Abstract
This study tested the hypothesis that aging significantly affects the influence of exogenous testosterone on neurobehavior in gonadally intact male mice. Groups of prepubertal and aged male mice received daily vehicle or testosterone propionate (TP; 2.5 or 5.0 mg/kg intraperitoneal [i.p.]) for 21 days. Behaviors were assessed on days 1 and 21. Weight gain was significant in prepubertal mice. Locomotion and rearing increased in prepubertal mice after first dose and decreased after last dose of TP. Rearing was suppressed in aged mice throughout. Suppression of grooming occurred in both age groups at day 21. Significant increase in working memory in both age groups was seen in the radial-arm maze (at specific doses) and in prepubertal mice in the Y-maze. Elevated plus maze test showed mixed anxiolytic/anxiogenic effects. Aged mice had higher serum testosterone. In conclusion, age is an important determinant for the influence of exogenous testosterone on behavior in gonadally intact male mice.
Keywords: androgens, age, central excitation, cognition, anxiety, novelty-induced behaviors
Introduction
Androgens such as testosterone are ligands that bind and activate nuclear androgen receptors through deoxyribonucleic acid (DNA) genomic mechanisms1 or alter neuron function through nongenomic interactions.2 Testosterone is an endogenous hormone and also a drug of abuse.3 It is a potent sex steroid produced by the testes in males and a precursor to estrogen synthesis from the ovary in females. In common with all androgens, testosterone has androgenic (masculinizing) and anabolic (muscle building) effects; hence, androgens are collectively known as anabolic androgenic steroids (AASs).
There is an increasing prevalence in the use of testosterone in hormone replacement therapy in elderly men who are predisposed to age-dependent testosterone deficiency due to decreasing testosterone levels associated with debility and reductions in total testosterone with normal aging.4 The use and abuse of androgenic steroids in adolescents is also increasing rapidly;5 testosterone in this age group is used largely for enhancement of physical appearance and athletic performance.6 However, with repeated use of AASs, individual psyche and behavior seem to be strongly affected, with noticeable induction of aggressive behaviors and hostility. Mood disturbances such as depression and hypomania are also documented.7 Abuse of AASs is a potential global epidemic. According to Sagoe et al, the global lifetime prevalence rate of AAS use is 3.3%, with nonmedical use of AASs fast becoming a public health issue of deep concern.8 An emerging increase in use of testosterone in young, gonadally intact men in addition to older gonad-deficient men implies a new area of focus for exogenous testosterone research. In addition, more effort needs to be put into studying age-related differences in response to testosterone administration.
Over the years however, extensive research has been conducted on the behavioral consequences of testosterone administration9,10 in rodents,11 nonrodents,12–15 and gonadally intact16,17 and gonadectomized animals.17,18 Numerous researchers have also studied the effects of either endogenous or exogenous testosterone on various behavioral paradigms such as16,18–24 sexual behaviors,25–27 obesity or lipid profile,28,29 and gender differences.30–32 With regard to age-related difference in testosterone effects, Chambers et al27 studied the effects of brain androgen binding/metabolism, testosterone, and sexual behavior in intact and gonadectomized old and young male Fischer 344 rats and reported significant differences in sexual behaviors but no significant difference in testosterone effects on aromatase activity. Laroche et al33 studied how shipping during the prepubertal period or adulthood influenced steroid hormone response in male and female mice and concluded that a reduction in behavioral response to exogenous testosterone administration is seen in prepubertal mice compared to adult mice, irrespective of gender.
Most of the studies conducted dealt with effects of either prenatal or prepubertal testosterone administration on adult behaviors; however, a dearth of literature exists in relation to the effect of exogenous testosterone administration (in two separate age groups) on novelty-induced behaviors, emotionality, and spatial working memory in mice. The rationale for this study is the need for a better understanding of the behavioral changes that accompany testosterone supplementation at physiologic (<3 mg/kg/day) and supraphysiologic doses (>3 mg/kg/day)34 in gonadally intact animals. We believe that gonadally intact animals model a significant subset of testosterone users in real life. Over the years, research had concentrated on the effects of exogenous testosterone post-gonadectomy; however, many testosterone users are gonadally intact. In this study, we investigated the effect of age, testosterone administration, and their interactions in gonadally intact prepubertal and aged male mice.
Methods
Drugs
Olive oil (Goya) and testosterone propionate (TP; Hubei-Datong Biochemical Company Limited) were given as i.p. injections of specific doses of testosterone.
Subjects
A total of 220 inbred Swiss albino male mice (Empire Breeders) were used in this study. Mice were housed in plastic cages measuring 41 cm × 31 cm × 26 cm (10 mice in each cage). Housing is a temperature-controlled quarters (22°C–25°C) with 12 hours of light daily. Mice had free access to food and water, except during the behavioral tests. All procedures were conducted in accordance with the approved institutional protocols and within the provisions for animal care and use prescribed in the scientific procedures on living animals, European Council Directive (EU 2010/63).
Experimental method
The 220 Swiss albino male mice were divided into two main age-related (prepubertal [postnatal day (PND) 29] and aged [18 months old]) groups, with 110 mice in each group. Mice in each age-related group were randomly divided into three groups of 10 animals each for the open-field test (30), four groups of 10 animals each for the cognition tests (40), and four groups of 10 animals each for the anxiety-related test (40). Control groups received i.p. injection of vehicle (olive oil), test groups received doses of TP (2.5 and 5.0 mg/kg/day) daily for a period of 21 days, while animals in the fourth group received a standard drug (diazepam at 0.5 mg/kg for the anxiety test and scopolamine at 1 mg/kg for cognition test). Animals were weighed weekly. Behavioral testing after acute dose was carried out 30 minutes after administration of first dose of vehicle, testosterone, or standard drug, while behavioral testing after subchronic dose was carried out 30 minutes after last dose of vehicle, testosterone, or standard drug. Blood samples were collected from 30 mice (animals used in the open-field test) one day after completion of behavioral tests to assay circulating total testosterone levels. Testosterone or vehicle was administered in the morning, and blood samples were collected two to three hours after completion of behavioral tests. Animals were euthanized using diethyl-ether, and blood was collected via a cardiac puncture.
Behavioral testing
The behavioral models used were the open-field, Y-maze, radial-arm, and elevated plus maze (EPM). Behavioral tests were conducted in a quiet room between the hours of 8 a.m. and 2 p.m. On each of the test days, mice were transported in their home cages to the testing room and allowed 30 minutes to acclimatize before testing; for all behavioral tests, at least 5 minutes was allowed between testing each individual animal to ensure the maze was completely dry and residual odor of ethanol had been dispersed. At the beginning of the behavioral tests, each animal was placed in the apparatus, and its behavior was videotaped for subsequent analysis. After testing, each mouse was removed from the maze and returned to its home cage, and all interior surfaces were cleaned thoroughly with 70% ethanol and then wiped dry to remove any trace of odor.
Open field
Novelty-induced behaviors were recorded in the open field (horizontal locomotion [line crossing], rearing, and grooming) over a 20-minute period.35 The open-field box is a rectangular arena comprised of a hard floor measuring 36 cm × 36 cm × 26 cm and made of white-painted wood with its floor divided by permanent red markings into 16 equal squares. The mice were placed in the center of the field and covered by a small dome that was then removed at the beginning of video recording of their activities.
Spatial memory test
Spontaneous alternation (a measure of spatial working memory) was measured using a Y-maze made up of three equally spaced arms (120°, 41 cm long, and 15 cm high). Each mouse was placed in one of the arm compartments and allowed to move freely until its tail completely entered another arm. An alternation is defined as entry into all three arms consecutively, and the number of maximum spontaneous alternations is the total number of arms entered minus two. The percentage alternation is calculated as (actual alternations/maximum alternations) × 100.36
Working memory in the radial-arm maze was measured as sequential arm entries before error. The apparatus is made up of eight equidistantly spaced arms, each about 33 cm long, and all radiating from a small circular central platform. Working memory was assessed when the mouse enters each arm a single time. Reentry into the arms would result in a working memory error.36 For each animal, the Y-maze and radial-arm maze testing each lasted five minutes.
Anxiety test
Anxiety-related behaviors (time spent in the open) were measured in the EPM.37 The EPM is plus shaped, with two open arms measuring 25 cm × 5 cm × 5 cm lying across from each other and perpendicular to two closed arms measuring 25 cm × 5 cm × 16 cm with a center platform (5 cm × 5 cm × 0.5 cm). The closed arms are enclosed by two high walls (16 cm), while the open arms have no side walls. Animals are placed in the central platform facing the closed arm and behaviors recorded for five minutes. The observer retired behind a screen to be obscured from the mouse’s view. The criterion for arm visit was considered only when the animal decisively moved all its four limbs into an arm.
Testosterone assay
Plasma was separated by centrifugation and stored at −20° until assayed. Total serum testosterone was measured using commercially available radioimmunoassay kits (Tianjin Medical & Bioengineering Co., Ltd.), following the manufacturer’s instructions. The kits had a lower limit of detection of 0.04 ng/mL, with intra- and interassay coefficients of variation of less than 5%. Mice in the vehicle-administered prepubertal group showed total testosterone levels that were not detectable (below the detection limit for the assay, 0.04 ng/mL); for this group, serum testosterone levels were recorded as 0.04 ng/mL to allow for statistical comparisons.
Statistical analysis
Data were analyzed using Chris Rorden’s ezANOVA for windows, version 0.98. Hypothesis testing was performed using analysis of variance (ANOVA). All data were first tested for normality and variance homogeneity, prior to statistical analysis. Having been found to be normally distributed and variances homogeneous, we tested the hypothesis that patterns of behaviors exhibited by gonadally intact male mice following administration of testosterone are dependent on age using ANOVA. Multifactorial ANOVA was used to test the effects of three main factors: dose, age (prepubertal vs aged), and duration of administration (acute vs subchronic) on behaviors in the open-field, Y-maze, radial-arm maze, and EPM, while two-factor ANOVA was used to analyze the main effect of dose and age on markers of blood testosterone levels. Tukey’s honest significant difference test was used for within- and between-group comparisons. Results are expressed as mean ± standard error of mean (SEM), P values less than 0.05 were considered statistically significant.
