Abstract
Mesenchymal stromal cells (MSCs) have been the object of extensive research for decades, due to their intrinsic clinical value. Nonetheless, the unambiguous identification of a unique in vivo MSC progenitor is still lacking, and the hypothesis that these multipotent cells could possibly arise from different in vivo precursors has been gaining consensus in the last years. We identified a novel multipotent cell population in human adult bone marrow that we first named Mesodermal Progenitor Cells (MPCs) for the ability to differentiate toward the mesenchymal lineage, while still retaining angiogenic potential. Despite extensive characterization, MPCs positioning within the differentiation pathway and whether they can be ascribed as possible distinctive progenitor of the MSC lineage is still unclear. In this study, we describe the ex vivo isolation of one novel bone marrow subpopulation (Pop#8) with the ability to generate MPCs. Multicolor flow cytometry in combination with either fluorescence-activated cell sorting or magnetic-activated cell sorting were applied to characterize Pop#8 as CD64brightCD31brightCD14neg. We defined Pop#8 properties in culture, including the potential of Pop#8-derived MPCs to differentiate into MSCs. Gene expression data were suggestive of Pop#8 in vivo involvement in hematopoietic stem cell niche constitution/maintenance. Pop#8 resulted over three logs more frequent than other putative MSC progenitors, corroborating the idea that most of the controversies regarding culture-expanded MSCs could be the consequence of different culture conditions that select or promote particular subpopulations of precursors.
Introduction
Mesenchymal stromal cells (MSCs) have been the object of extensive research [1] for their intrinsic clinical value, due to multilineage differentiation capacity as well as involvement in hematopoiesis, immunoregulation, and growth factor/cytokine secretions [2–4]. A limitation is the very low number of cells in the tissue of origin that forced to use in vitro expansion protocols to achieve feasible amounts of cells for infusion or transplantation. However, there is increasing evidence that in vitro expansion induces drastic changes in phenotype and biological properties of MSCs, with significant possible implications for therapy [5–7]. Research aimed to shed light on MSC origin failed to identify an unambiguous unique in vivo progenitor, whereas the hypothesis that MSCs could possibly arise from different precursors is gaining consensus [8–11].
For a number of years our studies have focused on the optimization of MSC culture conditions suitable for clinical application. When fetal bovine serum (FBS) was replaced by autologous serum in cultures from human bone marrow (hBM), we noticed the emergence of a small population of cells with distinct morphology [12]. They presented rounded fried egg-like shape compared to the usual spindle-shaped morphology of MSCs, were highly refringent, showed firm plastic adherence after trypsin digestion, and retained angiogenic potential.
Notably, reverting to FBS-supplemented medium, MSC-like cells growing to confluence were obtained. We named this cell population mesodermal progenitor cells (MPCs) [12] for their in vitro characteristics of both mesenchymal and endothelial progenitor. Subsequently, we were able to define selective culture conditions, including commercial pooled human AB-type serum (PhABS) as supplement to generate MPCs at high grade of purity [13]. Our highly reproducible isolation protocol allowed the characterization of MPC morphological and biological properties.
MPCs showed to be nestin-positive, slow cycling, and Ki-67-negative, with chromosomes characterized by long telomeres. They expressed pluripotency-associated transcription factors Oct-4 and Nanog, at a difference with MSC master regulators Runx2 and Sox9 [14,15]. Phenotypically, MPCs expressed Endoglin (CD105) at a lower level than MSCs while lacking CD73, CD90, CD166, and the other markers typical of the mesenchymal phenotype [16]. They showed a different pattern of adhesion molecules with respect to standard cultured MSCs, being characterized by consistent expression of PECAM (CD31), integrins αL (CD11a), αM (CD11b), αX (CD11c), and particularly integrin β2 (CD18) that specifically sustain podosome-like structures.
MPCs rapidly differentiated into MSCs in standard commercial MSC expansion media, throughout an intermediate stage of differentiation activating Wnt5/Calmodulin cell signaling, replacing podosome-like structures, lowering adhesion on activated and nonactivated endothelium, and losing all angiogenic properties [17,18]. While the definition of specific MPC selective culture conditions allowed to definitively demonstrate the mesengenic and angiogenic potential of these cells, convincing data on MPC differentiation toward other mesodermal lineages are still lacking. Thus, we recently proposed a revision of the terminology, introducing a new definition of these cells as “Mesangiogenic Progenitor cells,” maintaining the acronym MPCs [19].
MPCs represent an attractive cell population with promising clinical applications. However, we believe that a detailed investigation about MPC origin in vivo is needed to identify putative precursors and to clarify MPC/MSC lineage relationship(s). In this study, we analyze the expression of MPC/MSC common antigen CD105 and differentially expressed antigen CD31 in ex vivo isolated hBM fractions. Integrating these results with multiparametric cell characterization, we managed to unambiguously describe a unique specific bone marrow subpopulation able to generate MPCs in selective culture conditions.
Materials and Methods
Immunomagnetic fractioning of hBM mononuclear cells
Donors and sample collection
The study has been performed according to the declaration of Helsinki and the local ethics committee of “Azienda Ospedaliero-Universitaria Pisana” approved the protocol for human bone marrow (hBM) blood sample collection. After written informed consent, hBM aspirates were obtained from 37 patients undergoing orthopedic surgery for hip replacement (13 M/14 F, median age 64). Briefly, a 20-mL syringe containing 500 I.U. of heparin was used to aspirate 10 mL of fresh bone marrow immediately after femoral neck osteotomy and before femoral reaming. Samples were processed soon after.
Isolation, fractioning, and plating of hBM mononuclear cells
Fresh bone marrow samples were diluted 1:4 in Dulbecco's modified phosphate-buffered saline (D-PBS; Life Technologies, Carlsbad, CA) and gently layered on Ficoll-Paque™ PREMIUM (GE Healthcare, Uppsala, Sweden). Samples were centrifuged at 400g for 25 min and hBM mononuclear cells (MNCs) were harvested at the interface, filtered on 50 μm filters, and washed twice in D-PBS. MSCA-1+ immunomagnetic depletion by the Anti-MSCA-1 (W8B2) MicroBeads Kit (human) was performed on autoMACS™ Pro Separator (Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer's instructions. MSCA-1neg fraction was then incubated with anti-CD105 MicroBeads (human) (Miltenyi Biotec) and processed for separation running sensitive positive selection program.
The three fractions: MSCA-1+, CD105+MSCA-1neg, and CD105negMSCA-1neg were cytofluorimetrically evaluated for the expression of CD105, MSCA-1, and CD90. Aliquots were cytocentrifuged on glass slides to perform May–Grünwald–Giemsa stain and cultural properties were assayed in Dulbecco's modified Eagle's medium (DMEM)/10%PhABS and MesenPRO® RS (Life Technologies). To create permissive attaching conditions, cells were plated in tissue culture (TC) treated 24-well plates instead of using hydrophobic plastics as reported [13]. After 7 and 14 days of culture, wells were microscopically scanned by three independent operators and scored as follows: 0, no attached cells; 1, fewer than 20 cells/field; 2, 21–100 cells/field; 3, over 100 cells/field; and 4, cells at confluence. Scores were attributed considering either the MPC- or MSC-like morphology.
The same protocol was applied to obtain CD31+ fraction after MSCA-1 depletion, and morphological/cultural properties were assayed as above. Three further samples from donors (1 M/2 F, median age 64) were fractionated first performing monocytic/macrophagic and granulocytic lineage-positive cell depletion. hBM-MNCs were incubated with FITC-conjugated anti-CD14 and anti-CD66abce. Unbounded antibodies were removed by cell washing and anti-FITC MicroBeads-conjugated (Miltenyi Biotec) antibodies applied for depletion on autoMACS Pro Separator, according to the manufacturer's instructions. CD14/CD66neg fraction was then incubated with anti-CD31 PE and, after cell washing, incubated with anti-PE MicroBeads-conjugated and processed for sensitive positive selection. The three fractions, CD14/CD66+, CD31+CD14negCD66neg, and CD31negCD14/CD66neg, were then processed as described above.
Multicolor flow cytometry analysis of hBM-MNCs
Donors
Freshly isolated hBM-MNCs from 20 donors (10 M/10 F, median age 65) were processed for seven-color flow cytometry analysis, incubating cells for 30 min at 4°C with a set of fluorochrome-conjugated antibodies. After cell washing with MACSQuant Running Buffer (Miltenyi Biotec), samples were acquired on MACSQuant Flow Cytometer (Miltenyi Biotec) and analyzed by MACSQuantify Analysis Software (Miltenyi Biotec). Unstained and FMO (fluorescence minus one) tests were also processed to set gates for the analysis.
