Abstract
Gamma aminobutyric acid (GABA)-expressing interneurons are the major inhibitory cells of the cerebral cortex and hippocampus. These interneurons originate in the medial ganglionic eminence (MGE) and lateral ganglionic eminence of the ventral forebrain during embryonic development and show reduced survival and function in a variety of neurological disorders, including temporal lobe epilepsy. We and others have proposed that embryonic stem cell (ESC)–derived ventral forebrain progenitors might provide a source of new GABAergic interneurons for cell-based therapies. While human ESCs (hESCs) are readily differentiated in vitro into dorsal telencephalic neural progenitors, standard protocols for generating ventral subtypes of telencephalic progenitors are less effective. We now report efficient derivation of GABAergic progenitors using an established hESC reporter line that expresses green fluorescent protein (GFP) under the control of an endogenous NKX2.1 promoter. GABAergic progenitors were derived from this hESC line by a modified monolayer neural differentiation protocol. Consistent with sonic hedgehog (SHH)-dependent specification of NKX2.1-positive progenitors in the embryonic MGE, we show a dose-dependent increase in the generation of NKX2.1:GFP-positive progenitors after SHH treatment in vitro. Characterization of NKX2.1:GFP-positive cells confirms their identity as MGE-like neural progenitors, based on gene expression profiles and their ability to differentiate into GABAergic interneurons. We are also able to generate highly enriched populations of NKX2.1:GFP-positive progenitors, including cells with telencephalic identity, by fluorescence-activated cell sorting. These hESC-derived ventral forebrain progenitors are suitable candidates for cell-based therapies that aim at replacing dysfunctional or damaged cortical or hippocampal GABAergic interneurons.
Introduction
Gamma aminobutyric acid (GABA)-expressing interneurons comprise 20% of total cortical neurons [1] and form the main inhibitory populations of neurons in the mammalian nervous system. These neurons represent a diverse group, and subtypes are further categorized based on electrophysiological properties, expression of neuropeptide and calcium binding proteins, regional locations, morphology, and synaptic targets (reviewed in Ref. [2]). The GABA-synthesizing enzyme glutamic acid decarboxylase (GAD) is expressed by all GABAergic interneurons, and the calcium binding proteins calbindin (CB), calretinin (CR), or parvalbumin (PV) [2] are expressed individually or in combination with the neuropeptides somatostatin (SST), neuropeptide Y (NPY), cholecystokinin, and vasoactive intestinal peptide (VIP) [3,4].
Studies in rodents have shown that GABAergic interneuron progenitors of the forebrain are generated in a group of ventral telencephalic structures of the embryonic brain called the medial and caudal ganglionic eminences (MGE and CGE, respectively) and in the preoptic area [5–7]. These progenitors migrate tangentially from the ventricular zone into the neocortex and hippocampus, where they terminally differentiate into a variety of interneuron subtypes [8,9].
The ganglionic eminences are divided into their respective compartments based on discrete domains of transcription factor expression [10,11]. The proper gene expression patterns depend on interacting cell signaling pathways and are necessary for specifying different interneuron subtypes and their migration routes. Fate mapping analyses of progenitors from the various ventral forebrain regions demonstrated that MGE progenitors give rise predominantly to SST- and PV-positive subtypes, while the CGE generates mainly VIP- and CR-positive interneurons [4,10].
The homeodomain-containing transcription factor NKX2.1 is required for specification of MGE progenitors, as NKX2.1 mutant mice demonstrate a shift in patterning of this structure toward CGE-specific cell types [5]. In addition, NKX2.1 is necessary for activating the transcription factor Lhx6, which is required for generating the PV- and SST-expressing interneurons [12]. As MGE-derived progenitors destined for the cortex mature, Nkx2.1 expression is down-regulated, while Nkx2.1 is expressed up to the time of neuronal maturity in those progenitors that are fated to become striatal interneurons [13]. Nkx2.1-positive MGE derivatives are also a source of basal forebrain cholinergic projection and interneurons [14]. In addition, Nkx2.1 is expressed by certain subtypes of diencephalic progenitors, including those fated to become hypothalamic neurons [15].
Induction of Nkx2.1 expression depends on sonic hedgehog (SHH) signaling from mesendodermal structures underlying the MGE [16]. Higher levels of SHH signaling occur in the dorsal MGE relative to the ventral MGE, as indicated by higher expression of the SHH responsive gene Gli1 in this region [17]. This differential response to SHH leads to the predominant generation of progenitors of SST-positive neurons in the dorsal MGE and progenitors of PV-positive neurons in the ventral MGE [18]. Continued SHH signaling maintains Nkx2.1 expression until the progenitors exit the cell cycle, as mice deficient in SHH during this period of neurogenesis display reduced Nkx2.1 expression and go on to develop reduced numbers of neocortical SST-, PV-, and NPY-positive interneurons [19]. Conditional loss of SHH signaling via removal of the pathway effector Smoothened transforms the MGE progenitors into CGE progenitors that express the homeobox transcription factor Gsx2, and give rise to CR-expressing interneurons [20].
A second transcription factor in the Nkx family, Nkx6.2, is another SHH-responsive gene and is expressed predominantly in the dorsal MGE [21]. Consistent with this expression pattern, NKX6.2-positive MGE progenitors give rise largely to SST-expressing interneurons, though a smaller fraction of NKX6.2-positive progenitors become PV-positive interneurons [22].
Our understanding of the origins of cortical interneurons is largely based on rodent models. While some mechanisms of GABAergic interneuron specification are similar in the human and rodent nervous systems, including the expression patterns of two other ventral transcription factors, DLX2 and MASH1 [23], it is important to note that there are some species-specific differences. For example, Nkx2.1 is initially expressed in the ventral forebrain and preoptic area of the human fetal brain as early as 5 gestational weeks; however, by 20 gestational weeks, expression extends from the MGE to the neocortical ventricular and subventricular zones [24]. The authors of these studies suggest that secondary sites of interneuron generation emerge later in development due to increased complexity of the human neocortex and an increased demand for interneurons.
