% of our participants would continue to be eligible to receive an ICD at 90 days. An 8% ICD implantation rate for primary sudden cardiac death prevention, as was seen in the Pokorney study, suggested a marked underutilization of proven therapy and may explain the higher mortality rate in those without ICD implantation.
Risk scores derived from our prediction models demonstrated that those with the highest risk profile had a 9% and 4% probability of EF recovery to >35% and ≥50%, respectively, whereas those with the lowest risk profile had a 90% and 50% probability of EF recovery to >35% and ≥50%. Randomized studies have shown that alerts to physicians to consider ICDs in post-MI patients can increase appropriate primary prevention ICD implantation rates by 12-fold (10); however, these alerts are rarely used in practice. A risk score predicting those most likely to have a persistently low EF may focus attention on those at highest risk and frame the ICD discussion with the patient at the time of discharge to ensure follow-up. It is unlikely that this risk score will replace a follow-up echocardiogram; however, it is clear from previous studies that follow-up echocardiograms and ICD implantation are underused (9). Regardless of a patient’s risk of persistent severe LV dysfunction, we recommend following current guideline recommendations to delay ICD implantation until an EF of ≤35% is demonstrated 40-days post AMI (90 days if revascularization occurs).
Risk factors for persistent LV dysfunction identified in our study largely agree with various findings from prior work. Systolic function and troponin levels at the time of MI have been shown to have strong associations with subsequent functional recovery (4,6,11). Prior studies have reported an association between BNP elevation and adverse remodeling at 4 months (increase in LV end-diastolic volume by 20%), a finding not repeated in our study (12,13). In these prior studies, the mean EF at the time of MI was higher (55% to 46% vs. 28.8% in our study), and mean BNP was lower (195 ± 109 pg/ml and 137 ± 118 pg/ml vs. 1,054 ± 1,735 pg/ml here). These reports may describe a fundamentally different population of patients than our cohort.
In our study, VF or cardiac arrest at presentation predicted near normal functional recovery. This may appear paradoxical given the association of VF/arrest with higher levels of troponin in both the PREDICTS and VEST cohorts (p < 0.01 and p = 0.04, respectively). Patients who had experienced VF or cardiac arrest may have had myocardial stunning, leading to a low EF assessment at enrollment (though this does not explain higher troponin elevations in these participants). Alternatively, VF may be a marker for ischemia with spontaneous reperfusion (troponin release kinetics differ under conditions of spontaneous reperfusion, non-reperfusion, or when intervention is performed) (14). Animal models demonstrate that spontaneous VF is more likely in ischemia-reperfusion than under ischemia alone (15). One could also speculate that those who suffer VF with spontaneous reperfusion are more likely to survive long enough to present to the hospital compared to those who had VF with no reperfusion (making resuscitation less likely). Finally, it should be noted that VF occurred in 20% of the participants in our study, higher than the 11% incidence of VF at the time of MI reported in other studies (16). Our study specifically enrolled MI patients with EF ≤35%, in which one would expect a higher occurrence of VF.
Study Limitations
Strengths of this study included the prospective collection of a broad range of clinical data, the multicenter design, data collected soon after an acute MI, a validation cohort with identical inclusion criteria, and baseline data collection to the derivation cohort. An important weakness of the validation cohort (VEST Registry) was that follow-up echocardiograms were performed at the discretion of the clinician, rather than as part of a pre-defined study protocol (as was done in the derivation cohort, PREDICTS); this could be an important source of selection bias. There may be measured and unmeasured confounders that influenced the clinicians’ decision to order the follow-up echocardiogram. Likely confounders that were measured include significant lower peak troponin, less frequent PCI, higher levels of BNP, and fewer apical wall motion abnormalities in the registry. Because the inclusion and exclusion criteria are identical for PREDICTS and VEST Registry, the presence of significant differences in covariates may indicate informative censoring. Validation in an external cohort is needed to better estimate the models predictive capacity. Variables and alternative models identified during model selection may have significant predictive power in this and other cohorts.
An important covariate that was not available to us was time to revascularization. All sites in the study were major cardiovascular care centers with on-call interventionalists. In the era of reporting door-to-balloon time measures of quality, it can be assumed that most PCIs were performed within a few hours of presentation. We did not have information regarding LV function prior to the index MI, or the occurrence of staged revascularization after initial hospitalization. These variables, if known, could act as powerful predictors of left ventricular recovery.
Conclusions
Recovery of systolic function to an EF >35% occurs in the majority of patients who present with severe systolic dysfunction at the time of MI. Clinical variables at the time of acute myocardial infarction can predict the probability of EF recovery to greater than 35% as well as the probability of recovery to near normal systolic function.