Antibodies
Antibodies anti-CD31 PE-Cy7, anti-CD18 PE or APC, anti-CD51 FITC, anti-CD64 FITC, anti-CD14 FITC, PE or PerCP-Cy5.5, anti-CD66abce FITC, anti-CD34 VioBlue®, anti-CD38 FITC, anti-CD44 PE, anti-CD45 VioBlue® or APC-Vio770, anti-CD3 FITC or PerCP-Cy5.5, anti-CD20 FITC or PerCP-Cy5.5, anti-CD15 FITC, anti-CD16 PerCP-Cy5.5, anti-HLA-DR VioBlue®, anti-CD86 VioBlue®, anti-CD80 VioBlue®, anti-CD90 FITC and PerCP-Cy5.5, anti-CD133 APC, anti-CD138 PE, anti-CD140a PE, anti-CD146 FITC, and anti-MSCA-1 PE were from Miltenyi Biotec. Anti-STRO-1 AlexaFluor® 647, anti-CD73 PE-Cy7, anti-CD105 PerCP-Cy5.5, anti-CD140b PE, anti-CD56 APC, anti-CD309 PerCP-Cy5.5, anti-CD115, anti-CD144 PE, anti-SSEA-3 FITC, and anti-SSEA-4 FITC were from BioLegend (San Diego, CA). Anti-CD271 PE, anti-CD11b PE, anti-CD11c PE, and anti-CD36 APC were from BD Biosciences (San Jose, CA).
Fluorescence-activated cell sorting of hBM-MNCs
hBM-MNCs were stained with anti-CD18 PE, anti-CD31 PE-Cy7, and a cocktail of FITC-conjugated antibodies, including anti-CD3, anti-CD20, anti-CD14, and anti-CD66abce. After 30 min at 4°C cells were washed with prerefrigerated autoMACS Running Buffer and processed for cell sorting on S3™ Cell Sorter (Bio-Rad, Hercules, CA) equipped with ProSort® Software (Bio-Rad) applying “Purity” sort mode. Sorted cells were then cytocentrifuged on glass slides to perform May Grünwald–Giemsa and cytochemical staining or alternatively plated in DMEM/10%PhABS using TC 24-well plates. After 5 days of culture, cells were exposed to mesengenic and angiogenic differentiating conditions, tested for acetylated low-density lipoprotein (Ac-LDL) uptake and phagocytosis, or detached by TrypLE Select® (Life Technologies) digestion, and analyzed by flow cytometry, as described below.
Nestin detection was performed on sorted cells seeded in two-well Lab-Tek Permanox® Chamber Slides (Thermo Fisher Scientific, Waltham, MA). Fluorescence-activated cell sorting (FACS) was extended to hematopoietic lineage antigens using a cocktail of CD64-FITC, CD31-PE/Cy7, and CD14-PE antibodies. Within CD64brightCD31bright population, CD14neg and CD14+ cells were separated on S3 Cell Sorter and processed as described above.
Cell cultures
MPC selective cultures
Freshly sorted cells underwent MPC-selective culture in DMEM supplemented with 1% of GlutaMAX®, penicillin and streptomycin (Life Technologies), and 10% of PhABS of U.S. origin from Lonza (Walkersville, MD). Cultures were maintained at 37°C and 5% CO2 for 5–6 days. Cells were then harvested by TrypLE Select digestion and applied for cell characterization and differentiation potential evaluation, as described below.
Mesengenic differentiation
To induce differentiation into MSCs, freshly sorted cells and the MPCs derived from them were first cultured for 7 days in MesenPRO Reduced Serum (RS) Medium (Life Technologies), and then terminally differentiated into adipocytes and osteocytes using StemMACS® AdipoDiff and StemMACS® OsteoDiff Media from Miltenyi Biotec, respectively, for 21 days. Media were refreshed every 48 h. In parallel, cells were detached by TrypLE Select digestion and washed twice in MACSQuant Running Buffer (Miltenyi Biotec) for flow cytometry.
Angiogenic differentiation assessment of sprouting angiogenesis in three-dimensional culture
Three-dimensional (3D) spheroids were generated by the hanging drop method [20,21] with 1.5 × 105 of sorted cells or CD64brightCD31brightCD14neg-derived MPCs per drop and incubated overnight at 37°C in 5% CO2 for cell aggregation. Spheroids were then gently applied onto Matrigel® thick gel layer and cultured in EGM-2 medium. Sprouting was evaluated after 24 h and after 7 days by measuring the distance between last invading cell and the spheroid edge. Thereafter, Matrigel was melted incubating 3D cultures at 4°C for 2 h and diluted 10 mL of prerefrigerated D-PBS. Spheroids were harvested by centrifugation at 150g and enzymatically dissociated by adding TrypLE Select to obtain single cell suspensions, and standard capillary-like tube (CLT) formation assay was then performed on these cells [22].
Hematopoietic colony-forming assay
From 1.5 × 105 to 2.0 × 105 of freshly sorted CD64brightCD31brightCD14neg and CD64brightCD31brightCD14+ were seeded in 4 mL of StemMACS® HSC-CFU medium (Miltenyi Biotec). Using a sterile 1-mL syringe fitted with a 16-gauge blunt-end needle, the suspension was mixed and an aliquot of 2 mL was put into two 35-mm Petri dishes. Cultures were incubated at 37°C and 5% CO2 for 12 to 14 days.
Mononuclear phagocyte differentiation
Freshly sorted CD64brightCD31brightCD14neg cells were seeded 2 × 104 cells/cm2 in 24-well plates and cultured in DMEM/10%PhABS with or without 50 ng/mL of human recombinant granulocyte/macrophage colony-stimulating factor (hGM-CSF), for 5–6 days. At the end of the culture time, media were replaced by fresh DMEM/10%PhABS and 10 μL/mL of Indian ink were added. Cultures were incubated at 37°C and 5% CO2 for additional 4 h, extensively washed with fresh culture medium, and put back into the incubator. The day after, bright-field microscopic pictures were taken applying inverted DM IRB Leica microscope (Leica, Wetzlar, Germany), equipped with LAS image acquisition software (Leica).
Cell and culture characterization
Morphological and cytochemical staining
Morphological May Grünwald–Giemsa staining was performed on Wescor Aerospray® 7120 automatic slide stainer (ELITech Group, Puteaux, France). Cytochemical staining was carried out by α-naphthyl acetate esterase and the Tartrate-Resistant Acid Phosphate (TRAP) Detection Kit (Sigma-Aldrich, Saint Louis, MO), according to the manufacturer's instructions, and counterstained with Gill's Hematoxylin. Peroxidase activity was revealed by benzidine oxidation [23] and slides counterstained by Giemsa reagent diluted 1:10.
Flow cytometry characterization of cultured cell populations
Freshly isolated MPCs and differentiated MSCs were processed for flow cytometry analysis, incubating cells for 30 min at 4°C with a set of fluorochrome-conjugated antibodies, including: anti-CD31 PE-Cy7, anti-CD18 APC, anti-CD14 FITC, anti-CD66abce FITC, anti-CD34 VioBlue, anti-CD90 FITC, anti-CD146 FITC, and anti-MSCA-1 PE were from Miltenyi Biotec. Anti-STRO-1 AlexaFluor 647, anti-CD73 PE-Cy7, and anti-CD105 PerCP-Cy5.5 were from BioLegend. The anti-CD11c PE antibody was from BD Bioscience. After cell washing with MACSQuant Running Buffer (Miltenyi Biotec), samples were acquired on MACSQuant Flow Cytometer (Miltenyi Biotec) and analyzed by MACSQuantify Analysis Software (Miltenyi Biotec). Proper isotype controls (all from Miltenyi Biotec) were also employed to set the positive regions of the histograms.
Lipid droplet accumulation
Cells were cultured under adipogenic conditions, medium removed, and wells washed twice with prewarmed D-PBS. Cells were then incubated with 200 nM Nile Red (Life Technologies) for 10 min at 37°C in the dark.
Calcium deposits
Osteogenic induced cultures were processed by the OsteoImage™ Mineralization Assay Kit (Lonza), according to the manufacturer's instructions. Wells were washed twice with D-PBS and pictures taken using the inverted fluorescence DM IRB Leica microscope (Leica), equipped with LAS image acquisition software (Leica).