Many studies have demonstrated that ventral forebrain neural progenitors can be generated from mouse embryonic stem cells (ESCs) [25–28]; however, the production of MGE-specific progenitors from human ESCs (hESCs) occurs at a very low frequency. Li et al. [29] have shown that there are high levels of WNT signaling active during hESC neural differentiation, resulting in the generation of predominantly dorsal forebrain progenitors. This bias toward dorsal progenitors can be reversed by treating cultures with either the WNT antagonist DKK1 or with SHH [29]. Recently, Ma et al. demonstrated the ability to direct differentiation of hESCs into lateral ganglionic eminence-like progenitors and, ultimately, DARPP32-positive medium spiny neurons by treatment with relatively low levels of SHH [30]. In addition to dependence on levels of SHH signaling, specification of GABAergic progenitors in vitro may require precise delivery of SHH at particular stages of differentiation. A recent study showed that the time when SHH treatment is initiated in cultures is critical to promoting the production of ventral progenitors, as treatment of cells that have progressed too far along the neuroectoderm lineage appears to decrease the derivation of GAD67-positive interneurons [27].
Recently, a hESC line was generated containing the green fluorescent protein (GFP) sequence introduced into the NKX2.1 locus [31]. This reporter line allows for the identification and isolation of MGE-like neural progenitors, derived from hESCs, based on GFP expression driven by the endogenous NKX2.1 promoter. Goulburn et al. [31] characterized NKX2.1:GFP-positive cells, derived by embryoid body-based differentiation, and confirmed their basal forebrain identity. When further differentiated, these cells matured into GABAergic neurons, both in vitro and after transplantation into the neonatal mouse cortex. The in vitro neural differentiation protocol used in this study focused on the role of retinoic acid and fibroblast growth factor 2 (FGF2) in generating NKX2.1-positive cells, which resulted in only about 10% NKX2.1:GFP-positive cells out of the total cell population [31].
We aimed at increasing the yield of NKX2.1:GFP-positive cells by exposing neural progenitors to prolonged SHH signaling during in vitro neural differentiation. We now demonstrate efficient generation of NKX2.1-positive ventral forebrain progenitors, including telencephalic derivatives, using the hES3-NKX2.1:GFP cell line and a modified monolayer neural differentiation protocol. We also observed a striking SHH dose-dependent generation of NKX2.1:GFP-positive progenitors and enrichment of these GABAergic progenitors by fluorescence activated cell sorting (FACS). Further characterization of the gene and protein expression profiles of the NKX2.1:GFP-positive cells reveals a strong similarity to in vivo MGE progenitors, supported by their ability to mature into GABAergic interneurons. These data demonstrate our ability to isolate large quantities of hESC-derived MGE-like progenitors that are likely to be useful for cell-based regenerative medicine.
Materials and Methods
hESC culture and neural differentiation
H9 and hES3-NKX2.1:GFP hESCs [31] were routinely cultured on mitomycin C-treated mouse embryonic fibroblasts (MEFs; 2×104 cells/cm2) and passaged mechanically by scoring and picking fragments of colonies. The hESC medium consisted of Dulbecco's modified Eagle medium nutrient mixture F-12 (Sigma) supplemented with knockout serum replacement (KSR; Gibco), nonessential amino acids (NEAAs; Gibco), l-glutamine (Gibco), penicillin/streptomycin (Gibco), 2-mercaptoethanol (Sigma), and FGF2 (CONNStem; 8 ng/μL). hES3-NKX2.1:GFP hESCs were modified to include constitutive red fluorescence protein expression by transduction with a lentiviral vector containing mCherry driven by a ubiquitin promoter and conveying neomycin resistance (gift of Alex Lichtler, University of Connecticut Health Center). After viral transduction, all healthy colonies were passaged onto DR-4 MEFS (GlobalStem) and selected in G418 (Geneticin; Gibco).
For neural differentiation, hESC colonies were switched from culture in hESC medium to N2B27 medium supplemented with noggin (R&D Systems; 500 ng/mL) 1–2 days after passage. N2B27 medium consisted of neurobasal medium with N2, B27, and insulin-transferrin-selenium supplements, l-glutamine, and penicillin/streptomycin. N2B27 medium with noggin was changed every other day. After 8–10 days, differentiating colonies were mechanically passaged using a StemPro EZ-Passage tool (Life Technologies) onto laminin-coated substrates. For continued culture and differentiation into more mature neural progenitors, cells were mechanically passaged a second time after another 7 days of differentiation. After this point, cells could be passaged as a single-cell suspension with trypsin. When trypsinized, the ROCK inhibitor Y27632 (Calbiochem) was added to N2B27 at a 10 μM final concentration for at least one day after passaging. ROCK inhibitor is widely used to aid in cell survival when cultures are passaged as single cells.
To enrich for NKX2.1-positive ventral neural progenitors, recombinant human SHH with an N-terminus modification (rhSHH-N; R&D Systems) was added to the neural differentiation cultures on day 6 at final concentrations of either 250 or 500 ng/mL.
Fluorescence activated cell sorting
hES3-NKX2.1:GFP neural differentiation cultures were trypsinized and resuspended in N2B27 medium supplemented with 10 μM Y27632 at a concentration of 0.5–2.0×107 cells/mL and kept on ice until just before FACS. Immediately before FACS, the cells were pelleted and resuspended at the same concentration in Hank's balanced salt solution supplemented with 2% fetal calf serum (FCS), 20 mM glucose, penicillin/streptomycin, and 10 μM Y27632. Cells were then filtered through 45 μm mesh and treated with propidium iodide to facilitate elimination of dead and dying cells from the sort. H9-derived embryonic stem cell-derived neural progenitors (ESNPs) at a similar stage of neural differentiation were used as negative gating controls. Cells were sorted using an FACSVantage flow cytometer fitted with a 100 μm nozzle and analyzed with FACS Diva software (BD Biosciences). FACS sorted cells were collected in N2B27 medium supplemented with 20% FCS, 20 mM glucose, and 10 μM Y27632 and kept on ice until plating. After FACS, cells were pelleted, resuspended in N2B27 media supplemented with epidermal growth factor (EGF; 20 ng/mL), FGF2 (20 ng/mL), and 10 μM Y27632, and plated onto laminin-coated tissue culture dishes or glass chamberslides at no less than 7.5×104 cells/cm2. For differentiation into more mature neurons, FACS-isolated cells were cultured for 10 days in neurobasal medium supplemented with B27, NEAAs, l-glutamine, cyclic adenosine monophosphate (1 μM), brain-derived neurotrophic factor (10 ng/mL), glial cell-derived neurotrophic factor (10 ng/mL), and ascorbic acid (200 μM).