Results
Effect of TP on body weight
Figure 1 shows the effect of TP on body weight. Effect was measured as percentage change in body weight and was defined as the difference between the final and initial body weights divided by initial weight multiplied by 100. Two-factor ANOVA revealed a significant main effect of testosterone dose (F(2,54) = 286, P < 0.001), age of mice (F(1,54) = 4503, P < 0.001), and very strong interactions between testosterone dose × age (F(2,54) = 489, P < 0.001). Pairwise comparisons of the effects of age (prepubertal vs aged) on weight revealed that prepubertal mice gained weight significantly compared to aged mice, following administration of either vehicle or testosterone.
Figure 1.
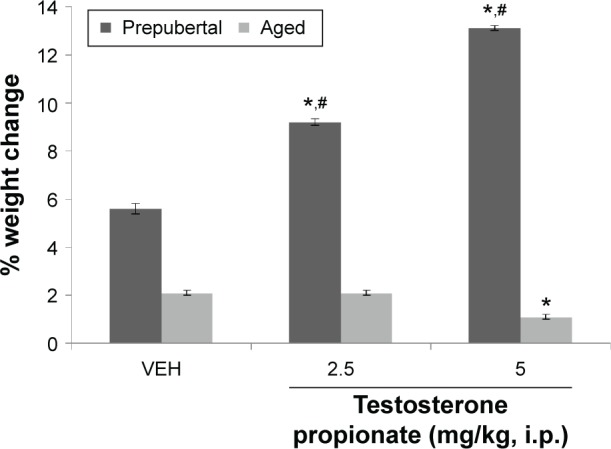
Effect of TP on body weight.
Notes: Each bar represents mean ± SEM, number of animals per group (n) = 10; *P < 0.05 vs VEH and #P < 0.05 prepubertal vs aged.
Abbreviation: VEH, vehicle.
Pairwise comparisons of testosterone dose against vehicle revealed a significant increase in weight in prepubertal mice at 2.5 mg/kg of TP (t(18) = 13.94, P < 0.001) and 5 mg/kg of TP (t(18) = 29.43, P < 0.001), while in aged mice, increase in body weight was significantly lesser at 5 mg/kg (t(18) = 7.07, P < 0.001) compared to corresponding vehicle-treated groups. At 2.5 mg/kg, no significant difference was seen.
Effect of TP on novelty-induced behaviors
Figures 2–4 show the effects of TP on horizontal locomotion, rearing, and grooming, respectively. MANOVA with TP dose, age, and duration of administration (acute behavioral test vs subchronic behavioral tests) as main factors revealed a significant main effect of TP dose (F(2,108) = 211, P < 0.001), duration of administration (F(1,108) = 1418, P < 0.001), and age (F(1,108) = 760, P < 0.001), with strong interactions between testosterone dose × duration of administration (F(2,108) = 292, P < 0.001), TP dose × age (F(2,108) = 55.8, P < 0.001), duration of administration × age (F(1,108) = 2832, P < 0.001), and TP × duration of administration × age (F(2,108) = 966, P < 0.001).
Figure 2.
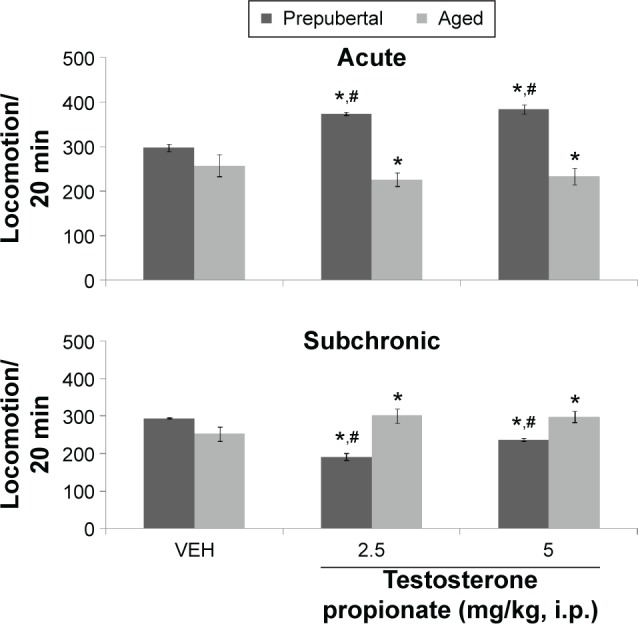
Effects of TP on open-field locomotor activity.
Notes: Each bar represents mean ± SEM; *P < 0.05 vs VEH and #P < 0.05 prepubertal vs aged.
Abbreviation: VEH, vehicle.
Figure 3.
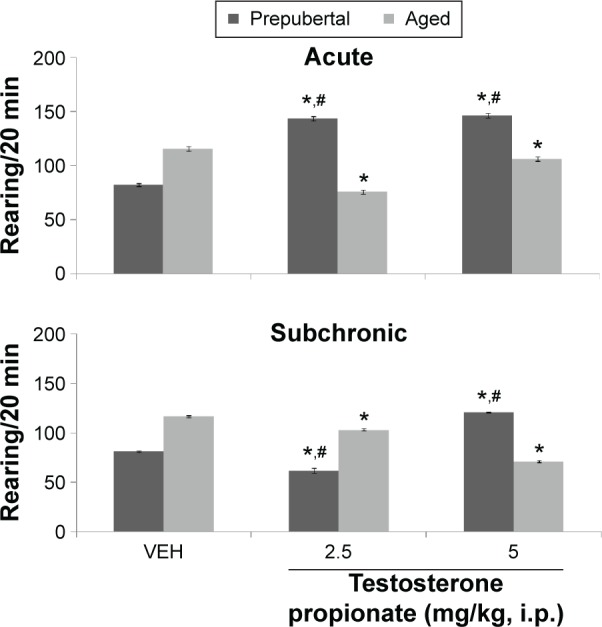
Effects of TP on rearing activity.
Notes: Each bar represents mean ± SEM; *P < 0.05 vs VEH and #P < 0.05 prepubertal vs aged.
Abbreviation: VEH, vehicle.
Figure 4.
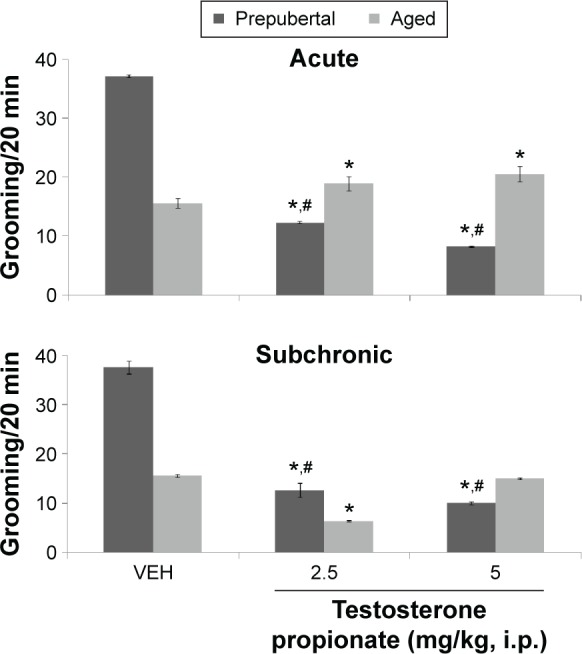
Effects of TP on grooming.
Notes: Each bar represents mean ± SEM; *P < 0.05 vs VEH and #P < 0.05 prepubertal vs aged.
Abbreviation: VEH, vehicle.
Pairwise comparisons of testosterone dose against vehicle following behavioral tests conducted after first dose (hereafter referred to as acute behavioral tests) of either vehicle or TP revealed a significant increase in horizontal locomotion at 2.5 mg/kg of TP (t(18) = 43.92, P < 0.001) and 5 mg/kg of TP (t(18) = 36.54, P < 0.001) in prepubertal mice and a significant reduction at 2.5 mg/kg of TP (t(18) = 6.88, P < 0.001) and 5 mg/kg of TP (t(18) = 9.07, P < 0.001) in aged mice compared to corresponding vehicle groups. Tests conducted after last dose of vehicle or testosterone (hereafter referred to as subchronic behavioral tests) revealed a significant decrease in locomotor activity at 2.5 mg/kg of TP (t(18) = 43.66, P < 0.001) and 5 mg/kg of TP (t(18) = 8.67, P < 0.001) in prepubertal mice and a significant increase at 2.5 mg/kg of TP (t(18) = 34.23, P < 0.001) and 5 mg/kg of TP (t(18) = 10.15, P < 0.001) in aged mice compared to corresponding vehicle-treated groups.
Pairwise comparison of age-related effects revealed a significant increase in locomotor activity at 2.5 mg/kg of TP (t(18) = 60.67, P < 0.001) and 5 mg/kg of TP (t(18) = 37.64, P < 0.001) in prepubertal mice compared to aged mice with acute behavioral tests and a significant decrease at 2.5 mg/kg of TP (t(18) = 56.43, P < 0.001) and 5 mg/kg of TP (t(18) = 4.63, P < 0.002) with subchronic behavioral tests; however, there was no significant difference in the groups administered vehicle in either prepubertal or aged animals. Comparison of effects due to duration of administration (acute behavioral test vs subchronic behavioral tests) revealed a significant increase in locomotor activity at 2.5 mg/kg of TP (t(18) = 75.11, P < 0.001) and 5 mg/kg of TP (t(18) = 27.53, P < 0.001; acute vs subchronic behavioral tests) in prepubertal mice and a significant decrease in locomotor activity at 2.5 mg/kg of TP (t(18) = 37.16, P < 0.001) and 5 mg/kg of TP (t(18) = 2.16, P < 0.045; acute vs subchronic behavioral tests) in aged mice.