Nestin detection
Cells in chamber slides were fixed for 15 min in 4% paraformaldehyde solution and subsequently permeabilized by 0.05% Triton X-100 for 30 min. Immunofluorescence was carried out using mouse monoclonal antibodies against Nestin (Abcam, Cambridge, United Kingdom) and the Goat Anti-Mouse SFX Kit (Life Technologies), according to the manufacturer's instructions, using AlexaFluor® 488 anti-mouse IgG. Slides were then stained with Phalloidin AlexaFluor® 555-conjugated (Life Technologies) for 30 min to reveal F-actin organization. Nuclei were detected by ProLong® Gold Antifade Reagent with 4′,6-diamidino-2-phenylindole (DAPI; Life Technologies). Pictures were taken using standard fluorescence DM RB (Leica) and LAS AF image acquisition software (Leica).
Ac-LDL uptake
Cultures of MPCs from CD64brightCD31brightCD14neg population were incubated for 4 h at 37°C with 5 μg/mL AlexaFluor 488-conjugated Ac-LDL (Life Technologies) in DMEM/1% bovine serum albumin. Cells were then washed twice and pictures taken as described above using inverted fluorescence microscope.
Gene expression analysis
Samples of FACS sorted CD64brightCD31brightCD14neg and CD64brightCD31brightCD14+ cells from seven patients (3 M/4 F, median age 63), together with five samples of MPC cultured cells were processed for gene expression analysis of PECAM, NESTIN, SPP1, NANOG, and OCT-4A (NCBI Gene: NM_000442, NM_006617, NM_001040060, NM_024865, and NM_002701). Total RNA was extracted using the RNeasy Micro Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's instructions. One hundred nanograms of RNA samples were retrotranscribed applying the QuantiTect Reverse Transcription Kit (Qiagen GmbH) and 2 μL samples of 10-fold cDNA dilutions were amplified by quantitative real-time polymerase chain reaction, using iCycler-iQ5 Optical System (Bio-Rad) and SsoAdvanced SYBR Green SuperMix (Bio-Rad). Samples were run in duplicate.
All primer pairs (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd) were from Sigma-Aldrich. Relative quantitative analysis was performed following 2−ΔΔCt Livak method [24]. Normalization was performed by using GAPDH and ATP5B (NCBI Gene: NM_002046 and NM_001686) housekeeping genes. Statistical analysis was carried out by two-tailed t-test, and results were reported as mean ± standard deviation.
Results
MPC progenitors were found in a specific CD31+ fraction from hBM-MNCs
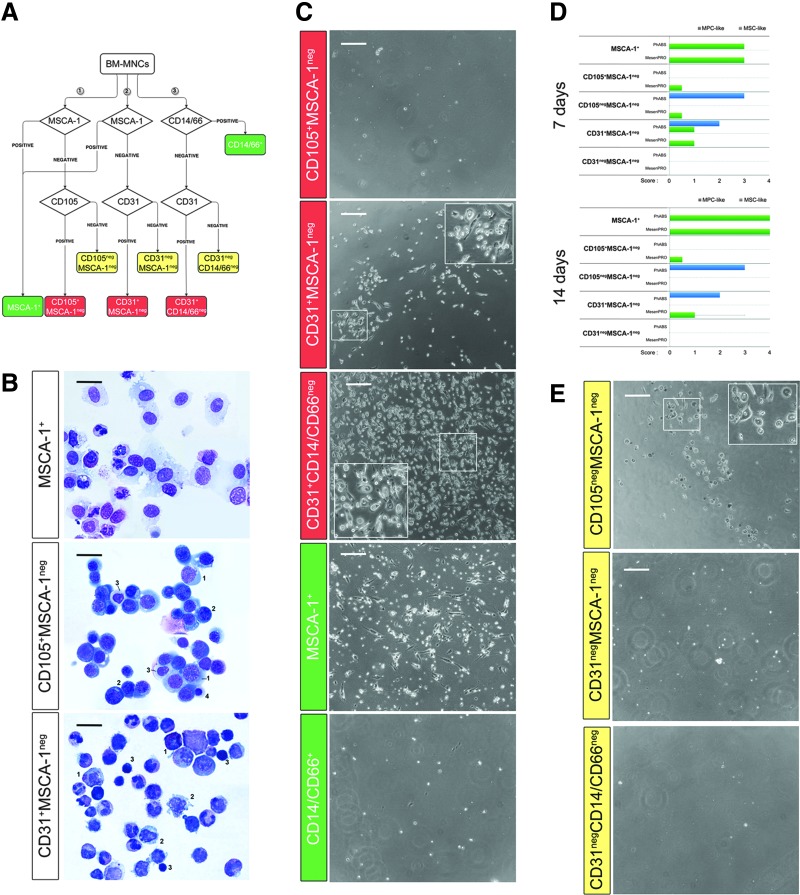
Two-step immunomagnetic hBM-MNC cell separation allowed us to obtain three different fractions expected to include MPC progenitors: CD105+MSCA-1neg, CD31+MSCA-1neg, and CD31+CD14/CD66neg (red boxed in Fig. 1A). Cell cultures were also set up from MSCA-1+ MSC progenitors as positive control, whereas CD14/66+ fraction was deemed as negative control (green boxed in Fig. 1A). Double negative cell fractions were also taken in consideration and assayed throughout the experiments (yellow boxed in Fig. 1A).
FIG. 1.
Morphological characterization of hBM-MNC immunomagnetic fractions. Two-step immunomagnetic hBM-MNC cell separation gave rise to six fractions, including CD105+MSCA-1neg, CD31+MSCA-1neg, CD31+CD14negCD66neg (red boxes in A), double negative fractions (yellow boxes in A), MSCA-1+-positive control, and CD14/66+-negative control (green boxes in A). May Grünwald–Giemsa staining of MSCA-1+-positive control revealed the cell stromal appearance. CD105+ fraction included erythroid elements as pronormoblasts (1), basophilic (2), polychromatophilic (3), and orthochromic (4) normoblasts. CD31+MSCA-1neg fraction appeared to be constituted by myeloid (1), monocytoid (2), and lymphoid (3) elements (B, black scale bar: 50 μm). Under MPCs/MSCs permissive culture conditions, at day 7 MPC-like cells were detected in CD31+ fractions, as well as in the CD105negMSCA-1neg (C–E, white scale bar: 200 μm). hBM-MNC, human bone marrow mononuclear cell; MPCs, mesodermal progenitor cells; MSCs, mesenchymal stromal cells.
MSCA-1+ fraction showed a percentage of CD105+MSCA-1+CD90bright cells of 56.6% ± 4.3% (n = 7). This population was characterized by very large cells with abundant faint cytoplasm, large regular elliptical nuclei showing finely dispersed chromatin with nucleoli occasionally evident. Significant leukocyte contamination was observed. Conversely, CD105+MSCA-1neg fraction was exclusively constituted by CD105+MSCA-1negCD90neg cells (97.5% ± 1.6%; n = 4) showing different morphologies very similar to the progenitors of the erythroid lineage, from pronormoblasts to orthochromic normoblasts. CD31+MSCA-1neg fraction (purity: 97.7% ± 2.7%, n = 4) featured a large variety of myeloid progenitors, elements of monocytic/macrophagic appearance, and cells from the lymphoid lineage (Fig. 1B). CD31+CD14/CD66neg cells (purity: 96.8% ± 3.4%, n = 4) resulted highly similar to the CD31+ cells obtained after MSCA-1 depletion, with a mild but evident increase of the lymphoid counterpart (data not shown).
CD105+MSCA-1neg cells cultured under MPC-selective conditions showed complete lack of adherence to plastic and were easily washed out at media change. Conversely, after 7 days under MPC-selective culture conditions, MSCA-1-depleted CD31+ cells showed strong adherence, with over 20 rounded refringent MPC-like cells/field detected (median score = 2, n = 3, Fig. 1C, blue bars in Fig. 1D) with score unvaried within 14 days of culture (Supplementary Fig. S1A). In DMEM/10%PhABS CD31+CD14/CD66neg fraction generated considerably more MPC-like cells as compared with CD31+MSCA-1neg fraction (median score = 4, n = 3). Among double negative populations, more than 100 MPC-like cells per microscopic fields were detected, after 7 days, only in the CD105negMSCA-1neg fraction cultured in DMEM/10%PhABS (median score = 3, n = 4, Fig. 1D, E). Cells maintained vitality at day 14 of culture without signs of proliferation (Supplementary Fig. S1A). MPC-like cells generated from both CD31+MSCA-1neg and CD105negMSCA-1neg were able to differentiate into MSC-like cells when DMEM/10%PhABS was replaced with MesenPRO RS and culture extended for further 14 days (Supplementary Fig. S1B).