Immunocytochemistry/immunohistochemistry
Cultured cells were fixed with 3.7% formaldehyde in phosphate buffered saline (PBS) for 10 min followed by permeabilization with 0.5% PBS-Triton X-100. Cells were then blocked for one hour at room temperature in 2% bovine serum albumin/5% Serum/0.1% PBS-Triton X-100 before incubation with primary antibodies diluted in blocking solution overnight at 4°C. Secondary antibody incubations were done for one hour at room temperature in blocking solution. The following antibodies were used: MAP2 (Sigma; mouse, 1:1000), GFP (Life Technologies; rabbit, AlexaFluor 488-conjugated, 1:1000), Ki67 (Abcam; rabbit, 1:1000), Pax6 (DSHB; mouse, 1:50), NKX2.1 (TTF1; Abcam; rabbit, 1:250), CR (Chemicon; rabbit, 1:1000), phospho-histone H3 (pH3; Chemicon; rabbit, 1:500), STT (Chemicon; rat, 1:100), GAD65 (Abcam; mouse, 1:400), Musashi-1 (Abcam; rabbit, 1:400), and CB (Swant; rabbit, 1:1000). All fluorescent secondary antibodies (Alexa Fluor-conjugated; Life Technologies) were used at 1:1000. Cells were counterstained with Hoechst 33342 before mounting in gelvatol. The cells were imaged using a Nikon Eclipse Ti with NIS Elements software. For quantification of marker expression in cultured cells, four or five random fields within each well of a chamberslide were imaged, and the percentages of GFP-positive cells expressing each marker were calculated. At least 100 GFP-positive cells per marker were counted.
Reverse transcriptase polymerase chain reaction and quantitative polymerase chain reaction
FACS isolated cells were pelleted and immediately resuspended in Trizol reagent (Life Technologies). RNA was isolated according to the manufacturer's protocol with the addition of three extra chloroform extractions before RNA precipitation. One microgram of total RNA was DNase-treated using the Turbo DNA-free kit (Applied Biosystems) and reverse transcribed into cDNA using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems). Polymerase chain reaction (PCR) was perfomed using REDTaq ReadyMix PCR Reaction Mix (Sigma) on an Applied Biosystems 2720 Thermal Cycler.
Quantitative reverse transcriptase polymerase chain reaction (RT-PCR) was performed with TaqMan Gene Expression Assays using cDNA generated earlier. The following assays were run: NKX2.1 (Hs00968940_m1), PAX6 (Hs01088112_m1), FOXG1 (Hs01850784_s1), LHX6 (Hs01030943_m1), and NKX6.2 (Hs00752986_s1). GAPDH (Hs99999905_m1) and β-ACTIN (Hs99999903_m1) were included as endogenous controls. Assays were run on an Applied Biosystems 7300 Real-Time PCR System, and data were analyzed using Applied Biosystems SDS version 1.2 software. Data from both populations were first normalized to GAPDH, and gene expression levels in the NKX2.1:GFP-positive population were then compared relative to those of the NKX2.1:GFP-negative population.
Results
In vitro differentiation of H9 and hES3-NKX2.1:GFP hESCs into ventral forebrain neural progenitors
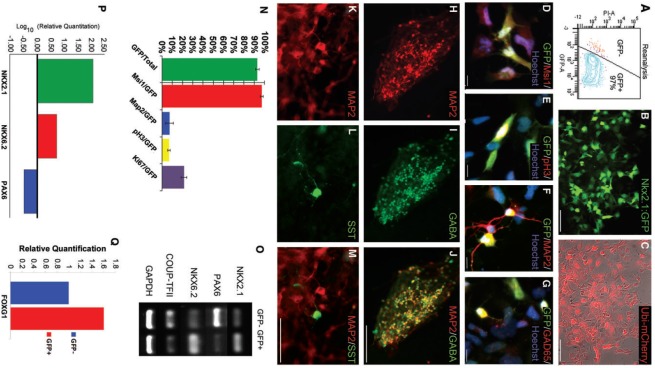
We have established a modified monolayer neural differentiation protocol, which involves treatment with the BMP antagonist noggin [32], to generate neural stem cells and neurons. To initiate neural differentiation, hESCs, grown in colonies on MEF feeders, were switched from ESC medium into nutrient-poor N2B27 medium, supplemented with noggin (Fig. 1A). Inclusion of noggin from the onset of differentiation directs the culture toward neuroectoderm and inhibits the generation of endoderm and mesoderm derivatives. After about 6 days, the differentiating colonies compacted and the cells at the edges of colonies began to elongate and resemble bipolar neuroepithelial cells (Fig. 1B). At around 10 days, colonies were mechanically passaged by splitting into pieces and plating onto laminin-coated tissue culture dishes. Within 2 days of passaging, immature rosette structures emerged, composed of radially arranged bipolar cells that expressed the early human neuroectoderm marker Pax6 (Fig. 1B). These rosette neural stem cells (NSCs) are highly proliferative and, over another week of differentiation, formed dense cell layers. Mechanical passaging at this point allowed us to generate mature rosettes with tight apical cell contacts surrounding a central lumen. Neural progenitors within rosettes at this stage expressed the NSC marker nestin (Fig. 1B). After ∼3 weeks of differentiation, cells were enzymatically passaged using trypsin, to yield enriched cultures of mature ESNPs.
FIG. 1.