Figure 3 shows the effect of TP on rearing activity. MANOVA revealed a significant main effect of TP dose (F(2,108) = 18.6, P < 0.001), duration of administration (F(1,108) = 78.9, P < 0.001), and age (F(1,108) = 41.4, P < 0.001), with strong interactions between TP × duration of administration (F(2,108) = 13.4, P < 0.006), TP dose × age (F(2,108) = 121, P < 0.001), duration of administration × age (F(1,108) = 35.6, P < 0.001), and TP dose × duration of administration × age (F(2,108) = 67.1, P < 0.001). Pairwise comparison of testosterone dose against vehicle following behavioral tests conducted after first dose (acute behavioral tests) of either vehicle or TP revealed a significant increase in rearing at 2.5 (t(18) = 25.92, P < 0.001) and 5 mg/kg (t(18) = 26.40, P < 0.001) in prepubertal mice and a decrease at 2.5 (t(18) = 10.82, P < 0.001) and 5 mg/kg (t(18) = 3.30, P < 0.004) in aged mice compared to corresponding vehicle-treated groups. Tests conducted after last dose (subchronic behavioral tests) of vehicle or TP showed an decrease in rearing at 2.5 (t(18) = 31.68, P < 0.001) and an increase at 5 mg/kg (t(18) = 41.79, P < 0.001) in prepubertal mice and a decrease at 2.5 (t(18) = 5.33, P < 0.005) and 5 mg/kg (t(18) = 3.41, P < 0.031) in aged mice compared to corresponding vehicle groups.
Comparison of age-related effects revealed a significant increase in rearing activity at 2.5 (t(18) = 18.38, P < 0.001) and 5 mg/kg (t(18) = 13.83, P < 0.001) of TP in prepubertal mice compared to aged mice with acute behavioral tests and a significant decrease at 2.5 (t(18) = 8.31, P < 0.001) and an increase at 5 mg/kg (t(18) = 43.31, P < 0.001) in prepubertal compared to aged mice following subchronic behavioral tests; however, there was no significant difference in the groups administered vehicle in either prepubertal or aged animals. Comparison of effects due to duration of administration (acute behavioral test vs subchronic behavioral tests) revealed a significant increase in rearing activity at 2.5 (t(18) = 19.61, P < 0.001) and 5 mg/kg (t(18) = 11.67, P < 0.001; acute vs subchronic behavioral tests) in prepubertal mice. A significant decrease in rearing activity at 2.5 (t(18) = 8.48, P < 0.001) and an increase at 5 mg/kg (t(18) = 16.22, P < 0.001; acute vs subchronic behavioral tests) was seen in aged mice.
Figure 4 shows the effect of TP on grooming behavior. MANOVA revealed a significant main effect of TP dose (F(2,108) = 4253, P < 0.001), duration of administration (F(1,108) = 202, P < 0.001), and age (F(1,108) = 1378, P < 0.001), with strong interactions between TP dose × duration of administration (F(2,108) = 246, P < 0.006), TP dose × age (F(2,108) = 4127, P < 0.001), duration of administration × age (F(1,108) = 938, P < 0.001), and TP dose × duration of administration × age (F(2,108) = 210, P < 0.001). Pairwise comparisons of the effect of testosterone dose against vehicle in behavioral tests after first dose (acute behavioral tests) of either vehicle or TP revealed a significant decrease in grooming at 2.5 (t(18) = 70.94, P < 0.001) and at 5 mg/kg (t(18) = 94.04, P < 0.001) in prepubertal mice and an increase at 2.5 (t(18) = 8.58, P < 0.001) and 5 mg/kg (t(18) = 11.31, P < 0.001) in aged mice compared to corresponding vehicle-treated groups. Tests conducted after the last dose (subchronic behavioral tests) of either vehicle or TP revealed a significant decrease in grooming at 2.5 (t(18) = 72.17, P < 0.001) and 5 mg/kg (t(18) = 77.25, P < 0.001) in prepubertal mice and at 2.5 mg/kg in aged mice compared to corresponding vehicle-treated groups. However, in aged mice at 5 mg/kg, no significant difference was seen.
Comparison of age-related effects revealed a significant increase in grooming in prepubertal compared to aged mice at baseline (vehicle) following both acute and subchronic behavioral tests. Administration of testosterone decreased grooming at 2.5 (t(18) = 23.67, P < 0.001) and 5 mg/kg (t(18) = 41.00, P < 0.001) in prepubertal mice compared to aged mice with acute behavioral tests and resulted in a significant increase at 2.5 (t(18) = 22.56, P < 0.001) and a decrease at 5 mg/kg (t(18) = 4.74, P < 0.002) in prepubertal compared to aged mice with subchronic behavioral tests. The significant difference seen previously is also reflected in a significant mean absolute difference measured when grooming scores of vehicle-treated prepubertal or aged mice were subtracted from corresponding age-matched grooming scores following TP at either 2.5 mg/kg (22.54 prepubertal vs −3 aged) or 5 mg/kg (26.27 prepubertal vs −4.45 aged) following acute behavioral tests and at 2.5 mg/kg (22.72 prepubertal vs 8.36 aged) or 5 mg/kg (25.10 prepubertal vs 0.55 aged) with subchronic behavioral tests. Comparison of effects due to duration of administration (acute behavioral test vs subchronic behavioral tests) revealed a significant decrease in grooming at 5 mg/kg (t(18) = 29.00, P < 0.001; acute vs subchronic behavioral tests) in prepubertal mice and a significant increase in grooming at 2.5 (t(18) = 51.51, P < 0.001) and 5 mg/kg (t(18) = 17.90, P < 0.001; acute vs subchronic behavioral tests) in aged mice.
Effect of TP on spatial working memory tasks
Figures 5 shows the effects of TP on arm entry before first error in the radial-arm maze. MANOVA revealed a significant main effect of TP dose (F(3,144) = 278, P < 0.001), duration of administration (F(1,144) = 11.8, P < 0.001), and age (F(1,144) = 11.8, P < 0.001), with strong interactions between TP dose × duration of administration (F(3,144) = 12.7, P < 0.001), TP dose × age (F(3,144) = 43.00, P < 0.001), and TP dose × duration of administration × age (F(3,144) = 44.5 P < 0.001), with no significant interaction of duration of administration × age (F(1,144) = 0.240, P < 0.626). Pairwise comparisons of the effect of testosterone dose against vehicle in behavioral tests conducted after first dose (acute behavioral tests) of vehicle, scopolamine control, or TP revealed significant decrease in radial-arm maze memory task scores (measured as number of arm entry before first error) in groups administered scopolamine (animal model of memory loss; t(18) = 5.69, P < 0.001) and an increase at 2.5 mg/kg (t(18) = 8.50, P < 0.001) in prepubertal mice, while in aged mice, there was a significant decrease in the scopolamine control group score (t(18) = 3.97, P < 0.001) and a significant increase at 5 mg/kg (t(18) = 13.38, P < 0.001) compared to corresponding vehicle groups. At 5 mg/kg (prepubertal) and 2.5 mg/kg (aged), no significant difference was seen. In comparison to scopolamine control however, there was a significant increase in radial-arm maze memory task scores at 2.5 (t(18) = 15.29, P < 0.001; t(18) = 3.35, P < 0.004) and 5 mg/kg (t(18) = 5.30, P < 0.001; t(18) = 15.25, P < 0.001) in prepubertal and aged mice, respectively. Tests conducted after last dose (subchronic behavioral tests) of vehicle, scopolamine, or TP revealed a significant decrease in radial-arm maze memory task score in groups administered scopolamine (t(18) = 5.66, P < 0.001; t(18) = 4.99, P < 0.001) and an increase at 2.5 (t(18) = 15.56, P < 0.001; t(18) = 6.53, P < 0.001) and 5 mg/kg (t(18) = 13.80, P < 0.001; t(18) = 5.82, P < 0.001) in both prepubertal and aged mice, respectively. In comparison to scopolamine control, radial-arm memory task scores also increased significantly at 2.5 (t(18) = 21.21, P < 0.001; t(18) = 15.91, P < 0.001) and 5 mg/kg (t(18) = 18.60, P < 0.001; t(18) = 12.32, P < 0.001) in both prepubertal and aged mice, respectively. Pairwise comparison of age-related effects revealed a significant increase in radial-arm maze memory task scores at 2.5 mg/kg (t(18) = 9.26, P < 0.001) and a decrease at 5 mg/kg (t(18) = 17.40, P < 0.001) in prepubertal mice compared to aged mice after acute behavioral tests, and no significant difference seen at any doses of TP after subchronic behavioral tests. There was no significant difference in the groups administered vehicle (baseline) in either prepubertal or aged animals. Comparison of effects due to duration of administration (acute behavioral test vs subchronic behavioral tests) revealed a significant decrease in memory task scores at 5 mg/kg (t(18) = 10.61, P < 0.001; acute vs subchronic) in prepubertal mice. A significant decrease in memory task score was seen at 2.5 mg/kg (t(18) = 51.51, P < 0.001) and an increase at 5 mg/kg (t(18) = 3.39, P < 0.003) in aged mice.
Figure 5.
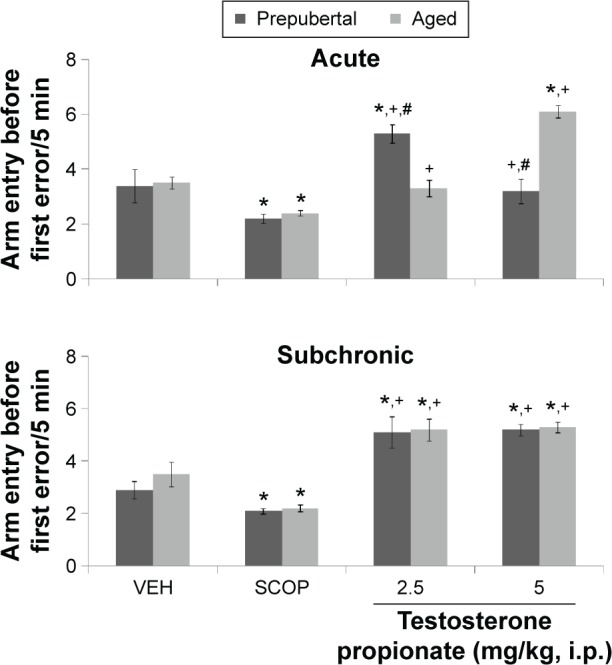
Effects of TP on radial-arm maze memory task.