After 7 days of culture of MSCA-1+ fraction in DMEM/10%PhABS, attached, flat multibranched cells were detected (Fig. 1C, D). These slightly proliferating cells were subconfluent at day 14, with evident signs of spontaneous mesengenic differentiation (Supplementary Fig. S1C). As expected, negative control population CD14/CD66+ was never able to generate adherent cultures (Fig. 1C).
When bone marrow-derived cell fractions were cultured directly in MesenPRO RS medium, confluent MSC-like spindle-shaped cells were obtained only from MSCA-1+ fraction (median score = 3, n = 7 after 7 days, median score = 4, n = 7 after 14 days; Fig. 1D and Supplementary Fig. S1D, E). Those cells were able to differentiate toward the mesengenic lineage after culturing in StemMACS OsteoDiff or StemMACS AdipoDiff medium (data not shown), confirming their MSC nature.
Few attached cells of undefined morphology were found when seeding CD105negMSCA-1neg (median score = 0.5, n = 4) and CD31+MSCA-1neg (median score = 1, n = 3) cells in MesenPRO RS medium (Fig. 1D). Those small condensed cells showed neither MPC-like nor MSC-like features and remained firmly attached without a sign of proliferation. Therefore, they were considered negative.
In conclusion, positive selection for markers CD105 and MSCA-1 did not allow isolation of bona fide MPC precursors that appear to reside specifically in CD31+CD14negCD66neg fraction.
A specific CD31+ subpopulation generated MPCs in culture
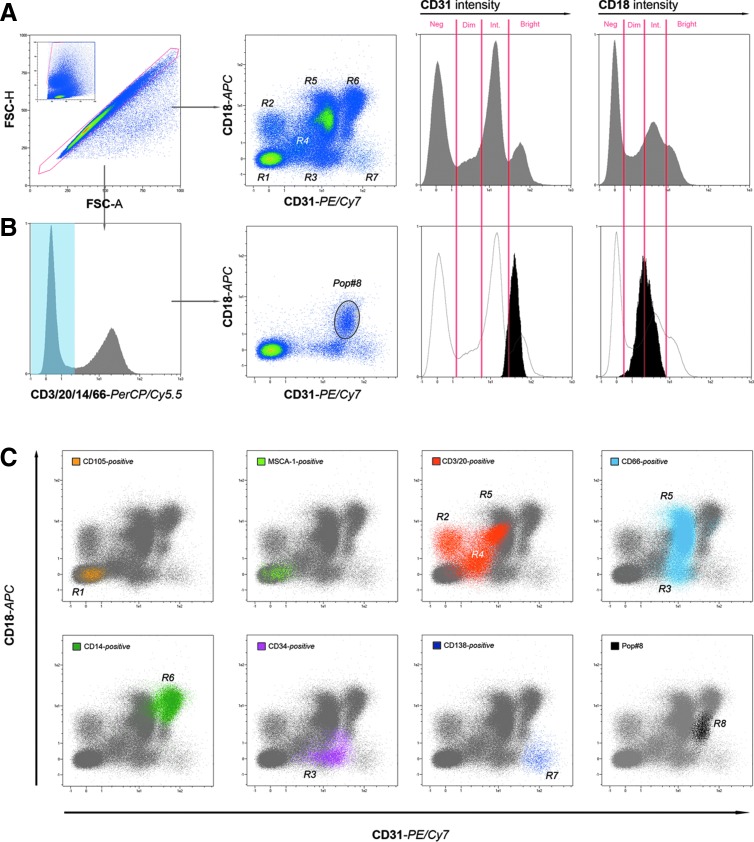
Immunomagnetic selection experiments indicated that CD31+ fraction was the candidate for further investigation. To analyze the large CD31+ bone marrow compartment seven-color flow cytometry was carried out using specific markers. In particular, Integrin-β2 (CD18) is known to be highly expressed on the surface of MPCs isolated in culture [17], although widely distributed within the hematopoietic compartment in vivo. CD31 and CD18 both results expressed nonhomogeneously, and we scored samples at four different levels: negative, dim, intermediate, and bright (Fig. 2A). CD18 versus CD31 plotting cytogram allowed the identification of eight distinct regions (R1–R8) of even distribution (Table 1 and Fig. 2A). Extending the analysis of CD18 versus CD31 plotting cytogram to specific bone marrow subpopulations (Table 2), we gated the experiments for the expression of lineage-specific markers (Supplementary Fig. S2A).
FIG. 2.
Pop#8 identification by lineage exclusion. Applying multicolor flow cytometry of freshly isolated hBM-MNCs, events generated by single cells were selected on the basis of scatter signals and plotted on CD18 versus CD31 density plot. Seven regions were identified (R1–R7) according to a combination of four levels of CD31/CD18 intensity: Neg (negative), Dim (low level of expression), Int (intermediate level of expression), and Bright (high level of expression) (A). Gating on lineage-negative cells (not expressing CD3, CD20, CD14, or CD66), CD18 versus CD31 density plot revealed in all samples a residual CD31brightCD18dim/int population that we defined as unknown population #8 (Pop#8) (B). Backgating strategy showed that most of the well-acquainted hBM populations, such as CD105+ (yellow dots), MSCA-1+ (light green dots), mature lymphocytes (orange dots), granulocytes (light blue dots), HSCs (pink dots), and plasma cells (violet dots) occupied one or more of the expected R1–R7 regions (C). Conversely, Pop#8 (black dots) occupied one distinct region (R8). HSCs, hematopoietic stem cells.
Table 1.
Events Distribution in CD31 Versus CD18 Dot Plots
| Region | Populations | CD31 intensity | CD18 intensity |
|---|---|---|---|
| R1 | MSCs and erythroid progenitors | Negative | Negative |
| R2 | Subpopulation of lymphoid cells | Negative | Dim |
| R3 | HSCs, EPCs, small subpopulation of myeloid cells | Intermediate | Negative |
| R4 | Subpopulation of lymphoid cells | Dim | Intermediate |
| R5 | Most of myeloid cells | Intermediate | Bright |
| R6 | Monocytoid cells and macrophages | Bright | Bright |
| R7 | Plasmacells | Bright | Negative |
| R8 | Pop#8 | Intermediate–bright | Intermediate |
Eight separate regions were identified and characterized by combinations of CD31 and CD18 intensity of expression.
EPCs, endothelial precursor cells; HSCs, hematopoietic stem cells; MSCs, mesenchymal stromal cells.
Table 2.
Distinct hBM Subpopulations Occupied Different Regions in CD18 Versus CD31 Plot
| Population | Lineage marker | Region | CD31 intensity | CD18 intensity |
|---|---|---|---|---|
| Mesenchymal/erythroid | CD105+ | R1 | Negative | Negative |
| BM-MSCs | MSCA-1+ | R1 | Negative | Negative |
| Lymphoid | CD3/CD20+ | R2, R4, R5 | Negative–dim–intermediate | Dim–intermediate–bright |
| Myeloid | CD66+ | R3, R5 | Intermediate | Negative–bright |
| Monocytoid | CD14+ | R6 | Bright | Bright |
| Plasmacytoid | CD138+ | R7 | Bright | Negative |
| HSCs/EPCs | CD34+ | R3 | Intermediate | Negative |
| Pop#8 | LinnegCD34negCD31+CD18+ | R8 | Bright– | Dim–intermediate |
Distribution of hBM subpopulations as defined by specific immunophenotypes resulted specific with the exception of lymphocytes that segregated into three different regions and myeloid cells that spread seamlessly from R3 to R5.
BM-MSCs, bone marrow–mesenchymal stromal cells; hBM, human bone marrow.
As expected, CD105+ and MSCA-1+ cells were detected within the CD31negCD18neg region (R1). Lymphocytes and their precursors, marked by the expression of CD3 or CD20, were mainly distributed among CD31negCD18dim (R2), CD31dimCD18int (R4), and CD31intCD18bright (R5). Myeloid CD66+ cells mostly resulted in CD31intCD18bright (R5), a small percentage was characterized by fading CD18 intensity, and a subpopulation was CD18neg (R3). The monocytic–macrophagic lineage, marked for the expression of CD14, occupied exclusively the CD31brightCD18bright region (R6), whereas hematopoietic stem cells (HSCs) and endothelial precursor cells (EPCs), both CD34+ cells, were plotted in R3. The remaining CD31brightCD18neg (R7) rare population revealed almost exclusively constituted by CD138+ elements, ascribable to normal bone marrow plasma cells (Supplementary Fig. S2A and Fig. 2C).