Monolayer neural differentiation of H9 and hES3-NKX2.1:GFP hESCs. (A) For neural differentiation of H9 hESCs, cells on MEF feeder cells are switched from ESC medium into N2B27 medium supplemented with 500 ng/mL of the BMP antagonist noggin. Blue lines indicate mechanical passaging. Red line indicates enzymatic passaging with trypsin. (B) After one week of monolayer neural differentiation, colonies compact and cells at the edges of the colonies exhibit an elongated morphology. After mechanical passaging onto poly-l-lysine/laminin-coated substrates, immature neural rosettes containing Pax6-positive cells emerge. Mature rosettes, with nestin-positive neural progenitors at the periphery, develop after ∼18 days of neural differentiation. Enriched cultures of mature neural progenitors are generated after enzymatic passaging. Scale bar in day 6 panel is 50 μm. Scale bars in days 12, 18, and 21 are 100 μm. (C) Quantification of cell types present in mature neural differentiation cultures, after trypsin passaging, reveals a majority of neural stem cells (Msi1, 88%), small populations of extraembryonic endoderm and mesoderm (TROMA1 and SMA, respectively), and 0.1% TRA-181-positive undifferentiated hESCs. Dorsal PAX6-positive cells make up about 56% of the total population, while ventral progenitors, expressing NKX2.1, make up about 3%. Error bars represent standard deviation between four individual differentiation cultures. (D) hESC-derived NSCs express markers of dorsal and ventral telencephalic progenitors. Ventral transcription factors Mash1 and NKX2.1 and the dorsal transcription factor PAX7 are detected by RT-PCR analysis at various stages of neural differentiation. (E) Differentiation of hES3-NKX2.1:GFP ESCs using the monolayer protocol results in enriched populations of ESNPs and follows the same progression of events. After about 3 weeks of differentiation, in the absence of exogenous growth factors, NKX2.1:GFP-positive cells emerge within neural rosette structures. Scale bars are 100 μm. GFP, green fluorescent protein; hESCs, human embryonic stem cell; MEFs, mouse embryonic fibroblasts; SMA, smooth muscle actin; ESNPs, embryonic stem cell-derived neural progenitors; NSCs, neural stem cells; RT-PCR, reverse transcriptase polymerase chain reaction. Color images available online at www.liebertpub.com/scd
Differentiation of H9 hESCs using this protocol resulted in the efficient generation of NSCs with diverse regional identities, which arose in neural rosettes. After ∼30 days of neural differentiation, cultures comprised of almost 90% musashi-1-positive ESNPs, while small fractions of the population were non-neural cells, including TROMA-1-positive extraembryonic endoderm, smooth muscle actin-positive mesoderm, and TRA-181-positive undifferentiated hESCs (5.94%, 0.85%, and 0.11%, respectively; Fig. 1C). We determined by RT-PCR analysis that ESNPs generated with this protocol expressed markers of dorsal and ventral forebrain progenitors, including the telencephalic marker FOXG1, the dorsal marker PAX7, and the ventral markers NKX2.1 and MASH1 (Fig. 1D). After 30 days of differentiation, however, only about 3% of the total population were labeled with an antibody against NKX2.1 (Fig. 1C). We differentiated the hES3-NKX2.1:GFP cells using this protocol and observed rosette and ESNP differentiation as described for the H9 cell line (Fig. 1E). The hES3-NKX2.1:GFP hESCs appear to undergo monolayer neural differentiation as efficiently as H9 hESCs, despite their origination from the hES3 parental cell line and the inherent differences that exist between hESC lines. Small populations of NKX2.1:GFP-positive cells appeared within rosettes after ∼3 weeks of in vitro differentiation (Fig. 1E). Analyses of individual cultures after 4 weeks of differentiation showed that the percentage of NKX2.1:GFP-positive cells ranged from <1% to 10% of the total population, and typically the percentage was in the lower end of this range.
In vitro characterization of NKX2.1:GFP-positive cells
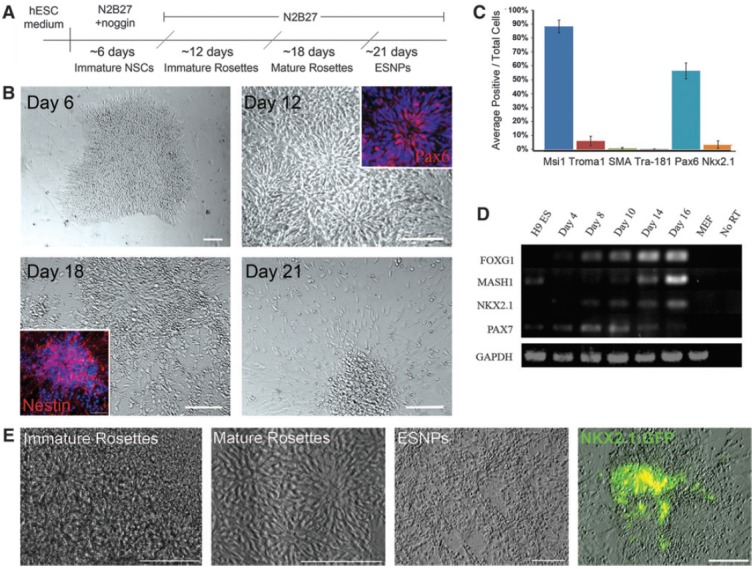
We characterized the NKX2.1:GFP-positive cells that emerged in our cultures by immunocytochemistry at two time points; first, when cultures were mainly composed of highly proliferative NSCs in rosette structures and later, once neuronal differentiation was observed. GFP-positive cells comprised just less than 10% of total cells at day 23 of differentiation in these experiments (Fig. 2A). Of these GFP-positive cells, almost 50% were labeled with an antibody against the cell proliferation marker Ki67 (Fig. 2B, B′), and about 90% expressed the neural stem cell marker musashi-1 (Fig. 2C, C′). Roughly 30% of GFP-positive cells were MAP2-positive neurons (Fig. 2D, D′). These data suggest that the NKX2.1:GFP-positive cells at this stage of neural differentiation are largely proliferative neural progenitors. Using immunocytochemistry, the NKX2.1 protein was detected in about 85% of NKX2.1:GFP-positive cells, confirming the accuracy of GFP reporter expression (Fig. 2E, E′). The slightly lower percentage of NKX2.1-positive cells based on antibody recognition versus GFP expression is likely due to the observation that GFP is a very stable protein and may remain present even after down-regulation of NKX2.1 itself in some cells. Expression of PAX6, which initially labels all early human neuroectoderm, later becomes restricted to the developing dorsal telencephalon of the embryo [33]. Consistent with mutually exclusive expression observed in vivo for the dorsal telencephalic progenitor marker PAX6 and the MGE-specific marker NKX2.1, we did not observe any NKX2.1:GFP-positive cells that were labeled with Pax6 (Fig. 2F, F′).
FIG. 2.