Notes: Each bar represents mean ± SEM; *P < 0.05 vs VEH, +P < 0.05 vs SCOP, and #P < 0.05 prepubertal vs aged.
Abbreviations: VEH, vehicle; SCOP, scopolamine.
Figure 6 shows the effects of TP on percentage alternation in the Y-maze. MANOVA revealed a significant main effect of TP dose (F(3,144) = 1850, P < 0.001), duration of administration (F(1,144) = 293, P < 0.001), and age (F(1,144) = 309, P < 0.001), with strong interactions between TP dose × duration of administration (F(2,144) = 51.8, P < 0.001), TP dose × age (F(2,144) = 758, P < 0.001), duration of administration × age (F(2,144) = 174, P < 0.001), and TP dose × duration of administration × age (F(3,144) = 99.4, P < 0.001). Pairwise comparisons of the effect of testosterone dose and vehicle in behavioral tests conducted after first dose (acute behavioral tests) of vehicle, scopolamine, or TP groups revealed a significant decrease in Y-maze memory task scores (measured as % alternation) in groups administered scopolamine (t(18) = 7.17, P < 0.001) and an increase at 2.5 mg/kg of TP (t(18) = 4.55, P < 0.001) and 5 mg/kg of TP (t(18) = 19.52, P < 0.001) in prepubertal mice; it also showed a significant decrease at 2.5 (t(18) = 49.60, P < 0.001) and 5 mg/kg (t(18) = 10.84, P < 0.001) of TP in aged mice compared to respective vehicle administered groups. In comparison to scopolamine control, there was a significant increase in Y-maze memory task scores at 2.5 (t(18) = 11.85, P < 0.001; t(18) = 25.62, P < 0.001) and 5 mg/kg (t(18) = 23.69, P < 0.001; t(18) = 33.70, P < 0.001) in both prepubertal and aged mice, respectively. Tests conducted after the last dose (subchronic behavioral tests) of vehicle, scopolamine, or TP revealed a significant decrease in memory task scores in groups administered scopolamine (t(18) = 19.40, P < 0.001; t(18) = 76.55, P < 0.001) and an increase at 2.5 mg/kg of TP (t(18) = 20.44, P < 0.001; t(18) = 42.98, P < 0.001) and 5 mg/kg of TP (t(18) = 13.48, P < 0.001; t(18) = 24.70, P < 0.001) in pre-pubertal mice and aged mice, respectively, when compared to vehicle. In comparison to scopolamine control however, there was a significant increase in Y-maze memory tasks scores at 2.5 (t(18) = 47.84, P < 0.001; t(18) = 21.35, P < 0.001) and 5 mg/kg (t(18) = 37.93, P < 0.001; t(18) = 32.87, P < 0.001) in prepubertal and aged mice, respectively. Pairwise comparison of age-related effects revealed a significant decrease in Y-maze memory tasks at 2.5 (t(18) = 7.41, P < 0.001) and 5 mg/kg (t(18) = 3.08, P < 0.007) in prepubertal mice compared to aged mice with acute behavioral tests and a significant increase at 2.5 (t(18) = 27.26, P < 0.001) and 5 mg/kg (t(18) = 4.24, P < 0.001) in prepubertal mice compared to aged mice with subchronic behavioral tests. There was no significant difference in the groups administered vehicle in either prepubertal or aged mice. Comparison of effects due to duration of administration of TP (acute behavioral test vs subchronic behavioral tests) revealed a significant increase in Y-maze memory task scores at 2.5 (t(18) = 19.38, P < 0.001) and a decrease at 5 mg/kg (t(18) = 9.42, P < 0.001; acute vs subchronic) in prepubertal mice and a significant increase in memory task sores at 2.5 (t(18) = 15.59, P < 0.001) and 5 mg/kg (t(18) = 13.58, P < 0.003; acute vs subchronic) in aged mice.
Figure 6.
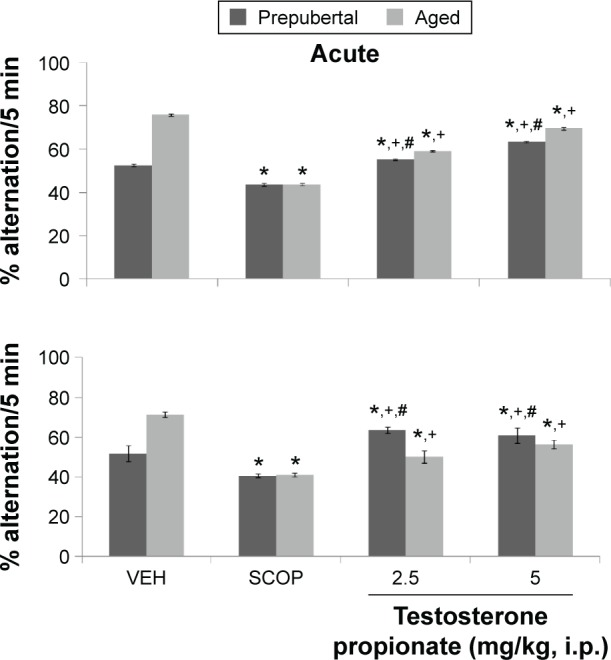
Effects of TP on Y-maze memory task.
Notes: Each bar represents mean ± SEM; *P < 0.05 vs VEH, +P < 0.05 vs SCOP, and #P < 0.05 prepubertal vs aged.
Abbreviations: VEH, vehicle; SCOP, scopolamine.
Effect of TP on anxiety behavior
Figure 7 shows the effect of TP on percentage time spent in the open arm of the EPM. MANOVA revealed a significant main effect of TP dose (F(3,144) = 21,962, P < 0.001), duration of administration (F(1,144) = 1439, P < 0.001), and age (F(1,144) = 547, P < 0.001), with strong interactions between TP dose × duration of administration (F(2,144) = 712, P < 0.001), TP dose × age (F(2,144) = 975, P < 0.001), duration of administration × age (F(2,144) = 309, P < 0.001), and TP dose × duration of administration × age (F(3,144) = 2015, P < 0.001). Pairwise comparisons of the effect of testosterone and vehicle in behavioral tests carried out after first dose (acute behavioral tests) of vehicle, diazepam, or TP showed a significant increase in percentage open arm time in groups of animals administered diazepam (an animal model of anxiolysis) at (t(18) = 90.02, P < 0.001) and following TP at 2.5 (t(18) = 35.18, P < 0.001) with a decrease at 5.0 mg/kg (t(18) = 5.4, P < 0.001) in prepubertal mice. In aged mice, there was a significant increase in open arm time with diazepam (t(18) = 112.75, P < 0.001) and following TP at 5 mg/kg (t(18) = 67.10, P < 0.001), at 2.5 mg/kg no significant difference was seen. Tests carried out after last dose (subchronic behavioral tests) of vehicle, diazepam, or TP revealed a significant increase in open arm time in groups administered diazepam (t(18) = 127.39, P < 0.001; t(18) = 97.94, P < 0.001) and at 2.5 mg/kg (t(18) = 5,43, P < 0.001; t(18) = 8.79, P < 0.001) of TP in both prepubertal and aged mice, respectively. At 5 mg/kg, there was a significant increase in open arm time for prepubertal mice (t(18) = 5.43, P < 0.001) and a decrease for aged mice (t(18) = 11.40, P < 0.001). In comparison to diazepam control, open arm time decreased at 2.5 mg/kg (t(18) = 73.50, P < 0.001; t(18) = 150.43, P < 0.001) and 5 mg/kg (t(18) = 105.75, P < 0.001; t(18) = 165.22, P < 0.001) in prepubertal and aged mice, respectively, following acute behavioral tests. Following subchronic behavioral tests, open arm time also decreased at 2.5 mg/kg (t(18) = 117.22, P < 0.001; t(18) = 126.84, P < 0.001) and 5 mg/kg (t(18) = 117.22, P < 0.001; t(18) = 165.22, P < 0.001) compared to respective diazepam-treated groups. Pairwise comparison of age-related effects revealed a significant increase in percentage time spent in the open arm at 2.5 (t(18) = 48.38, P < 0.001; t(18) = 15.29, P < 0.001) and a decrease at 5 mg/kg (t(18) = 75.89, P < 0.002; t(18) = 15.28, P < 0.001) in prepubertal mice compared to aged mice following both acute and subchronic behavioral tests, respectively. There was no significant difference in the groups administered vehicle, either prepubertal or aged. Comparison of effects due to duration of administration (acute behavioral test vs subchronic behavioral tests) revealed a significant increase in open arm time at 2.5 (t(18) = 45.17, P < 0.001) and a decrease at 5 mg/kg (t(18) = 10.34, P < 0.001; acute vs subchronic tests) in prepubertal mice. A significant decrease in open arm time at 2.5 (t(18) = 48.38, P < 0.001) and an increase at 5 mg/kg (t(18) = 93.34, P < 0.001; acute vs subchronic tests) was seen in aged mice.
Figure 7.
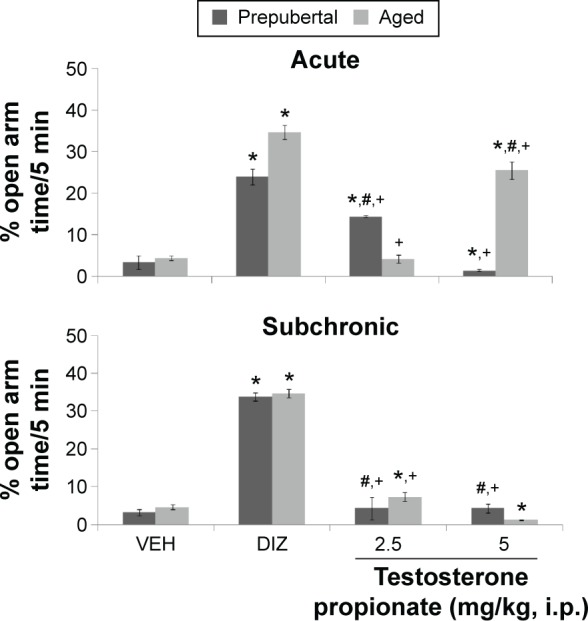
Effects of TP on anxiety behavior in the elevated plus maze.