Gating on lineage-negative cells (CD34neg, CD3/20neg, CD14neg, and CD66neg, HSCs/EPCs CD18neg populations and plasma cells) CD18 versus CD31 density plot, revealed in all samples, a residual CD31brightCD18dim/int population we defined as “unknown population #8” (Pop#8) (Fig. 2B), this population resulted around 1% of the total BM-MNCs (1.27% ± 0.60%; n = 17) without significant variations, related to donor's age or sex. Backgating revealed a separated and specific region for Pop#8 on the plot (R8), adjacent to the monocytoid, myeloid, and CD34+ populations (Fig. 2C). To the best of our knowledge, a population with similar features has not been characterized yet. Pop#8 is the only CD31+/CD18+ population not attributable to any of the main bone marrow subpopulations. Therefore, it appeared to be a reasonable candidate to represent the ex vivo progenitor of MPCs.
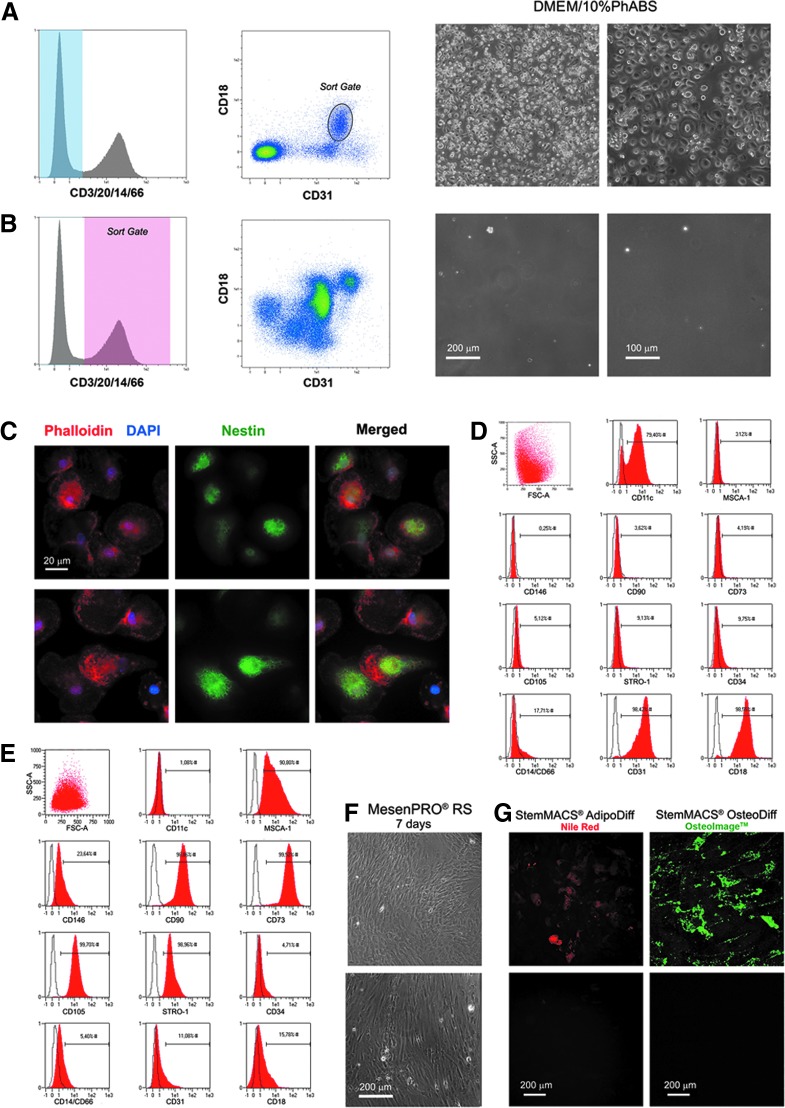
To verify our hypothesis, FACS sorting was performed on freshly isolated hBM-MNCs after incubation with FITC-conjugated anti-CD3/CD20/CD14/CD66abce, PE-conjugated anti-CD18, and PE-Cy7-conjugated anti-CD31. Pop#8 sorting protocol included in hierarchical order: (1) selection of cellular events on forward scatter (FSC) versus side scatter (SSC) plot, (2) selection of single-cell events on FSC-A versus FSC-H plot, (3) exclusion of FITC-positive events, and (4) selection of CD31brightCD18dim/int events on PE versus PE-Cy7 plot, excluding HSCs/EPCs in R3, and plasma cells in R7. CD3/20/14/66+ cells were sorted in parallel, as control. Pop#8 (median purity of 97.4% ± 1.6%, n = 6) was the only population able to generate adherent MPC-like cells, characterized by the typical MPC fried egg-shape shown in PhABS cultures (Fig. 3A, B). Pop#8 differentiation potential was tested and derived cultured cells were definitively characterized as MPCs for the presence of a high number of dispersed podosomes, positive stain for Nestin (Fig. 3C), and typical MPC CD31+, CD18+, and CD11c+ immunophenotype (Fig. 3D).
FIG. 3.
Pop#8 exclusive in vitro generation of MPCs. After 5 days in MPC-selective culture conditions (DMEM/10%PhABS) Pop#8 generated MPC-like cells characterized by the peculiar fried egg-shaped morphology (A). CD3/20/14/CD66+ cells were sorted in parallel, as control population (B). Immunofluorescence analysis of Pop#8-derived MPCs, revealed podosome-like actin organization (red in C) and intense positivity to nestin (green in C). The MPC phenotype was confirmed by flow cytometry (D). After 7 days of culture under mesengenic-differentiating conditions (MesenPRO® RS medium), Pop#8-derived MPCs gave rise to confluent cells showing MSC phenotype (E) and morphology (F). To confirm the mesenchymal nature of Pop#8 MPC-derived MSCs, cultures were further exposed to adipogenic or osteogenic stimuli. Lipid droplet (red in G) accumulation and calcium deposition (green in G) were detected only in terminally differentiated cultures (G, upper panels). No fluorescence signals were detected in cultures maintained in MesenPRO RS medium (G) (lower panels). DMEM, Dulbecco's modified Eagle's medium; PhABS, pooled human AB-type serum; RS, reduced serum.
Moreover, Pop#8-derived MPCs showed the ability to differentiate into MSCs once switched from DMEM/10%PhABS to MesenPRO RS medium, consistently with MPCs isolated by plastic adherence [18]. After 7 days of mesengenic differentiation, cells expressed MSC-associated markers CD105, CD90, CD73, STRO-1, and MSCA-1, whereas CD31, CD18, and CD11c expression was rapidly lost during differentiation (Fig. 3E). Typical spindle-shaped fibroblastoid cell morphology was evident as well as the ability to reach confluence within a week (Fig. 3F). F-actin resulted reorganized in the characteristic stress fibers usually reported in MSCs, whereas podosome-like structures were lost. Moreover, differentiation was associated to reduced nestin expression (data not shown).
MSC typical features of differentiated cultures were further confirmed by inducing terminal differentiation into adipogenic or osteogenic lineages. After 21 days under adipogenic stimuli, lipid droplet accumulation was easily detectable by red fluorescence, whereas osteogenic stimuli led to high deposition of mineralized matrix, revealed by intense green florescence (Fig. 3G, upper panels). On the other side, cultures kept in MesenPRO RS medium for 21 days showed no spontaneous differentiation (Fig. 3G, lower panels).
Characterization of Pop#8
May Grünwald–Giemsa staining of freshly sorted Pop#8 showed distinctive morphological features. Cells were 30–40 μm in diameter with abundant densely basophilic cytoplasm sometimes revealing intense dot-patterned acidophilic stain. Nuclei appeared of regular shape with condensed chromatin, rarely showing mild invaginations. Evident nucleoli were frequently found (Supplementary Fig. S3A). All other CD31 versus CD18 plot cell populations revealed the morphologies expected for related lineages (Supplementary Fig. S2B). Pop#8 retained high peroxidase activity (dark brown in Supplementary Fig. S3B), from very low to absent naphthyl-acetate esterase activity, and absent tartrate-resistant acid phosphatase activity, as shown by cytoenzymatic assays.
Immunophenotyping showed that none of the antigens mostly applied for perspective isolation of MSCs was expressed in Pop#8 (Table 3A). In detail, Pop#8 showed no expression of CD105 and MSCA-1 antigens, thus confirming the results from the immunomagnetic selection; no expression of CD271 (LNGFR), STRO-1, SSEA-3, SSEA-4, or CD146 (MCAM), as well as platelet-derived growth factor receptor chains (CD140a, CD140b) or Integrin αV (CD51). Moreover, Pop#8 did not express common endothelial-associated antigens CD309 (KDR), CD144 (VE-cadherin), alongside to CD34 and CD133 (Supplementary Fig. S3C and Table 3A). MPC highly expressed CD11c (Integrin αX) and CD11b (Integrin αM) were not found in Pop#8, but they were rapidly induced in DMEM/10%PhABS culture (Supplementary Fig. S3C and Fig. 3D).