In vitro characterization of unsorted NKX2.1:GFP-positive cells. (A) Quantification of individual marker expression as a percentage of GFP-positive cells in differentiation on day 23 neural cultures. Data are presented as the average of three experiments with error bars representing standard error of the mean. (B, B′) About half of the NKX.2.1:GFP-positive cells in day 23 neural differentiation cultures labeled with the cell proliferation marker Ki67. (C, C′) The majority of GFP-positive cells expressed the neural stem cell marker Msi1. (D, D′) A population of GFP-positive cells were MAP2-positive neurons at this stage. (E, E′) Co-labeling of NKX2.1:GFP-positive cells with an antibody against the NKX2.1 protein itself confirms the reliability of the GFP reporter. (F, F′) Expression of NKX2.1:GFP and the dorsal telencephalic progenitor marker PAX6 are mutually exclusive. (G, G′) At 31 days of differentiation, 35% of NKX2.1:GFP-positive cells have matured into MAP2-positive neurons. (H, H′) Clusters of GFP-positive cells expressed the neurotransmitter GABA. (I, I′) NKX2.1:GFP-positive cells give rise to SST-expressing neurons. (J, J′) Expression of the GABA synthesizing enzyme GAD65 was also detected in some GFP-positive cells. (K, K′) A population of GFP-positive cells were also positive for the calcium binding protein CB. (L, L′) CR expression was only observed in NKX2.1:GFP-negative cells. Nuclei are counterstained with Hoechst (blue). All scale bars are 10 μm. GABA, gamma aminobutyric acid; SST, somatostatin; CB, calbindin; CR, calretinin. Color images available online at www.liebertpub.com/scd
After another 7 days of differentiation, the proportion of GFP-positive cells that co-expressed MAP2 had increased to 34% (Fig. 2G, G′). To assess the ability of NKX2.1:GFP-positive cells to differentiate into GABAergic interneurons in vitro, we performed immunocytochemistry for the calcium binding proteins CB and CR, and the neuropeptide SST, in addition to GABA and its synthesizing enzyme GAD65. Dense clusters of GABA- and GAD65-expressing GFP-positive cells were observed at this stage of differentiation, dispersed throughout the cultures, and this heterogeneous distribution precluded unbiased quantification of their frequency (Fig. 2H, H′ and Fig. 2J, J′, respectively). We also observed GFP-positive cells that were SST- or CB-positive (Fig. 2I, I′ and Fig. 2K, K′), indicating that hESC-derived NKX2.1-positive progenitors were able to mature into the appropriate interneuron subtypes that characteristically develop in the basal telencephalon. Quantification of the population of GFP-positive cells showed that SST-positive cells represented about 19%, and CB-positive cells represented 7.5%. Strikingly, despite observing GFP-negative/CR-positive cells, we did not find any GFP-positive cells that expressed CR (Fig. 2L, L′). Since CR-positive interneurons are predominantly generated from the NKX2.1-negative CGE, in vivo, this further supports the MGE progenitor-like characteristics of NKX2.1:GFP-positive cells.
Efficient generation of NKX2.1:GFP-positive neural progenitors in a dose-dependent response to SHH
NKX2.1 expression is induced in early neuroepithelial cells of the ventral telencephalon by SHH signaling from the underlying mesendoderm [16]. While low levels of SHH signaling have been documented during neural differentiation in hESC cultures, high levels of WNT signaling are also present, which result in the generation of predominantly dorsalized progenitors [29]. Treating cultures with WNT antagonists or SHH was previously shown to shift cell fate specification and generation of NKX2.1-positive cells [29]. To increase the percentage of GFP-positive cells, and allow for their efficient isolation by FACS, we treated differentiating cultures with rhSHH beginning at 6 days of neural differentiation. This time point was chosen, as it coincides with the emergence of neuroepithelial-like cells expressing PAX6, an early human neuroectoderm marker [33], at the periphery of differentiating hESC colonies. We hypothesize that these early NSCs are still in a naïve state and have not yet acquired regional identity, making them receptive to patterning cues. Treatment of the NKX2.1:GFP reporter line with 250 ng/mL SHH for 10 days resulted in an increase of GFP-positive cells from less than 3% to about 36%, demonstrating the appropriate response to ventralizing signals and up-regulation of the SHH responsive gene NKX2.1 (data not shown).
We assayed the generation of NKX2.1:GFP-positive cells in response to differing concentrations of SHH over time. No GFP-positive cells were observed in untreated cultures at 10 days of differentiation (Fig. 3A, A′). At this same time point, but after 4 days of treatment with SHH, GFP-positive cells were first observed in 250 ng/mL treated cultures (Fig. 3B, B′) with a higher percentage observed in cultures treated with 500 ng/mL (Fig. 3C, C′). GFP-positive cells were still absent after 17 days of differentiation in control cultures (Fig. 3D, D′), while the number of GFP-positive cells appeared to increase significantly in both 250 and 500 ng/mL cultures (Fig. 3E–F′). The morphology of cells and colonies did not appear to differ between control and SHH-treated cultures, with neural rosettes forming in all conditions (Fig. 3A–C, D–F). Flow cytometric analysis of differentiation day 18 cultures confirmed that in response to SHH treatment, there was a dose-dependent increase in NKX2.1:GFP-positive cells. In control cultures, there were no GFP-positive cells (0.0% of propidium iodide-negative live cells, Fig. 3G). In contrast, 16.7% of total cells (28.9% of live, Fig. 3H) in 250 ng/mL SHH cultures were GFP positive, and this percentage increased to 25.5% of total (46.7% of live, Fig. 3I) in cultures treated with 500 ng/mL SHH.
FIG. 3.