Notes: Each bar represents mean ± SEM, *P < 0.05 vs VEH, +P < 0.05 vs DIZ, and #P < 0.05 prepubertal vs aged.
Abbreviations: VEH, vehicle; DIZ, diazepam.
Effect of TP on locomotor activity in the Y-maze, radial-arm maze, and EPM
Figures 8–10 show the effect of TP on locomotor activity measured as total arm entry in the Y- and radial-arm maze and percentage time spent in the closed arm in the EPM, respectively.
Figure 8.
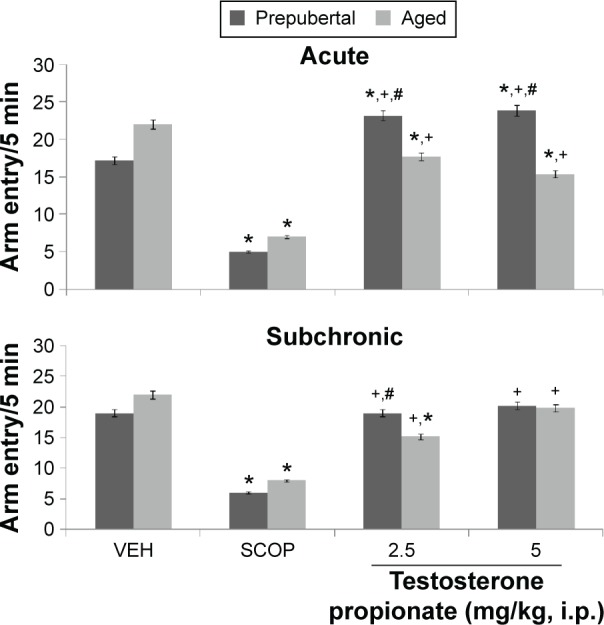
Effects of TP on Y-maze total arm entry.
Notes: Each bar represents mean ± SEM, *P < 0.05 vs VEH, #P < 0.05 vs prepubertal vs aged, and +P < 0.05 vs SCOP.
Abbreviations: VEH, vehicle; SCOP, scopolamine.
Figure 9.
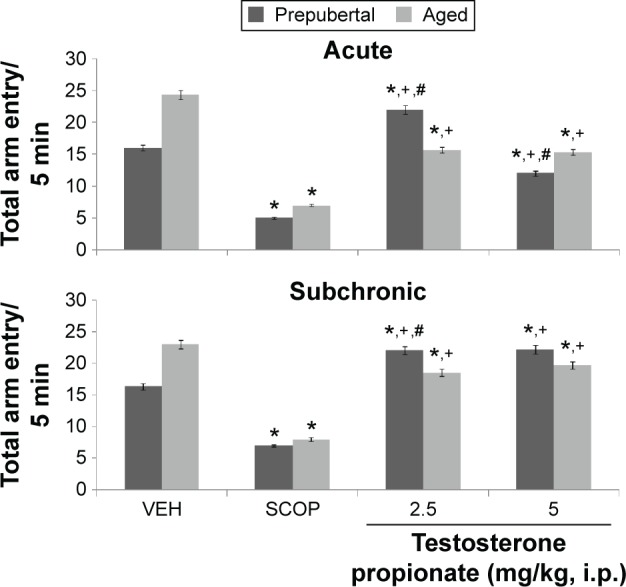
Effects of TP on radial-arm maze total arm entry.
Notes: Each bar represents mean ± SEM, *P < 0.05 vs VEH, #P < 0.05 vs prepubertal vs aged, and +P < 0.05 vs SCOP.
Abbreviations: VEH, vehicle; SCOP, scopolamine.
Figure 10.
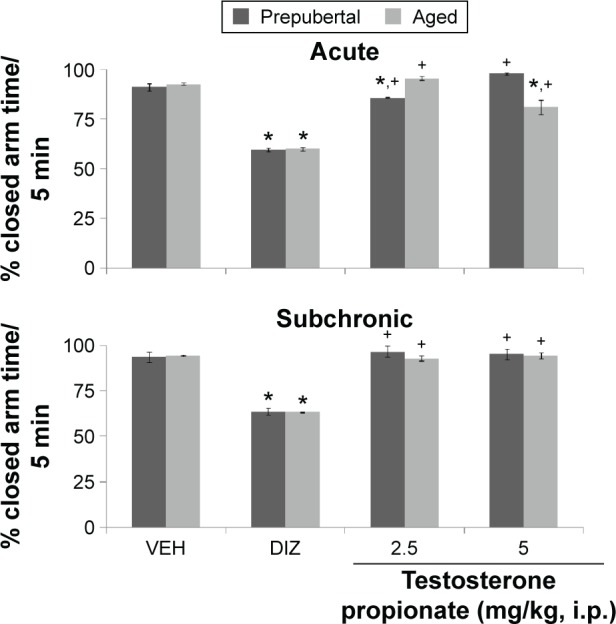
Effects of TP on percentage closed arm time.
Notes: Each bar represents mean ± SEM, *P < 0.05 vs VEH, #P < 0.05 vs prepubertal vs aged, and +P < 0.05 vs DIZ.
Abbreviations: VEH, vehicle; DIZ,diazepam.
Following exploration in the Y-maze, MANOVA revealed a significant main effect of TP dose (F(2,144) = 110.6, P < 0.001), duration of administration (F(1,144) = 31.5, P < 0.012), and age (F(1,144) = 123.54, P < 0.002), with strong interactions between TP dose × duration of administration (F(2,144) = 932, P < 0.001), TP dose × age (F(2,108) = 586, P < 0.001), duration of administration × age (F(2,144) = 195, P < 0.001), and TP dose × duration of administration × age (F(2,144) = 989, P < 0.001). Y-maze total arm entry decreased significantly with administration of scopolamine (t(18) = 189.53, P < 0.001; t(18) = 178.33, P < 0.001) in both prepubertal and aged mice. In prepubertal mice, Y-maze total arm entry increased significantly at 2.5 mg/kg of TP (t(18) = 13.53, P < 0.001) and 5 mg/kg of TP (t(18) = 16.11, P < 0.001) and decreased at 2.5 (t(18) = 15.52, P < 0.001) and 5.0 mg/kg (t(18) = 26.33, P < 0.001) in aged mice compared to respective vehicle-treated groups following acute behavioral tests. With subchronic behavioral tests, arm entry decreased significantly at 2.5 mg/kg (t(18) = 24.79, P < 0.001) in aged mice and showed no significant difference at 5 mg/kg in aged mice or any of the doses in prepubertal mice. In comparison to scopolamine control, there was a significant increase in Y-maze total arm entry at 2.5 (t(18) = 41.15, P < 0.001; t(18) = 65.32, P < 0.001) and 5 mg/kg (t(18) = 3.98, P < 0.011; t(18) = 73.10, P < 0.001) in both prepubertal and aged mice, respectively, following both acute and subchronic behavioral tests. Pairwise comparisons of the effects of age revealed significant increase at 2.5 (t(18) = 18.44, P < 0.001) and 5 mg/kg (t(18) = 29.85, P < 0.001) with acute behavioral test and at 2.5 mg/kg (t(18) = 12.04, P < 0.001) with subchronic behavioral tests in prepubertal compared to aged mice.
Following exploration in the radial-arm maze, MANOVA revealed a significant main effect of TP dose (F(2,144) = 125.00, P < 0.001), duration of administration (F(1,144) = 523, P < 0.001), and age (F(1,144) = 52.63, P < 0.012), with strong interactions between TP dose × duration of administration (F(2,144) = 58.4, P < 0.001), TP dose × age (F(2,144) = 156, P < 0.001), duration of administration × age (F(2,144) = 75.2, P < 0.001), and TP dose × duration of administration × age (F(2,144) = 45.2, P < 0.001). Total arm entry in the radial-arm maze decreased significantly with administration of scopolamine (t(18) = 155, P < 0.001; t(18) = 162, P < 0.001) in both prepubertal and aged mice. Radial-arm maze total arm entry increased significantly at 2.5 mg/kg (t(18) = 33.98, P < 0.001) and decreased at 5.0 mg/kg (t(18) = 14.00, P < 0.011) with acute behavioral tests in prepubertal mice while with aged mice, arm entry decreased at 2.5 (t(18) = 25.64, P < 0.001) and 5 mg/kg (t(18) = 38.01, P < 0.001). With subchronic behavioral tests, arm entry increased at 2.5 and 5 mg/kg (t(18) = 26.30, P < 0.001; t(18) = 42.43, P < 0.001), respectively, in prepubertal mice and decreased at 2.5 and 5 mg/kg (t(18) = 21.86, P < 0.001; t(18) = 45.12, P < 0.001), respectively, in aged mice. In comparison to scopolamine control, there was a significant increase in radial-arm maze total arm entry at 2.5 (t(18) = 55.25, P < 0.001; t(18) = 25.12, P < 0.001) and 5 mg/kg (t(18) = 54.00, P < 0.001; t(18) = 13.66, P < 0.001) in both prepubertal and aged mice, respectively, following both acute and subchronic behavioral tests. Pairwise comparisons of the effect of age revealed a significant increase in radial-arm maze total arm entry at 2.5 mg/kg (t(18) = 23.40, P < 0.001) and a decrease at 5.0 mg/kg (t(18) = 19.00, P < 0.001) following acute behavioral tests; with subchronic test, there was a significant increase at 2.5 and 5 mg/kg (t(18) = 14.33, P < 0.001 t(18) = 14.42, P < 0.001), respectively, in prepubertal compared to aged mice.