Table 3.
Pop#8 Immunophenotype
| A | B | ||
|---|---|---|---|
| Antigen | Intensity | Antigen | Intensity |
| CD34 | Negative | CD11b | Neg/dim |
| CD44 | Bright | CD11c | Negative |
| CD51 | Negative | CD13 | Neg/dim |
| CD56 | Negative | CD14 | Negative |
| CD105 | Negative | CD15 | Negative |
| CD133 | Negative | CD16 | Negative |
| CD140a/b | Negative | CD33 | Positive |
| CD144 | Negative | CD36 | Negative |
| CD146 | Negative | CD38 | Positive |
| CD271 | Negative | CD45 | Dim |
| CD309 | Negative | CD64 | Bright |
| STRO-1 | Negative | CD80 | Negative |
| SSEA-3 | Negative | CD86 | Negative |
| SSEA-4 | Negative | CD115 | Negative |
| HLA-DR | Positive | ||
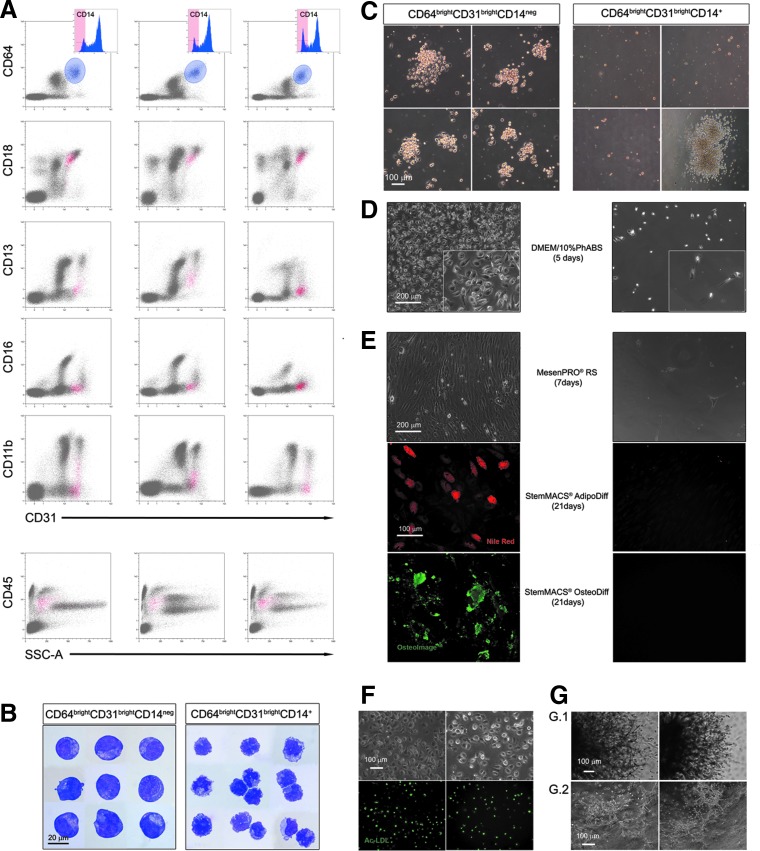
Immunophenotype analysis extended to the hematopoietic lineage antigens revealed low levels of CD45 and intense positivity for the myeloid marker CD64 (Table 3B). Thus, events were gated for bright expression of both CD31 and CD64. Within this population most cells showed to be either definitely CD14+ or CD14neg, with only a small portion of cells expressing CD14 at intermediate levels. In CD18 versus CD31 cytogram, the CD64brightCD31brightCD14neg population (1.38% ± 0.30%; n = 11), plotted exclusively in the R8 region associated to Pop#8 cells (Fig. 4A). In CD45 versus SSC cytogram, CD64brightCD31brightCD14neg population plotted in the myeloblast/monoblast area, although CD16, CD11b, and CD13 resulted mainly negative or occasionally dim (Fig. 4A). Mature and immature monocyte markers CD36 and CD115 were not expressed in Pop#8. CD33, CD38, and HLA-DR were detected, whereas costimulatory proteins CD80 and CD86 were not present (Table 3B).
FIG. 4.
Pop#8 isolation by their unique CD64brightCD31brightCD14neg phenotype. Gating events for bright expression of both CD31 and CD64 (light blue gate) most cells showed to be CD14+. CD64brightCD31brightCD14neg minor population plotted exclusively in the R8 region of CD18 versus CD31 cytogram (red dots), confirming that this population was the Pop#8 population (three representative samples are presented in (A). CD45 was mildly expressed as well as CD13, CD16, and CD11b. CD64brightCD31brightCD14neg appeared to be large cells with blast-like morphology, in sharp contrast with the monocytic morphology showed by the CD14+ counterpart (B). Applying hematopoietic colony-forming assay, CD64brightCD31brightCD14neg generated clusters of large cells above few adherent MPC-like cells not detected culturing the CD14+ counterpart, where GM- or M-CFUs were occasionally reported (C). Consistently with LinnegCD31brightCD18dim/int, CD64brightCD31brightCD14neg cells generated MPCs (left panel) (D) when cultured in DMEM/10%PhABS. Those MPCs rapidly differentiated into MSCs, which retained their adipogenic and osteogenic potential (left panels) (E). The CD14+ counterpart failed to generate adherent cultures (right panels) (D, E). Pop#8-derived MPCs were also capable of Ac-LDL uptake (green in F) and angiogenic sprouting (G.1) that primed the cells to form capillary tube-like structures (G.2). Ac-LDL, acetylated low-density lipoprotein; GM-CFUs, granulocyte/macrophage colony-forming units; M-CFUs, macrophage colony-forming units.
CD64brightCD31brightCD14neg as well as LinnegCD31brightCD18dim/int, sorted Pop#8, showed cytomorphological traits of the myeloblast/monoblast lineage. Conversely, mature elements of the monocyte/macrophage compartment were easily identified in CD64brightCD31brightCD14+ fraction (Fig. 4B). Clonogenic test revealed that Pop#8 cells, despite their myeloblastic/monoblastic appearance, do not retain any hematopoietic colony-forming potential. In fact, after 14 days of culture in StemMACS HSC-CFU medium, only numerous clusters of <100 cells, were detectable. These clusters resulted constituted by aggregates of large rounded cells on top of few attached MPC-like cells. Rare uncolored hematopoietic colonies (median frequency of 6.2 ± 2.0/106 plated cells, n = 4) were instead detected applying CD64brightCD31brightCD14+, those colonies were classified as derived from GM-CFUs or M-CFUs only (Fig. 4C) and probably were generated from CD14dim myeloid precursors included in the CD14+ sort gate.
Phagocytic activity has been induced in Pop#8 cells cultured in the presence and also in the absence of hGM-CSF, as revealed by uptake of Indian ink particles (Supplementary Fig. S4).
CD64brightCD31brightCD14neg sorted Pop#8 were also able to generate MPCs, once cultured in DMEM/10%PhABS for 6 days (Fig. 4D, right panel). Those Pop#8-derived MPCs showed their mesangiogenic nature, differentiating in functional MSCs, which retain the adipogenic and osteogenic potential (Fig. 4E, right panels), and showing the ability to internalize Ac-LDL (Fig. 4F), as well as of sprouting angiogenesis from 3D spheroids once seeded on Matrigel (Fig. 4G.1). Cells from enzymatic digestion of sprouted spheroids generate CLT structures, in the secondary cultures on Matrigel (Fig. 4G.2). At a difference, CD64brightCD31brightCD14+ generated few loosely adherent MPC-like cells (Fig. 4D, left panel), unable to differentiate into functional MSCs once cultured in MesenPRO RS (Fig. 4E, left panels). Freshly sorted Pop#8 plated directly in MesenPRO RS did not generate any culture (data not shown), in accordance with immunomagnetic separation results on CD31+ fraction (Supplementary Fig. S1D). Similarly, ex vivo isolated Pop#8 failed to form cell aggregates during preparation of 3D spheroids (data not shown).
Gene expression analysis of Pop#8 showed that neither NESTIN nor SPP1 mRNA, highly expressed by cultured MPCs, were found. Conversely, NANOG and OCT4A were detected in Pop#8 as well as in CD64brightCD31brightCD14+ fraction, with no significant difference with cultured MPCs. PECAM (CD31) expression resulted less than one log lower in Pop#8 as compared with their CD14+ counterpart (1.75 ± 0.62 × 10−1 and 1.37 ± 0.17, P < 0.05, respectively; n = 3, Supplementary Fig. S5).