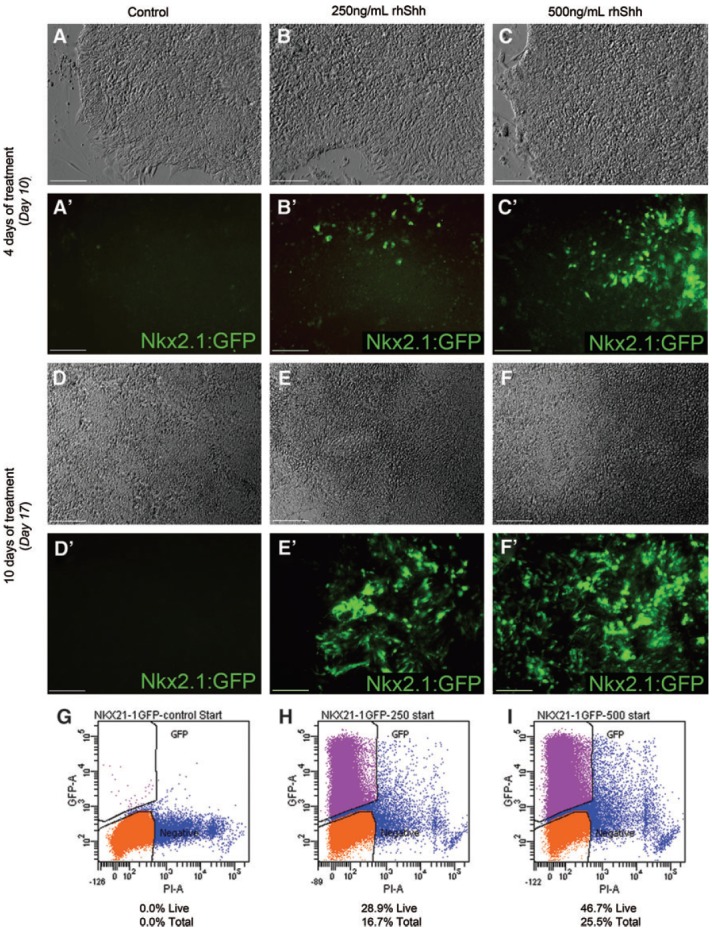
Treatment with rhSHH increases the percentage of NKX2.1:GFP-positive cells in a dose-dependent manner. (A–C) Phase images of representative neural differentiation cultures at day 10 of differentiation. (A′) No NKX2.1:GFP-positive cells are observed after 10 days of differentiation in control cultures. (B′) After 4 days of treatment with 250 ng/mL SHH, sparse patches of GFP-positive cells are observed. (C′) GFP-positive cells are more numerous in cultures treated with 500 ng/mL SHH after 4 days. (D–F) Phase images of representative differentiation cultures after 17 days of neural differentiation. (D′) NKX2.1:GFP-positive cells are not observed at day 17 of differentiation in the absence of SHH. (E′, F′) The number of GFP-positive cells has increased significantly after 10 days of treatment with SHH in both 250 and 500 ng/mL cultures, with many more positive cells observed in the 500 ng/mL SHH treated cultures. (G) Flow cytometric analysis of control cultures at day 18 confirms the absence of NKX2.1:GFP-positive cells at this time point. (H) 16.7% of the total cell population and 28.9% of the PI-negative live cells were GFP-positive in 250 ng/mL SHH treated cultures. (I) 25.5% of the total cell population and 46.7% of live cells were GFP positive in 500 ng/mL SHH treated cultures. Scale bars in (A–F′) are 100 μm. rhSHH, recombinant human sonic hedgehog; PI, propidium iodide. Color images available online at www.liebertpub.com/scd
Characterization of FACS-enriched NKX2.1:GFP-positive cells
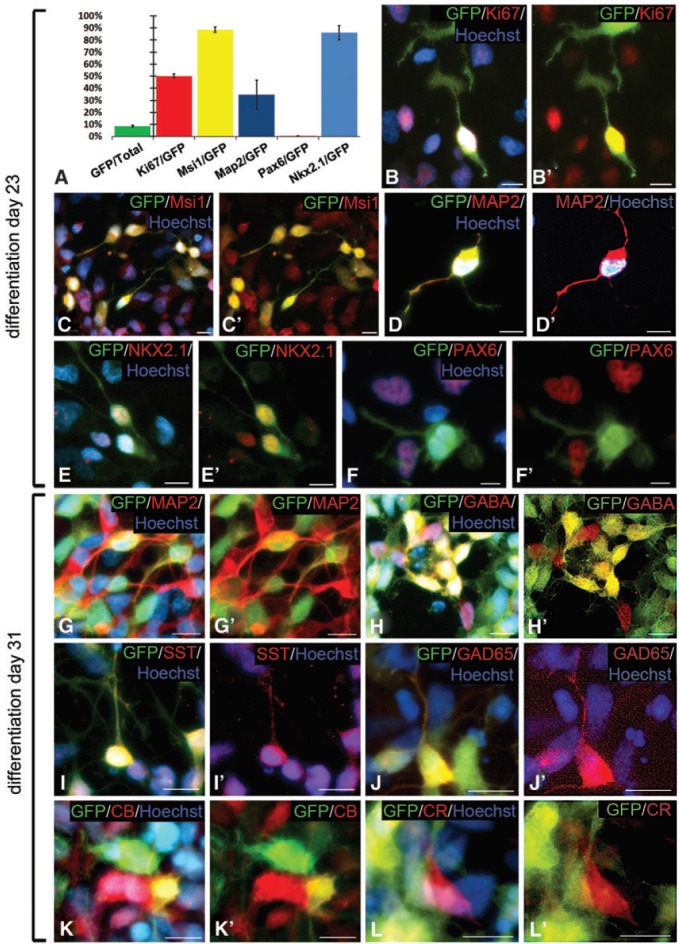
To further characterize the NKX2.1:GFP-positive cells, we isolated them by FACS and performed immunocytochemical staining and quantitative RT-PCR analyses. In hES3-NKX2.1:GFP neural differentiation cultures, which had been treated with 500 ng/mL SHH for 10 days and subjected to FACS, the isolated GFP-expressing progenitors were significantly enriched, ranging from 94% to 97% of live cells (Fig. 4A). Reanalysis of GFP-negative cells was typically 99%, supporting clean separation of the two populations by FACS. One day after FACS, isolated GFP-positive cells had normal bipolar neural progenitor morphology in culture and appeared healthy (Fig. 4B, C).
FIG. 4.
Characterization of FACS-enriched NKX2.1:GFP/Ubi-mCherry cells. (A) Reanalysis of the FACS-enriched NKX2.1:GFP-positive fraction post-sort shows enrichment to 97%. (B) Live FACS-enriched GFP-positive cells in culture, one day post-sort, exhibit strong GFP expression. (C) All cells have uniform mCherry expression and normal neural progenitor morphology. Scale bars in (B) and (C) are 100 μm. (D) The majority of isolated NKX2.1:GFP-positive cells express the neural stem cell marker Msi1. (E) Proliferative GFP-positive cells in M phase were detected by pH3 labeling. (F) A small population of FACS-enriched GFP-positive cells were MAP2-positive neurons. (G) A few GFP-positive cells co-labeled with GAD65, indicating the presence of some GABAergic interneurons in the FACS-enriched NKX2.1:GFP-positive population. Scale bars in (D–G) are 10 μm. (H–J) After 10 days of culture in differentiation medium post-sort, clusters of MAP2-positive cells co-label with GABA. (K–M) Some MAP2-positive neurons express the neuropeptide SST after 10 days of differentiation following FACS. Scale bars in (H–J) are 100 μm and in (K–M) are 50 μm. (N) Quantification of individual marker expression as a percentage of GFP-positive cells, one day post-sort. Data are presented as the average of four experiments for GFP, Msi1, and MAP2 and the average of two experiments for pH3 and Ki67. Error bars represent standard error of the mean. (O) RT-PCR analysis of FACS-enriched NKX2.1:GFP-negative and NKX2.1:GFP-positive cells. (P) qRT-PCR analysis, performed once, confirms enrichment of NKX2.1 and NKX6.2 and lower levels of PAX6 in the NKX2.1:GFP-positive population. (Q) Expression of FOXG1 measured by qPCR in NKX2.1:GFP-positive and -negative populations. Samples were normalized to GAPDH, and relative quantification of expression levels were compared with those of the NKX2.1:GFP-negative population. FACS, fluorescence activated cell sorting; pH3, phospho-histone H3; qRT-PCR.