Supplementary Material
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE
Most patients with severe left ventricular dysfunction in the acute phase of myocardial infarction exhibit improvement in LV function 90 days later. Prior MI, early ventricular fibrillation or cardiac arrest, peak serum troponin, and ejection fraction early after presentation are predictors of later myocardial recovery.
TRANSLATIONAL OUTLOOK
Prospective studies are needed to assess whether earlier implantation of automatic defibrillators in patients with a low likelihood of myocardial recovery improves survival post-MI.
Acknowledgments
Grants, Contracts and other Financial Support: *** NIH/NHLBI U01-HL089458 (Olgin); NIH/NHLBI U01-HL089145 (Pletcher); Medtronic ZOLL
Disclosures: Dr. Morin: Speaker's Bureau: Medtronic Inc; Biotronik, Zoll; Research Grant – Medtronic, Boston Scientific; Dr. Buxton: Research support, Medtronic, Biosense-Webster; Jeffrey Olgin: Grant: Zoll, Medtronic; Dr. Zweibel: Consultant: XXX; Speaker: XXXX
ABBREVIATIONS
- ACEI
angiotensin-converting enzyme inhibitor
- ARB
angiotensin receptor blocker
- CK
creatine kinase
- EF
ejection fraction
- ICD
implantable cardioverter-defibrillator
- VF
ventricular fibrillation
Appendix
PREDICTS and VEST Registry Site PI’s
Rajesh Malik, McLeod Health, Florence, SC
Daniel Morin, Ochsner Clinic, New Orleans, LA
Peem Lorvidhaya, Brown Medical School, Providence, RI
Steven Zweibel, Hartford Hospital, Hartford, CT
Gregory Jones, Wellmont Cardiovascular, Kingsport, TN
Samy Claude Elayi, University of Kentucky, Gill Heart Institute, Lexington, KY
Paul Mounsey, University of North Carolina, Chapel Hill, Chapel Hill, NC
Gopi Dandamudi, Geisinger Heart Institute, Danville, PA
Jay Gross, Montefiore Medical Center, Bronx, NY
Robert Phang, Albany Associates in Cardiology, Albany, NY
Lawrence Rosenthal, University of Massachusetts Hospital, Worcester, MA
Nicholas Skipitaris, Lenox Hill Hospital, New York, NY
Eric Rashba, Stony Brook, Stony Brook, NY
David Sandler, Oklahoma Heart Institute, Tulsa, OK
Soufian Almahameed, Carilion Clinic, Roanoke, VA
David Slotwiner, Long Island Jewish Hospital, New Hyde Park, NY
R. Lee Jobe, Wake Heart Research, Raleigh, NC
Lawrence Nair, Presbyterian Heart Group, Alberquerque, NM
Venkateshwar Gottipaty, Providence Cardiology, Columbia, SC
Jay Franklin, Baylor University Medical Center, Dallas, TX
Mauricio Arruda, University Hospital Case Medical Center, Cleveland, OH
Neal Kavesh, Watson Clinic, Lakeland, FL
Kara Quan, North Ohio Heart Center, Elyria, OH
Michael Stillabower, Christiana Care, Newark, DE
Aylmer Tang, Chambersburg Hospital, Chambersburg, PA
Tariq Salam, Cardiac Study Center, Tacoma, WA
Theofanie Mela , Massachusetts General Hospital, Boston, MA
Donald Hegland, Duke University, Durham, NC
William Miles, University of Florida, Gainesville, FL
Peter Lee, Dean Foundation, Madison, WI
Richard Wu, University of Texas Southwestern Medical Center, Dallas, TX
Michael Gen, Cardiovascular Consultants Heart Center, Fresno, CA
Emad Aziz, St. Luke’s-Roosevelt Hospital Center, New York, NY
Manoj Duggal, Advocate Christ Hospitial, Oak Lawn, IL
Gregory Marcus, U of California SF, San Francisco, CA
Duy Nguyen, University of Colorado Denver, Aurora, CO
Govindarajan Venkatesh, The Guthrie Clinic, Sayre, PA
Sandeep Joshi, St. Vincent Medical Group, Indianapolis, IN
Tony Simmons, Wake Forest University, Winston Salem, NC
Steven Compton, Alaska Heart Institute, Anchorage, Alaska
Ramakota Reddy, Sacred Heart Medical Center, Springfield, OR
Rajiv Chandra, Melbourne Cardiac Research Institute, Melbourne, FL
Paul Maccaro, Huntington Hospital, Huntington, NY
John Hayes, Marshfield Clinic, Marshfield, WI
Afzal ur Rehman, United Health Services, Johnson City, NY
Mina Chung, Cleveland Clinic, Cleveland, OH
Jonathan Weinstock, Tufts Medical Center, Boston, MA
Bruce Koplan, Brigham and Women’s Hospital, Boston, MA
Andrzej Przybylski, The Valley Hospital, Ridgewood, NJ
PREDICTS Steering Committee
Mark Hlatky, Stanford University, Stanford, CA
Derrick Exner, University of Calgary, Calgary, Alberta, Canada
Alfred Buxton, Beth Israel Deaconess Medical Center, Boston, MA