Following 5 minutes of exploration in the EPM, MANOVA revealed significant main effects of TP dose (F(3,144) = 21,007, P < 0.001), duration of administration (F(1,144) = 470, P < 0.011), and age (F(1,144) = 28.5, P < 0.001), with strong interactions between TP dose × duration of administration (F(3,144) = 165, P < 0.001), TP dose × age (F(3,144) = 585, P < 0.001), and TP dose × duration of administration × age (F(3,144) = 621 P < 0.001), with no significant interaction of duration of administration × age (F(1,144) = 5.8, P < 0.677). Percentage time in the closed arm of the EPM revealed a significant decrease following administration of diazepam (t(18) = 176.63, P < 0.001; t(18) = 82.60, P < 0.001) in prepubertal and aged mice, respectively. Closed arm time also increase at 2.5 (t(18) = 40.76, P < 0.001) and 5 mg/kg (t(18) = 12.97, P < 0.001) in prepubertal mice and at 5.0 mg/kg (t(18) = 35.55, P < 0.001) in aged mice with acute behavioral tests. Following subchronic behavioral tests, percentage time in the closed arm decreased in the groups administered diazepam (t(18) = 163.29, P < 0.001; t(18) = 95.69, P < 0.001) in prepubertal and aged mice, respectively, while no significant difference was seen at either doses of TP in either prepubertal or aged mice compared to respective vehicle-treated groups. In comparison to diazepam-treated groups, closed arm time increased at 2.5 mg/kg (t(18) = 123.04, P < 0.001; t(18) = 68.25, P < 0.001) and 5 mg/kg (t(18) = 165.75, P < 0.001; t(18) = 92.30, P < 0.001) in prepubertal and aged mice, respectively, following acute behavioral tests. Following subchronic behavioral tests, closed arm time also increased at 2.5 mg/kg (t(18) = 106.37, P < 0.001; t(18) = 109.34, P < 0.001) and 5 mg/kg (t(18) = 119.144, P < 0.001; t(18) = 155.39, P < 0.001) compared to respective diazepam-treated groups. Pairwise comparison of the effect of age showed no significant difference between prepubertal and aged mice at either of the doses.
Effect of TP on serum testosterone levels
Figure 11 shows the effect of subchronic intraperitoneal injection of TP on serum testosterone levels in prepubertal and aged mice. Two-factor ANOVA revealed a significant main effect of testosterone dose (F(2,18) = 319, P < 0.001), age (F(1,18) = 91.0, P < 0.001), and testosterone dose vs age interaction (F(2,18) = 22.6, P < 0.001). Serum testosterone levels increased (F(2,18) = 319, P < 0.001) at both doses of TP in both prepubertal and aged mice. Comparing effects seen in prepubertal to aged mice revealed significant increase at 2.5 mg/kg (t(6) = 8.25, P < 0.002) and 5.0 mg/kg (t(6) = 5.99, P < 0.010) in aged mice compared to prepubertal mice.
Figure 11.
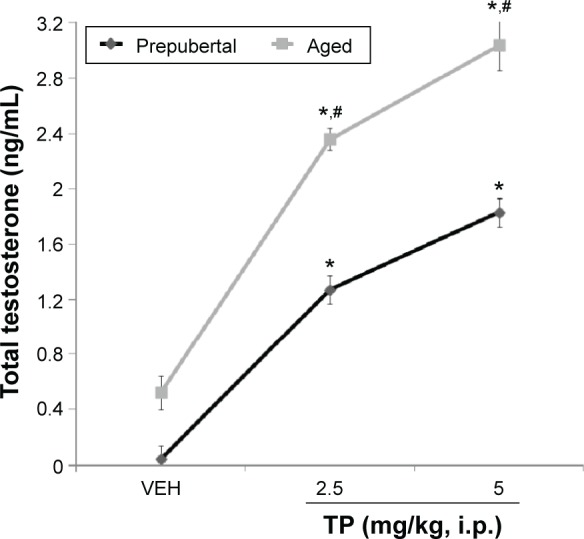
Effects of TP on serum testosterone level.
Notes: Each bar represents mean ± SEM; *P < 0.05 vs VEH and #P < 0.05 prepubertal vs aged.
Abbreviation: VEH, vehicle.
Discussion
Endogenous or exogenous testosterone influences brain behavior and chemistry through mechanisms that are both due to its organizational and activational effects.38 Concerning its effects, a number of human and animal studies have been published, with several conflicting and puzzling results largely due to variability in dosing, route of administration, state of the gonads, gender, and timing, all of which could considerably alter outcome.38 We set out to investigate the effects exogenous testosterone in gonadally-intact prepubertal and aged male mice with a view to testing the hypothesis that aging modulates the influence of exogenous testosterone on neurobehavior and serum testosterone levels.
The results of this study revealed alterations in behavioral response of gonadally intact male mice to i.p. injection of TP, which were influenced not only by age (prepubertal vs aged) but also by duration of administration (effects seen when behavioral tests were conducted after 1st dose compared to after the 21st dose of TP [acute versus subchronic]). The prepubertal period in male rodents extend from PNDs 28 to 70.39,40 This period however can be characterized using varying physiological parameters, such as physical features, levels of sex steroid, the attainment of sexual maturity, and reproductive capability.41 These variations make comparisons of research results difficult.
In this study, all animals administered TP gained weight over the 21-day period, although prepubertal mice gained more weight than aged mice at both doses. Studies have shown that administration of androgenic steroids could result in weight gain.42,43 The results seen in aged mice corroborate the results by Davies et al,44 who reported a decrease in weight in male obese Zucker rats following testosterone supplementation. Several human studies have also reported weight loss in elderly men on hormone replacement therapy.45 Testosterone therapy has been associated with weight reduction and increase in lean body mass,46,47 in obese elderly men with primary hypogonadism on hormone replacement therapy. Weight changes seen in prepubertal mice could be attributable to prepubertal growth spurts.48 In aged mice, a decrease in total body fat may be a plausible explanation for the changes seen, as an inverse association has been described between levels of testosterone and accumulation of body fat.49,50
Testosterone (endogenous or exogenous) has been reported to exert several behavioral traits in both human and animal studies,51 and these effects are mediated not only through the androgen or estrogen receptors but also through rapid nongenomic effects.52 In this study, administration of testosterone resulted in increase in horizontal locomotion and rearing in prepubertal mice with acute administration and a decrease with repeated administration, while in aged mice, the reverse was the case. Grooming behavior however showed a decrease in prepubertal mice and an increase in aged mice with acute administration, while with repeated administration, it decreased in both prepubertal and aged mice. Results of behavioral tests after acute TP administration in prepubertal mice and repeated administration of TP in aged mice point to cerebral cortical stimulation. It was also observed that repeated administration of testosterone in prepubertal rats had inhibitory effects on cerebral cortical stimulation. Central excitatory effect seen in prepubertal mice after acute administration could have been mediated via glutamatergic and dopaminergic systems. A few studies have indicated that testosterone supplementation particularly within the adolescence period may regulate dopamine neurotransmission.53 Purves-Tyson54 reported that exogenous testosterone during adolescence (between PNDs 45 and 60) in male rats increased dopamine synthesis and stimulated expression of dopamine receptor 2 messenger RNA (DR2 mRNA) in the midbrain. Dopaminergic transmission in the nucleus accumbens may have increased with testosterone supplementation since increase in dopaminergic transmission in the nucleus accumbens is associated with locomotor hyperactivity and increases in the caudate nucleus with increased rearing activity.55 Results of repeated administration however suggests possible upregulation of the Gamma aminobutyric acid (GABA) systems, which could be direct, as seen when testosterone supplementation in postnatal female rats resulted in upregulation of α2 subunit of the GABA receptor subunits in the cerebral cortex,56 or indirect via the effect of serotonin receptor in the amygdala, which have been shown to potentiate GABAergic inhibitory interneurons, resulting in central inhibition of neuronal activity.57 However, how TP specifically interacts with dopaminergic, glutamatergic, and serotonergic systems to modify novelty-induced behaviors in rodents is still a source of continuous research.
Testosterone has been reported to influence spatial cognition in male rats,58,59 especially in studies that have been modeled using reward or task-motivated paradigms, however, spatial-working memory model used in our study capitalizes on the propensity of mice to navigate toward novel spatial environments and remember where they have last visited. Numerous studies have documented the gender-related effects of testosterone on memory in rodents.23,58 Some of these studies involved spatial memory tasks that compared the performance of castrated male animals given TP to that of females, with many concluding that males tend to outperform females.60,61 Few studies have however tried to evaluate age-related differences in response among males. A number of human and rodent studies have reported that testosterone supplementation improves spatial working memory,62,63 while some reported no effect.23 In this study, working memory in the radial-arm maze increased in both prepubertal and aged mice, with prepubertal mice performing better than aged mice at the lower dose and aged mice performing better at the higher dose, this effect was sustained with repeated administration. Y-maze spatial working memory increased in prepubertal mice and showed no significant improvements in aged mice. Our results corroborate those by Spritzer et al,50 where a disparity in working memory effect of testosterone is seen with respect to model used, pointing to the possibility of task-dependent response with testosterone. Variations in accuracy of working memory measurement by the two tasks may also be responsible, although it is accepted that radial-arm reentry errors are a better index of working memory; other reasons may include age-related differences in receptor modulation effects of testosterone and its metabolites.60 Many of the models that have been used to study spatial memory including the radial-arm maze and Y-maze rely heavily on hippocampal function.15,62 Though the mechanisms underlying sex steroid effects on spatial memory are not entirely clear, both exogenous estradiol and testosterone have been shown to alter pyramidal spine density and connectivity in the cornus ammonis 1 region and possible cornus ammonis 3 in the hippocampus.15,62 However, higher endogenous testosterone levels have been associated with increased pyramidal cell activity in the amygdala and also with improved memory.64
Anxiety is reported to be the most sensitive behavioral response to testosterone.35 In this study, dose-related anxiolysis was noticed in both prepubertal and aged mice, although the anxiolytic effect reported was significantly less than that following administration of diazepam. Repeated administration of testosterone showed mixed responses. A number of researchers have investigated the effects of endogenous and exogenous testosterone on anxiety-related behaviors in rodents and humans,52,58,65–69 most of these studies reported anxiolytic effects of testosterone. Aikey et al69 reported anxiolysis in male mice following endogenous and exogenous testosterone in the EPM, although this effect was dose dependent. Fernandez-Guasti and Martinez-Mota68 also reported anxiolysis, this time in the marble-burying behavior test, while Van-Honk et al67 reported anxiolytic effects of testosterone administration in women. The results obtained in the previous studies are similar to what we observed in our study, a dose-related anxiolytic effect following acute behavioral tests. This result also corroborates what was reported by Frye et al,66 who observed anxiolytic effects in a number of anxiety-related behavioral paradigms following acute administration of testosterone in aged intact, male mice. Overall, with continuous administration of TP, mice spent less time in the open arm irrespective of their age. Anxiogenic effect of nandrolone, a synthetic testosterone derivative, has been reported following chronic administration,57,70 while some other studies have reported either no response or anxiolytic effects in some behavioral paradigms, which are not replicated in others.51 Though testosterone may have the ability to reduce anxiety or increase it, its effects and mechanisms are still being studied, and different mechanisms are being proposed and investigated.