When FACS-enriched NKX2.1:GFP-positive cells were immunostained one day after FACS, we found that on average 93% of the GFP-positive cells expressed Msi1 (Fig. 4D, N). Almost 7% of GFP-positive cells were labeled with pH3, a marker of proliferative cells in mitosis (Fig. 4E, N), and 21% were positive for Ki67 (Fig. 4N). The lower percentage of Ki67-positive cells at one day post-sort, as compared with that of unsorted GFP-positive cells, may be a result of FACS-enriched cells being in a state of reduced proliferation, as they recover from the stress induced by FACS. About 7% of FACS-isolated GFP-positive cells were labeled with MAP2 (Fig. 4F, N), and a few of these neurons were found to express GAD65 (Fig. 4G). Expression of more mature GABAergic interneuron markers such as SST, CB, and CR was not detected at this point, indicating that NKX2.1:GFP-positive cells isolated by FACS, at this stage of neural differentiation, are immature neural progenitors that have not yet acquired interneuron subtype specificity. However, continued culture of FACS-enriched NKX2.1:GFP-positive cells for 10 days in a differentiation medium designed for neuronal maturation resulted in clusters of MAP2-positive cells that co-label with GABA (Fig. 4H–J). Since these clusters were dense and multilayered, quantification was not possible. In addition, after this extended differentiation of FACS-enriched cells, we identified rare SST-positive neurons and cells expressing choline acetyltransferase, which also arise from the embryonic NKX2.1-positive progenitors [34] (Fig. 4K–M and data not shown).
To further characterize the progenitors generated using our modified monolayer neural differentiation protocol with SHH treatment, we compared the expression of regional transcription factors in FACS-enriched NKX2.1:GFP-positive and negative cells from day-23 500 ng/mL SHH treated cultures by RT-PCR (Fig. 4O). RT-PCR analysis confirmed enriched expression of NKX2.1 and very low levels of PAX6 in the GFP-positive sample, while very low levels of NKX2.1 and high levels of PAX6 expression were observed in a GFP-negative sample. Expression of LHX6, a direct target of NKX2.1 in postmitotic MGE progenitors, was not detected in these samples. However, its expression was seen in cells at a slightly later stage of differentiation (data not shown), further suggesting that GFP-positive cells isolated at this stage represent an immature MGE progenitor population.
Given our treatment of differentiating cells with SHH, we assayed for the expression of NKX6.2, another SHH-dependent transcription factor specific to progenitors, of the dorsal MGE. As expected, we detected significantly higher levels of NKX6.2 expression in the GFP-positive sample, as compared with GFP-negative cells (Fig. 4O). In addition, we investigated expression of the CGE-specific transcription factor COUP-TFII. Levels of COUP-TFII were enriched in GFP-negative cells and were very low in GFP-positive cells (Fig. 4O). Quantitative RT-PCR analyses for NKX2.1, NKX6.2, and PAX6 confirmed these patterns of gene expression (Fig. 4P). In line with our earlier findings of the regional identities adopted by ESNPs generated with this monolayer differentiation protocol, we detected expression of the telencephalic marker FOXG1 by both the NKX2.1:GFP-negative and -positive populations by quantitative RT-PCR and found FOXG1 to be slightly enriched in the GFP-positive cells compared with the GFP-negative population (Fig. 4Q). This observation is consistent with the NKX2.1-positive population, including cells with telencephalic identity. Taken together, these data suggest that our protocol promotes the generation of dorsal MGE-like progenitors from NKX2.1:GFP-positive cells.
Discussion
We have developed a modified monolayer neural differentiation protocol that promotes efficient differentiation of hESCs into MGE-like ventral forebrain progenitors. By using the hES3-NKX2.1:GFP reporter line, we were able to observe NKX2.1:GFP-positive progenitors, as they emerge, in live cultures. Initial rounds of neural differentiation with this cell line resulted in a very low percentage of cells expressing NKX2.1, consistent with our previous experiments with other hESC lines. Since SHH signaling is critical for the induction of NKX2.1 expression in the developing ventral forebrain, we differentiated hES3-NKX2.1 hESCs in medium containing rhSHH and demonstrated a dose-dependent increase in the number of ventral progenitors generated.
During embryonic development of the ventral telencephalon, higher levels of SHH signaling in dorsal regions of the MGE lead to the generation of predominantly SST-expressing interneurons, while PV-positive interneurons are generated in the ventral MGE [20]. In our analysis of unsorted NKX2.1:GFP-positive cells, which were derived from 500 ng/mL SHH-treated cultures, we observed GFP-positive cells that were co-labeled with SST. In addition, RT-PCR analysis of FACS-enriched GFP-positive cells showed expression of the SHH-dependent transcription factor Nkx6.2, which is expressed primarily in the dorsal-most regions of the developing MGE. Together, these data demonstrate the presence in our cultures of dorsal MGE-like progenitors that have the ability to differentiate into interneuron subtypes which are appropriate for the dorsal MGE. Further experiments, in which cultures are treated with a wider range of SHH concentrations, are necessary to determine whether hESC-derived ventral forebrain progenitors are patterned by SHH in the same way as progenitors in the embryonic MGE. For example, progenitors derived under low SHH conditions may give rise to PV-expressing neurons or cells typical of the CGE, such as CR-positive neurons.
Our characterization of unsorted and FACS enriched NKX2.1:GFP-positive cells after 23 days of differentiation revealed an apparently immature population of MGE-like progenitors. These cells are highly proliferative, based on Ki67 expression, and are predominantly Msi1-positive NSCs. Several pieces of evidence confirm the MGE identity of the NKX2.1:GFP-positive cells that we have derived using our modified monolayer neural differentiation protocol with SHH treatment. Immunocytochemistry and RT-PCR analysis showed that GFP-positive cells were negative for PAX6 expression, which is restricted in the embryonic brain to dorsal telencephalic progenitors. In addition, expression of the CGE-specific transcription factor Coup-TFII was considerably lower in FACS-enriched NKX2.1:GFP-positive cells when compared with the GFP-negative population. We also observed significantly higher levels of Nkx6.2 expression in GFP-positive cells by RT-PCR, indicating activity of other transcription factors that are exclusively expressed in the MGE.