Discussion
In this study, we describe the isolation by immunomagnetic fractioning of CD45lowCD64brightCD31brightCD14neg subpopulation from adult hBM with the ability to generate MPCs in culture. Notably, CD45 and CD31 antigens are routinely included in the depletion cocktails to enrich for MSC progenitors [25]. Our results, therefore, indicate that MPCs and MSCs belong to different lineages in vivo.
The hypothesis that MPC and MSC progenitors are set apart in different bone marrow subpopulations was supported by the failure to isolate MSC-like cells directly from CD31+ fraction even under permissive culture conditions (gas-treated instead of hydrophobic plastic), as previously described in unfractionated hBM [12]. No MPC-like cells were obtained from CD105+ fractions suggesting also that the expression of endoglin in MPCs would be early induced in vitro.
Multicolor flow cytometry analysis of freshly isolated hBM-MNCs confirmed CD105+ and MSCA-1+ events to be confined in the R1 (double negative) region of CD18 versus CD31 plot. Further characterization of the plot led to the identification of six different CD31+ subpopulations (R3, R4, R5, R6, R7, and R8; Fig. 2 and Table 1). The screening of FACS-sorted cell subpopulations for the ability to generate MPCs in culture allowed us to identify R8 fraction as the putative candidate for MPC progenitor in vivo. We named the novel population “Pop#8.” Apparently, Pop#8 seemed belonging to the myelocytic/monocytic lineage, and in details, these cells partially resemble precursors of monocytes and macrophages, considering their morphology, immunophenotype, and the ability to generate phagocytes. A genuine hematopoietic colony-forming potential has not been detected on Pop#8, however, it interestingly revealed signs of replication when culturing these cells in StemMACS HSC-CFU medium, which contains 30% of FBS, instead of the expected mesengenic differentiation [12,13]. This could be explained by a possible effect of the hematopoietic growth factors, as stem cell factors (SCF) and GM-CSF contained at high doses in the semisolid medium, on Pop#8 cells.
Quantitative analysis showed the frequency of Pop#8 (1.3%) to be consistent with reported frequency (0.9%) of cultured MPCs from hBM-MNCs [13]. MPCs derived from culturing Pop#8 in PhABS-containing medium resulted undistinguishable from MPCs from unfractionated hBM-MNCs. They showed the expected mesengenic and angiogenic differentiation potential, giving rise, respectively, to MSCs matching the ISCT criteria [26] and retaining the adipogenic/osteogenic differentiation capability, as well as of sprouting angiogenesis.
Therefore, we were able to obtain MSCs in vitro starting from the CD31+ compartment, which is usually excluded from the studies on prospective isolation of in vivo MSC progenitors. Further Pop#8 characterization revealed CD45 to be mildly expressed, whereas most of the antigens feasible for prospective isolation of MSCs from bone marrow [11] remained unexpressed. In particular, the lack of CD146 expression on ex vivo Pop#8, similar to what is reported for the in vitro MPCs, suggests that this cell population is distinct from the CD146bright clonogenic progenitor, residing in the subendothelial layer of sinusoids, identified by Sacchetti et al. [27]. Thus, Pop#8 could represent an inducible postnatal BM stromal progenitor that should be considered distinct from the determined self-renewing, multipotent progenitors for skeletal tissue, recently described as SSCs [28]. This is also suggested by the evidence that CD146neg Pop#8 could be easily induced in vitro into a CD146dim MSC-like osteogenic cell strain (Fig. 3E), where the low expression of CD146 results in accordance to the expression levels reported on the “nonstem” osteogenic cell populations [27].
Most of the information available on MSC biology is derived from in vitro studies making it difficult to determine the primary cells of origin. A multitude of ex vivo MSC progenitors, representing distinct and sometimes mutually exclusive populations, has been identified from different tissues [29,30]. In particular, for hBM-derived MSCs strong evidence indicates that the fibroblastoid colonies originally described by Friedenstein et al. [31] originated from the CD146+ pericytic population residing in the subendothelial layer of bone marrow sinusoids [27,32,33]. More recently, in vivo MSC progenitors were localized in the trabecular bone lining CD271+CD146neg cell population as well [34]. Stromal reticular cells also were shown to retain MSC-like adipogenic/osteogenic potential [35].
Therefore, cells with different immunophenotypes can generate MSCs in the perivascular, in the endosteum, as well as in the stromal bone marrow compartments. The search for the MSC progenitor is turning into a search for various MSC progenitors, with their mutual relationship still to be clarified. In this scenario, our results indicate that MSC-like cells can be indirectly propagated also from a distinctive CD146negCD271negCD31brightCD45low population; this also represents a peculiar feature of Pop#8. In fact, this cell population, conversely to other mesengenic or angiogenic progenitor cells isolated, showed neither CFU-F nor CFU-Hill properties when directly cultured in differentiating conditions, and only after induction in MPCs demonstrates its mesangiogenic potential.
Thus, in our hands Pop#8 represents the exclusive primary ex vivo precursor of MPCs that were shown to be in vitro progenitor of both mesenchymal and endothelial lineages [12,13]. This suggests a possible functional role of Pop#8 in the local maintaining of the HSC supporting microenvironment that include remodeling of both the microvascular plexus and the stromal compartment [36,37], acting as inducible progenitor, as hypothesized above. The precise anatomical localization of Pop#8 and serial transplantation experiments are necessary to clarify these aspects, and will be the object of future studies.
The demonstration that nestin+ perivascular MSCs are crucial in maintaining bone marrow HSC pool in a steady state [38] casts new light on the role of this intermediate filament protein previously associated to central nervous system development [39,40] and malignancy [41]. In murine and human bone marrow, nestin+ MSCs in close contact with HSCs at perivascular locations, in the endosteum and in the stroma, were found expressing HSC maintenance genes as CXCL12, SCF, and SPP1 [42]. Moreover, NESTIN expression was associated to angiogenesis in proliferating endothelial progenitors at a difference with mature endothelial cells [43]. Induction of SPP1 expression was also reported in the vascular remodeling process [44,45] and specifically in neointima formation [46].
Therefore, nestin and osteopontin appear to be good candidate markers for both the mesengenic and angiogenic remodeling processes. The idea is intriguing, in particular regard to tissues which are physiologically highly remodeled, like bone marrow. Notably, we found NESTIN and SPP1 to be consistently upregulated when Pop#8 were cultured to generate MPCs, further corroborating the hypothesis of a Pop#8 role as in vivo progenitor of the different cell populations involved in bone marrow microenvironment remodeling.
Moreover, the discovery of Pop#8 in hBM-MNCs strongly supports the idea that most of the controversies regarding culture-expanded MSCs could be the consequence of different culture conditions that select or promote particular subpopulations of precursors [47]. Pop#8 resulted from two to three logs more frequent than other putative MSC progenitors reported in hBM-MNCs, making it reasonable that specific culture systems (ie, different batches of human or bovine sera) could affect the percentage of coisolated MPCs. We believe the heterogeneous composition of cultures, including subpopulations of MPCs and MSCs to be responsible for the controversial data regarding angiogenic potential of MSC cultures, as previously reported [48].
In conclusion, in this study, we demonstrated that MPCs arise from a unique novel CD45lowCD64brightCD31brightCD14neg human bone marrow subpopulation (Pop#8), whose identification could represent a significant contribution to the debate on MSC in vivo progenitors. Further studies are needed to localize Pop#8 in vivo and to define its role on the homeostasis and pathology of bone marrow microenvironment.