From our study, effects of testosterone on anxiety behaviors are modulated by age, dose of testosterone, and duration of administration. Possible mechanisms of testosterone action on anxiety (in our opinion) could be both genomic and nongenomic. The acute anxiolytic effect seen is possible through rapid nongenomic mechanisms, while the anxiogenic effects seen with continuous administration is possible through genomic mechanisms. Earlier studies have shown that the anxiogenic or anxiolytic effects of testosterone could be mediated via androgen or estrogen receptors and also via rapid nongenomic effects or via interactions with GABA benzodiazepines receptors.57,69 Hodosy et al51 were of the opinion that both exogenous and endogenous testosterones exhibited anxiolysis, and this effect was blocked by administration of flutamide. Flutamide also resulted in anxiolysis in the open-field box, suggesting the possibility of a nonlinear relationship between genomic effects of testosterone and anxiety.
In this study, administration of TP resulted in a concomitant increase in plasma levels of total testosterone in both prepubertal and aged mice compared to vehicle. The increase was significantly higher in aged mice compared to prepubertal mice; however, values obtained for prepubertal mice were significantly lower than values for mature noncastrated adult males (5.2 ng/mL) but higher than testosterone levels in age appropriate males (1.8 ± 0.9 ng/mL).71 Generally, the serum testosterone levels for a male mouse typically range from 1.7 to 14.4 ng/mL with a mean of 6.78 ng/mL.
Conclusion
Exogenous testosterone administration results in age-related neurobehavioral responses; however, more studies are needed to clarify the mechanisms that underlie them.
Footnotes
ACADEMIC EDITOR: Lora Watts, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 2,104 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: OJO and AYO. Analyzed the data: AYO and OJO. Wrote the first draft of the manuscript: AYO and TAO. Contributed to the writing of the manuscript: TAO, ATO, TSB, and TO. Agree with manuscript results and conclusions: OJO, AYO, TOA, ATO, TSB, and TO. Jointly developed the structure and arguments for the paper: OJO and AYO. Made critical revisions and approved final version: AYO and OJO. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Rommets FFG. Testosterone: an overview of biosynthesis, transport, metabolism and non-genomic actions. In: Nieschlage E, Behre HM, editors. Testosterone: Action, Deficiency, Substitution. 3rd ed. Cambridge: Cambridge University Press; 2004. pp. 1–38. [Google Scholar]
- 2.Losel R, Wehling M. Non genomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4(1):46–55. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- 3.Wood RI, Stanton SJ. Testosterone and sport: current perspectives. Horm Behav. 2012;61(1):147–155. doi: 10.1016/j.yhbeh.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 5.Denham BE. The Anabolic Steroid Control Act of 2004: a study in the political economy of drug policy. J Health Soc Policy. 2006;22(2):51–78. doi: 10.1300/J045v22n02_04. [DOI] [PubMed] [Google Scholar]
- 6.Holland-Hall C. Performance-enhancing substances: is your adolescent patient using? Pediatr Clin North Am. 2007;54(4):651–662. doi: 10.1016/j.pcl.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Hartgens F, Kuipers H. Effects of androgenic-anabolic steroids in athletes. Sports Med. 2004;34(8):513–554. doi: 10.2165/00007256-200434080-00003. [DOI] [PubMed] [Google Scholar]
- 8.Sagoe D, Molde H, Andreassen CS, Torsheim T, Pallesen S. The global epidemiology of anabolic-androgenic steroid use: a meta-analysis and meta-regression analysis. Ann Epidemiol. 2014;24(5):383–398. doi: 10.1016/j.annepidem.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Retana-Marquez S, Bonilla-Jaime H, Vazquez-Palacios G, Martinez-Garcia R, Velzquez-Moctezuma J. Changes in masculine sexual behaviour, corticosterone and testosterone in response to acute and chronic stress in male rats. Horm Behav. 2003;44:327–337. doi: 10.1016/j.yhbeh.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Taylor GT, Womack S, Weiss J, Pitha J. Behaviour and physiological effects of supplemental episodes of testosterone: its precursors and metabolite in rats. Life Sci. 1990;47:1965–1971. doi: 10.1016/0024-3205(90)90409-k. [DOI] [PubMed] [Google Scholar]
- 11.Locklear MN, Kritzer MF. Assessment of the effects of sex and sex hormones on spatial cognition in adult rats using the Barnes maze. Horm Behav. 2014;66(2):298–308. doi: 10.1016/j.yhbeh.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wobber V, Herrmann E. The influence of testosterone on cognitive performance in Bonobos and Chimpanzees. Behaviour. 2015;152(3–4):407–442. [Google Scholar]
- 13.Van Hout AJ, Pinxten R, Darras VM, Eens M. Testosterone increases repertoire size in an open-ended learner: an experimental study using adult male European starlings (Sturnus vulgaris) Horm Behav. 2012;62(5):563–568. doi: 10.1016/j.yhbeh.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL. Gonadal hormones masculinize and defeminize reproductive behaviours during puberty in the male Syrian hamster. Horm Behav. 2004;45(4):242–249. doi: 10.1016/j.yhbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Butera PC, Czaja JA. Effects of intracranial implants of dihydrotestosterone in the reproductive physiology and behaviour of male guinea pigs. Horm Behav. 1989;23:424–431. doi: 10.1016/0018-506x(89)90054-8. [DOI] [PubMed] [Google Scholar]
- 16.Gibbs RB. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm Behav. 2005;48(3):268–277. doi: 10.1016/j.yhbeh.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macció DR, Calfa G, Roth GA. Oral testosterone in male rats and the development of experimental autoimmune encephalomyelitis. Neuroimmunomodulation. 2005;12(4):246–254. doi: 10.1159/000085656. [DOI] [PubMed] [Google Scholar]
- 18.Purves-Tyson TD, Boerrigterb D, Allen K, et al. Testosterone attenuates and the selective oestrogen receptor modulator, raloxifene, potentiates amphetamine-induced locomotion in male rats. Horm Behav. 2015;70:73–84. doi: 10.1016/j.yhbeh.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Khakpai F. The effect of opiodergic system and testosterone on anxiety behavior in gonadectomized rats. Behav Brain Res. 2014;15(263):9–15. doi: 10.1016/j.bbr.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Bayless DW, Darling JS, Daniel JM. Mechanisms by which neonatal testosterone exposure mediates sex differences in impulsivity in prepubertal rats. Horm Behav. 2013;64(5):764–769. doi: 10.1016/j.yhbeh.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Hawley WR, Grissom EM, Martin RC, Halmos MB, Bart CLS, Dohanich GP. Testosterone modulates spatial recognition memory in male rats. Horm Behav. 2013;63(4):559–565. doi: 10.1016/j.yhbeh.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Jianxin J, Lin K, Sha L, et al. Amelioratory effects of testosterone treatment on cognitive performance deficits induced by soluble Aβ1–42 oligomers injected into the hippocampus. Horm Behav. 2013;64(3):477–486. doi: 10.1016/j.yhbeh.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Spritzer MD, Fox EC, Larsen GD, Batson CG, Wagner BA, Maher J. Testosterone influences spatial strategy preferences among adult male rats. Horm Behav. 2013;63(5):800–812. doi: 10.1016/j.yhbeh.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrett-Connor E, Goodman-Gruen D, Patay B. Endogenous sex hormones and cognitive function in older men. J Clin Endocrinol Metab. 1999;84:3681–3685. doi: 10.1210/jcem.84.10.6086. [DOI] [PubMed] [Google Scholar]
- 25.James PJ, Nyby JG. Testosterone rapidly affects the expression of copulatory behavior in house mice (Mus musculus) Physiol Behav. 2002;75(3):287–294. doi: 10.1016/s0031-9384(01)00666-7. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa S, Robbins A, Kumar N, Pfaff DW, Sundaram K, Bardin CW. Effects of testosterone and 7 alpha-methyl-19-nortestosterone (MENT) on sexual and aggressive behaviors in two inbred strains of male mice. Horm Behav. 1996;30:74–84. doi: 10.1006/hbeh.1996.0011. [DOI] [PubMed] [Google Scholar]
- 27.Chambers KC, Thornton JE, Roselli CE. Age-related deficits in brain androgen binding and metabolism, testosterone, and sexual behavior of male rats. Neurobiol Aging. 1991;12(2):123–130. doi: 10.1016/0197-4580(91)90050-t. [DOI] [PubMed] [Google Scholar]
- 28.Wang C, Jackson G, Jones HT, et al. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care. 2011;34(7):1669–1675. doi: 10.2337/dc10-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frisch F, Sumida KD. Strength training does not alter the effects of testosterone propionate injections on high-density lipoprotein cholesterol concentrations. Metabolism. 1999;48(12):1493–1497. doi: 10.1016/s0026-0495(99)90235-4. [DOI] [PubMed] [Google Scholar]
- 30.Kyung PK, Jung EL, Ae SL, et al. Effect of gender differences on the regulation of renal ischemia-reperfusion-induced inflammation in mice. Mol Med Rep. 2014;9(6):2061–2068. doi: 10.3892/mmr.2014.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gouchie C, Kimura D. The relationship between testosterone levels and cognitive ability patterns. Psychoneuroendocrinology. 1991;16(4):323–334. doi: 10.1016/0306-4530(91)90018-o. [DOI] [PubMed] [Google Scholar]
- 32.Koyama Y, Nagao S, Ohashi K, Takahashi H, Marunouchi T. Effect of systemic and topical application of testosterone propionate on the density of epidermal Langerhans cells in the mouse. J Invest Dermatol. 1989;92(1):86–90. doi: 10.1111/1523-1747.ep13071282. [DOI] [PubMed] [Google Scholar]