Daniel Morin, Ochsner Clinic, New Orleans, LA
Stephen Hulley, UCSF, San Francisco, CA
Steven Zweibel, Hartford Hospital, Hartford, CT
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. J Am Coll Cardiol. 2013;61:e6–e75. doi: 10.1016/j.jacc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Hohnloser SH, Kuck KH, Dorian P, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–2488. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 3.Steinbeck G, Andresen D, Seidl K, et al. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009;361:1427–1436. doi: 10.1056/NEJMoa0901889. [DOI] [PubMed] [Google Scholar]
- 4.Chia S, Senatore F, Raffel OC, et al. Utility of cardiac biomarkers in predicting infarct size, left ventricular function, and clinical outcome after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2008;1:415–423. doi: 10.1016/j.jcin.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Hackel DB, Reimer KA, Ideker RE, et al. Comparison of enzymatic and anatomic estimates of myocardial infarct size in man. Circulation. 1984;70:824–835. doi: 10.1161/01.cir.70.5.824. [DOI] [PubMed] [Google Scholar]
- 6.Solomon SD, Glynn RJ, Greaves S, et al. Recovery of ventricular function after myocardial infarction in the reperfusion era: the healing and early afterload reducing therapy study. Ann Intern Med. 2001;134:451–458. doi: 10.7326/0003-4819-134-6-200103200-00009. [DOI] [PubMed] [Google Scholar]
- 7.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Saczynski JS, Lessard D, Spencer FA, et al. Declining length of stay for patients hospitalized with AMI: impact on mortality and readmissions. Am J Med. 2010;123:1007–1015. doi: 10.1016/j.amjmed.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pokorney SD, Miller AL, Chen AY, et al. Implantable Cardioverter-Defibrillator Use Among Medicare Patients With Low Ejection Fraction After Acute Myocardial Infarction. JAMA. 2015;313:2433–2440. doi: 10.1001/jama.2015.6409. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Gholami P, Turakhia MP, et al. Clinical reminders to providers of patients with reduced left ventricular ejection fraction increase defibrillator referral: a randomized trial. Circ Heart Fail. 2014;7:140–145. doi: 10.1161/CIRCHEARTFAILURE.113.000753. [DOI] [PubMed] [Google Scholar]
- 11.Oh PC, Choi IS, Ahn T, et al. Predictors of recovery of left ventricular systolic dysfunction after acute myocardial infarction: from the Korean acute myocardial infarction registry and Korean myocardial infarction registry. Korean Circ J. 2013;43:527–533. doi: 10.4070/kcj.2013.43.8.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi H, Yoo BS, Doh JH, et al. The optimal time of B-type natriuretic peptide sampling associated with post-myocardial infarction remodelling after primary percutaneous coronary intervention. Cardiovasc J Afr. 2013;24:165–170. doi: 10.5830/CVJA-2013-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Alvarez A, Sitges M, Delgado V, et al. Relation of plasma brain natriuretic peptide levels on admission for ST-elevation myocardial infarction to left ventricular end-diastolic volume six months later measured by both echocardiography and cardiac magnetic resonance. Am J Cardiol. 2009;104:878–882. doi: 10.1016/j.amjcard.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 14.Katus HA, Remppis A, Scheffold T, et al. Intracellular compartmentation of cardiac troponin T and its release kinetics in patients with reperfused and nonreperfused myocardial infarction. Am J Cardiol. 1991;67:1360–1367. doi: 10.1016/0002-9149(91)90466-x. [DOI] [PubMed] [Google Scholar]
- 15.Qin H, Walcott GP, Killingsworth CR, et al. Impact of myocardial ischemia and reperfusion on ventricular defibrillation patterns, energy requirements, and detection of recovery. Circulation. 2002;105:2537–2542. doi: 10.1161/01.cir.0000016702.86180.f6. [DOI] [PubMed] [Google Scholar]
- 16.Jabbari R, Engstrom T, Glinge C, et al. Incidence and risk factors of ventricular fibrillation before primary angioplasty in patients with first ST-elevation myocardial infarction: a nationwide study in Denmark. J Am Heart Assoc. 2015;4:e001399. doi: 10.1161/JAHA.114.001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.