Supplementary Material
Acknowledgment
Regione Toscana supported this work under “Bando Salute” grant.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Si YL, Zhao YL, Hao HJ, Fu XB. and Han WD. (2011). MSCs: biological characteristics, clinical applications and their outstanding concerns. Ageing Res Rev 10:93–103 [DOI] [PubMed] [Google Scholar]
- 2.Madrigal M, Rao KS. and Riordan NH. (2014). A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med 12:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma RR, Pollock K, Hubel A. and McKenna D. (2014). Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion 54:1418–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimarino AM, Caplan AI. and Bonfield TL. (2013). Mesenchymal stem cells in tissue repair. Front Immunol 4:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ankrum J. and Karp JM. (2010). Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol Med 16:203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S, Qu X. and Zhao RC. (2012). Clinical applications of mesenchymal stem cells. J Hematol Oncol 5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farini A, Sitzia C, Erratico S, Meregalli M. and Torrente Y. (2014). Clinical applications of mesenchymal stem cells in chronic diseases. Stem Cells Int 2014:306573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delorme B, Ringe J, Gallay N, Le Vern Y, Kerboeuf D, Jorgensen C, Rosset P, Sensebe L, Layrolle P, Haupl T. and Charbord P. (2008). Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood 111:2631–2635 [DOI] [PubMed] [Google Scholar]
- 9.Harichandan A. and Buhring HJ. (2011). Prospective isolation of human MSC. Best Pract Res Clin Haematol 24:25–36 [DOI] [PubMed] [Google Scholar]
- 10.Matsuoka Y, Nakatsuka R, Sumide K, Kawamura H, Takahashi M, Fujioka T, Uemura Y, Asano H, Sasaki Y, et al. (2015). Prospectively isolated human bone marrow cell-derived MSCs support primitive human CD34-negative hematopoietic stem cells. Stem Cells 33:1554–1565 [DOI] [PubMed] [Google Scholar]
- 11.Lv FJ, Tuan RS, Cheung KM. and Leung VY. (2014). Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells 32:1408–1419 [DOI] [PubMed] [Google Scholar]
- 12.Petrini M, Pacini S, Trombi L, Fazzi R, Montali M, Ikehara S. and Abraham NG. (2009). Identification and purification of mesodermal progenitor cells from human adult bone marrow. Stem Cells Dev 18:857–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trombi L, Pacini S, Montali M, Fazzi R, Chiellini F, Ikehara S. and Petrini M. (2009). Selective culture of mesodermal progenitor cells. Stem Cells Dev 18:1227–1234 [DOI] [PubMed] [Google Scholar]
- 14.Frith J. and Genever P. (2008). Transcriptional control of mesenchymal stem cell differentiation. Transfus Med Hemother 35:216–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Augello A. and De Bari C. (2010). The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther 21:1226–1238 [DOI] [PubMed] [Google Scholar]
- 16.Pacini S, Carnicelli V, Trombi L, Montali M, Fazzi R, Lazzarini E, Giannotti S. and Petrini M. (2010). Constitutive expression of pluripotency-associated genes in mesodermal progenitor cells (MPCs). PLoS One 5:e9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pacini S, Fazzi R, Montali M, Carnicelli V, Lazzarini E. and Petrini M. (2013). Specific integrin expression is associated with podosome-like structures on mesodermal progenitor cells. Stem Cells Dev 22:1830–1838 [DOI] [PubMed] [Google Scholar]
- 18.Fazzi R, Pacini S, Carnicelli V, Trombi L, Montali M, Lazzarini E. and Petrini M. (2011). Mesodermal progenitor cells (MPCs) differentiate into mesenchymal stromal cells (MSCs) by activation of Wnt5/calmodulin signalling pathway. PLoS One 6:e25600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montali M, Barachini S, Pacini S, Panvini FM. and Petrini M. (2016). Isolating mesangiogenic progenitor cells (MPCs) from human bone marrow. J Vis Exp. [Epub ahead of print]; e54225, DOI: 10.3791/54225(2016) [DOI] [PubMed] [Google Scholar]
- 20.Del Duca D, Werbowetski T. and Del Maestro RF. (2004). Spheroid preparation from hanging drops: characterization of a model of brain tumor invasion. J Neurooncol 67:295–303 [DOI] [PubMed] [Google Scholar]
- 21.Foty R. (2011). A simple hanging drop cell culture protocol for generation of 3D spheroids. J Vis Exp pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeCicco-Skinner KL, Henry GH, Cataisson C, Tabib T, Gwilliam JC, Watson NJ, Bullwinkle EM, Falkenburg L, O'Neill RC, Morin A. and Wiest JS. (2014). Endothelial cell tube formation assay for the in vitro study of angiogenesis. J Vis Exp e51312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham GS. (1918). Benzidine as a peroxidase reagent for blood smears and tissues. J Med Res 39:15–24.1 [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ. and Schmittgen TD. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 25.Modder UI, Roforth MM, Nicks KM, Peterson JM, McCready LK, Monroe DG. and Khosla S. (2012). Characterization of mesenchymal progenitor cells isolated from human bone marrow by negative selection. Bone 50:804–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D. and Horwitz E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317 [DOI] [PubMed] [Google Scholar]
- 27.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M. and Bianco P. (2007). Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131:324–336 [DOI] [PubMed] [Google Scholar]
- 28.Bianco P. and Robey PG. (2015). Skeletal stem cells. Development 142:1023–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buhring HJ, Battula VL, Treml S, Schewe B, Kanz L. and Vogel W. (2007). Novel markers for the prospective isolation of human MSC. Ann N Y Acad Sci 1106:262–271 [DOI] [PubMed] [Google Scholar]
- 30.Mabuchi Y, Houlihan DD, Akazawa C, Okano H. and Matsuzaki Y. (2013). Prospective isolation of murine and human bone marrow mesenchymal stem cells based on surface markers. Stem Cells Int 2013:507301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedenstein AJ, Petrakova KV, Kurolesova AI. and Frolova GP. (1968). Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 6:230–247 [PubMed] [Google Scholar]
- 32.Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C. and Canfield AE. (2004). Chondrogenic and adipogenic potential of microvascular pericytes. Circulation 110:2226–2232 [DOI] [PubMed] [Google Scholar]
- 33.Brighton CT, Lorich DG, Kupcha R, Reilly TM, Jones AR. and Woodbury RA., 2nd. (1992). The pericyte as a possible osteoblast progenitor cell. Clin Orthop Relat Res 287–299 [PubMed] [Google Scholar]
- 34.Tormin A, Li O, Brune JC, Walsh S, Schutz B, Ehinger M, Ditzel N, Kassem M. and Scheding S. (2011). CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood 117:5067–5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K. and Nagasawa T. (2010). The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 33:387–399 [DOI] [PubMed] [Google Scholar]
- 36.Doan PL. and Chute JP. (2012). The vascular niche: home for normal and malignant hematopoietic stem cells. Leukemia 26:54–62 [DOI] [PubMed] [Google Scholar]
- 37.Mendez-Ferrer S, Scadden DT. and Sanchez-Aguilera A. (2015). Bone marrow stem cells: current and emerging concepts. Ann N Y Acad Sci 1335:32–44 [DOI] [PubMed] [Google Scholar]
- 38.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN. and Frenette PS. (2010). Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466:829–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dahlstrand J, Lardelli M. and Lendahl U. (1995). Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain Res Dev Brain Res 84:109–129 [DOI] [PubMed] [Google Scholar]
- 40.Lendahl U, Zimmerman LB. and McKay RD. (1990). CNS stem cells express a new class of intermediate filament protein. Cell 60:585–595 [DOI] [PubMed] [Google Scholar]
- 41.Tohyama T, Lee VM, Rorke LB, Marvin M, McKay RD. and Trojanowski JQ. (1992). Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab Invest 66:303–313 [PubMed] [Google Scholar]
- 42.Pinho S, Lacombe J, Hanoun M, Mizoguchi T, Bruns I, Kunisaki Y. and Frenette PS. (2013). PDGFRalpha and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med 210:1351–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsuda Y, Hagio M. and Ishiwata T. (2013). Nestin: a novel angiogenesis marker and possible target for tumor angiogenesis. World J Gastroenterol 19:42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Infanger M, Shakibaei M, Kossmehl P, Hollenberg SM, Grosse J, Faramarzi S, Schulze-Tanzil G, Paul M. and Grimm D. (2005). Intraluminal application of vascular endothelial growth factor enhances healing of microvascular anastomosis in a rat model. J Vasc Res 42:202–213 [DOI] [PubMed] [Google Scholar]
- 45.Leen LL, Filipe C, Billon A, Garmy-Susini B, Jalvy S, Robbesyn F, Daret D, Allieres C, Rittling SR, et al. (2008). Estrogen-stimulated endothelial repair requires osteopontin. Arterioscler Thromb Vasc Biol 28:2131–2136 [DOI] [PubMed] [Google Scholar]
- 46.Li XD, Chen J, Ruan CC, Zhu DL. and Gao PJ. (2012). Vascular endothelial growth factor-induced osteopontin expression mediates vascular inflammation and neointima formation via Flt-1 in adventitial fibroblasts. Arterioscler Thromb Vasc Biol 32:2250–2258 [DOI] [PubMed] [Google Scholar]
- 47.Pacini S. (2014). Deterministic and stochastic approaches in the clinical application of mesenchymal stromal cells (MSCs). Front Cell Dev Biol 2:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pacini S. and Petrini I. (2014). Are MSCs angiogenic cells? New insights on human nestin-positive bone marrow-derived multipotent cells. Front Cell Dev Biol 2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.