- 33.Laroche J, Gasbarro L, Herman JP, Blaustein JD. Reduced behavioural response to gonadal hormones in mice shipped during the peripubertal/adolescent period. Endocrinology. 2009;150(5):2351–2358. doi: 10.1210/en.2008-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark AS, Henderson LP. Behavioral and physiological responses to anabolic-androgenic steroids. Neurosci Biobehav Rev. 2003;27:413–436. doi: 10.1016/s0149-7634(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 35.Onaolapo OJ, Onaolapo AY, Akanmu MA, Olayiwola G. Foraging enrichment modulates open field response to monosodium glutamate in mice. Ann Neurosci. 2015;22(3):162–170. doi: 10.5214/ans.0972.7531.220306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onaolapo OJ, Onaolapo AY, Akanni AA, Eniafe LA. Central depressant and nootropic effects of daytime melatonin in mice. Ann Neurosci. 2014;21(3):90–96. doi: 10.5214/ans.0972.7531.210304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onaolapo OJ, Onaolapo AY, Mosaku TJ, Onigbinde AO, Oyedele RA. Elevated plus maze and Y-maze behavioural effects of subchronic oral low dose monosodium glutamate in Swiss albino mice. IOSR J Pharm Biol Sci. 2012;3(4):21–27. [Google Scholar]
- 38.Celec P, Ostatníková D, Hodosy J. On the effects of testosterone on brain behavioural functions. Front Neurosci. 2015;9:12. doi: 10.3389/fnins.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adriani W, Macri S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology. 2002;27:212–224. doi: 10.1016/S0893-133X(02)00295-6. [DOI] [PubMed] [Google Scholar]
- 40.Schneider M. Adolescence as a vulnerable period to alter rodent behavior. Cell Tissue Res. 2013;354:99–106. doi: 10.1007/s00441-013-1581-2. [DOI] [PubMed] [Google Scholar]
- 41.Walker DM, Kirson D, Perez LF, Gore AC. Molecular profiling of postnatal development of the hypothalamus in female and male rats. Biol Reprod. 2012;87:129. doi: 10.1095/biolreprod.112.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bahrke MS, Yesalis CE. Abuse of anabolic androgenic steroids and related substances in sport and exercise. Curr Opin Pharmacol. 2004;4:614–620. doi: 10.1016/j.coph.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Van Marken Lichtenbelt WD, Hartgens F, Vollaard NB, Ebbing S, Kuipers H. Bodybuilders’ body composition: effect of nandrolone decanoate. Med Sci Sports Exerc. 2004;36:484–489. doi: 10.1249/01.mss.0000117157.06455.b0. [DOI] [PubMed] [Google Scholar]
- 44.Davies DD, Ruiz AL, Yanes LL, et al. Testosterone supplementation in male obese Zucker rats reduces body weight and improves insulin sensitivity, but increases blood pressure. Hypertension. 2012;59(3):726–731. doi: 10.1161/HYPERTENSIONAHA.111.180943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haider A, Yassin A, Doros G, Saad F. Effects of long-term testosterone therapy on patients with ‘diabesity’: results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. Int J Endocrinol. 2014;2014:683515. doi: 10.1155/2014/683515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Francomano D, Lenzi A, Aversa A. Effects of five-year treatment with testosterone undecanoate on metabolic and hormonal parameters in ageing men with metabolic syndrome. Int J Endocrinol. 2014;2014:527470. doi: 10.1155/2014/527470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdulmaged MT. Testosterone and weight loss: the evidence. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):313–322. doi: 10.1097/MED.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seung HH, Sung-Ho L. Differential growth of the reproductive organs during the peripubertal period in male rats. Balsaenggwa Saengsig. 2013;17(4):469–475. doi: 10.12717/DR.2013.17.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frederiksen L, Højlun K, Hougaard DM, et al. Testosterone therapy decreases subcutaneous fat and adiponectin in aging men. Eur J Endocrinol. 2012;166:469–476. doi: 10.1530/EJE-11-0565. [DOI] [PubMed] [Google Scholar]
- 50.Spritzer MD, Daviau ED, Coneeny MK, Engelman SM, Prince WT, Karlye N. Effects of testosterone on spatial learning and memory in adult male rats. Horm Behav. 2011;59(4):484–496. doi: 10.1016/j.yhbeh.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hodosy J, Zelmanová D, Majzúnová M, et al. The anxiolytic effect of testosterone in the rat is mediated via the androgen receptor. Pharmacol Biochem Behav. 2012;102(2):191–195. doi: 10.1016/j.pbb.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Filova B, Malinova M, Babickova J, et al. Effects of testosterone and estradiol on anxiety and depressive-like behaviour via a non-genomic pathway. Neurosci Bull. 2015;31(3):288–296. doi: 10.1007/s12264-014-1510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinclair D, Purves-Tyson TD, Allen KM, Weickert CS. Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain. Psychopharmacology (Berl) 2014;231(8):1581–1599. doi: 10.1007/s00213-013-3415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Purves-Tyson TD, Handelsman DJ, Double KL, Owens SJ, Bustamante S, Weickert CS. Testosterone regulation of sex steroid-related mRNAs and dopamine-related mRNAs in adolescent male rat substantia nigra. BMC Neurosci. 2012;13:95. doi: 10.1186/1471-2202-13-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Khatib IMH, Doè Kmeci I, Fujiwara M. Differential role of nucleus accumbens and caudate-putamen in mediating the effect of nomifensine and methamphetamine on ambulation and rearing of rats in the open-field test. Jpn J Pharmacol. 1995;67:69–77. doi: 10.1254/jjp.67.69. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L, Chang YH, Feldman AN, et al. The expression of GABA (A) receptor α2 subunit is upregulated by testosterone in rat cerebral cortex. Neurosci Lett. 1999;265(1):25–28. doi: 10.1016/s0304-3940(99)00193-7. [DOI] [PubMed] [Google Scholar]
- 57.Ambar G, Chiavegatto S. Anabolic-androgenic steroid treatment induces behavioural disinhibition and down regulation of serotonin receptor messenger RNA in the prefrontal cortex and amygdala of male mice. Genes Brain Behav. 2009;8:161–173. doi: 10.1111/j.1601-183X.2008.00458.x. [DOI] [PubMed] [Google Scholar]
- 58.Frye CA, Edinger KL, Lephart ED, Walf AA. 3 alpha-androstanediol, but not testosterone, attenuates age-related decrements in cognitive, anxiety and depressive behaviour of male rats. Front Aging Neurosci. 2010;2:15. doi: 10.3389/fnagi.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Naghdi N, Oryan S, Etemadi R. The study of spatial memory in adult male rats with injection of testosterone enanthate and flutamide into the basolateral nucleus of the amygdala in Morris water maze. Brain Res. 2003;972:1–8. doi: 10.1016/s0006-8993(03)02227-3. [DOI] [PubMed] [Google Scholar]
- 60.Harris AP, D’Eath RB, Healy SD. Sex differences or not, in spatial cognition in albino rats: acute stress is the key. Anim Behav. 2008;76:1579–1589. [Google Scholar]
- 61.Cherney ID, Brabec CM, Runco DV. Mapping out spatial ability: sex differences in way-finding navigation. Percept Mot Skills. 2008;107:747–760. doi: 10.2466/pms.107.3.747-760. [DOI] [PubMed] [Google Scholar]
- 62.Gibbs RB, Gabor R, Cox T, Johnson DA. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29:741–748. doi: 10.1016/S0306-4530(03)00118-5. [DOI] [PubMed] [Google Scholar]
- 63.Gibbs RB, Johnson DA. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology. 2008;149:3176–3183. doi: 10.1210/en.2007-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ackermann S, Spalek K, Rasch B, et al. Testosterone levels in healthy men are related to amygdala reactivity and memory performance. Psychoneuroendocrinology. 2012;37(9):1417–1424. doi: 10.1016/j.psyneuen.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 65.Roohbakhsh A, Akbar HM, Karim MD. Anxiolytic-like effect of testosterone in male rats: GABAC receptors are not involved. Iran J Basic Med Sci. 2011;14(4):376–382. [PMC free article] [PubMed] [Google Scholar]
- 66.Frye CA, Edinger K, Sumida K. Androgen administration to aged male mice increases anti-anxiety behaviour and enhances cognitive performance. Neuropsychopharmacology. 2008;33(5):1049–1061. doi: 10.1038/sj.npp.1301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van-Honk J, Peper JS, Schutter D. Testosterone reduces unconscious fear but not consciously experienced anxiety: implications for the disorders of fear and anxiety. Biol Psychiatry. 2005;58:218–225. doi: 10.1016/j.biopsych.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Fernandez-Guasti A, Martinez-Mota L. Anxiolytic-like actions of testosterone in the burying behaviour test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology. 2005;30:762–770. doi: 10.1016/j.psyneuen.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 69.Aikey JL, Nyby JG, Anmuth DM, James PJ. Testosterone rapidly reduces anxiety in male house mice (Mus musculus) Horm Behav. 2002;42:448–460. doi: 10.1006/hbeh.2002.1838. [DOI] [PubMed] [Google Scholar]
- 70.Rocha VM, Calil CM, Ferreira R, Moura MJ, Marcondes FK. Influence of anabolic steroid on anxiety levels in sedentary male rats. Stress. 2007;10(4):326–331. doi: 10.1080/10253890701281344. [DOI] [PubMed] [Google Scholar]
- 71.Takeo M, Yumiko Y, Tetsuo N. Age-associated changes in plasma testosterone levels in male mice and their relation to social dominance or subordinance. Horm Behav. 1981;15(3):238–245. doi: 10.1016/0018-506x(81)90013-1. [DOI] [PubMed] [Google Scholar]