Goulburn et al. [31] demonstrated two distinct stages of NKX2.1-positive progenitors in their differentiation cultures, based on co-expression or absence of E-cadherin, a cell surface protein that characterizes neuroepithelial cells. NKX2.1/E-cadherin double-positive cells were enriched in more immature neuroepithelial cell markers, while NKX2.1-positive cells that were E-cadherin negative expressed markers of more mature MGE neural progenitors, including Mash1, Lhx6, and GAD1. We did not detect expression of Lhx6 or Mash1 in our day 23 GFP-positive cells, suggesting that our NKX2.1:GFP-positive cells, at this stage, represent a relatively immature MGE progenitor population. Consistent with this idea, cultures at this stage of differentiation contain mainly neural rosettes, composed of neuroepithelial-like cells.
Our characterization of more mature NKX2.1:GFP-positive cells, in mixed populations with GFP-negative cells, revealed their ability to differentiate into GABAergic neurons, based on expression of GAD65, GABA, SST, and CB. While SST- and CB-expressing neurons represented 19% and 7.5% of NKX2.1:GFP-positive cells, respectively, after one month in culture, these cells were a small fraction of the total cell population. We did not detect any GFP-positive cells that also expressed CR, which is consistent with the in vivo generation of CR-positive neurons from CGE progenitors. Although the post-FACS NKX2.1-positive population consists predominantly of proliferative neural progenitors, on differentiation in vitro, these cells produce MAP2-positive clusters that include rare SST- or CHAT-positive neurons.
While the expression of appropriate neuropeptides and calcium binding proteins, in combination with expression of the telencephalic marker FOXG1 in NKX2.1:GFP-positive cells strongly suggests an MGE-like fate, we have not ruled out the presence of hypothalamic neurons in our cultures. Since Nkx2.1 is also involved in the specification of certain FOXG1-negative diencephalic progenitors that give rise to hypothalamic neurons, future experiments will investigate the generation of these neuronal subtypes which express both GABA and tyrosine hydroxylase [35]. Goulburn et al. have also shown that hESC-derived NKX2.1-positive progenitors can give rise to telencephalic oligodendrocytes expressing platelet-derived growth factor receptor alpha [31]. We have not determined whether our modified monolayer neural differentiation protocol generates these oligodendrocytes, and future work should investigate this population.
In our hands, FACS-enriched NKX2.1:GFP-positive cells have poor long-term survival after FACS, unless they are continually cultured in the presence of the basic FGF and EGF. These growth factors have previously been shown to stimulate proliferation and maintain NSC identity in cultured progenitors [36]. To fully differentiate GFP-positive cells into mature neurons, and allow identification of the interneuron subtypes they become, it is necessary to remove FGF and EGF from the culture medium. Co-culture of FACS-enriched GFP-positive cells with primary cultures of neocortical neurons may be required for complete maturation of these cells [19,37]. Goulburn et al. [31] demonstrated efficient differentiation of isolated NKX2.1:GFP-positive cells into GABA-positive neurons, after being cultured on dissociated neocortical neurons. Further, the presence of excitatory neurons in this co-culture system may allow for the formation of functional synapses between hESC-derived interneuron progenitors and the cortical neurons. These synaptic events and electrophysiological properties of the NKX2.1:GFP-positive cells may then be analyzed by patch-clamp recording.
Loss of GABAergic inhibitory interneurons is implicated in the pathophysiology of several neurological diseases, including temporal lobe epilepsy (TLE) [38–42]. Loss of inhibition occurs in part as a result of loss of PV- and SST-expressing interneurons within the hilus and CA1 regions of the hippocampus [38,43,44]. Cell-based therapies that are aimed at restoring these lost inhibitory cells are under development in animal models of epilepsy. Several studies have demonstrated effective reduction in the severity and total number of seizures observed, after transplantation of embryonic MGE-derived cells into rodent epilepsy models [45–48]. The use of embryonic MGE-derived cells for the treatment of human TLE presents several problems, however, including the lack of availability and uniformity of material for transplantation as well as ethical issues associated with obtaining this material. As an alternative, the generation of MGE-like progenitors from hESCs holds great potential.
Mouse ESNPs (mESNPs) derived using a monolayer neural differentiation protocol [49] have the ability to differentiate into GABAergic interneurons, including cells expressing CB, CR, and PV, in the hippocampus of pilocarpine-treated mice [50]. Some mESNPs transplanted in this epilepsy model had electrophysiological properties typical of hilar interneurons, and mESNP derivatives were morphologically similar to endogenous GABAergic interneurons [50]. We hypothesize that enrichment of ventral forebrain progenitors, derived from hESCs, will generate high percentages of PV- and SST-expressing neurons after transplantation into the mouse hippocampus. The success of cell-based therapies using hESC-derived ventral forebrain progenitors depends on their ability to mature in vivo, and establish functional connections to the host circuitry. Studies are currently underway to test the ability of hESC-derived NKX2.1-positive neural progenitors to differentiate into GABAergic interneurons after transplantation into the mouse hippocampus.
Our data thus far demonstrate an efficient means for differentiating and isolating NKX2.1-positive ventral forebrain progenitors from hESCs. Using a modified monolayer neural differentiation protocol supplemented with SHH treatment, we show that the generation of NKX2.1:GFP-positive cells is dependent on SHH activity and that the percentage of GFP-positive cells generated increases with SHH concentration. In vitro characterization of NKX2.1:GFP-positive cells reveals their similarity to corresponding progenitors in vivo, in terms of their gene expression profiles and ability to mature into GABAergic interneurons. By demonstrating the derivation of large populations of MGE-like progenitors from hESCs, this work represents progress in the development of hESC-based cell therapies for the replacement of inhibitory GABAergic interneurons.
Acknowledgments
The authors would like to thank Dr. Andrew Elefanty at Monash University in Victoria, Australia, for providing the hES3-NKX2.1:GFP hESCs. They also thank Diane Gran and Evan Jellison at the University of Connecticut Flow Cytometry Center for assistance with FACS. This work was supported by grants from the Connecticut Stem Cell Initiative to L.B.G and J.R.N.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hendry SH. Schwark HD. Jones EG. Yan J. Numbers and proportions of GABA-immunoreactive neurons in different areas of monkey cerebral cortex. J Neurosci. 1987;7:1503–1519. doi: 10.1523/JNEUROSCI.07-05-01503.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freund T. Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 3.Markram H. Toledo-Rodriguez M. Wang Y. Gupta A. Silberberg G. Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 4.Fishell G. Rudy B. Mechanisms of inhibition within the telencephalon: “where the wild things are.”. Annu Rev Neurosci. 2011;34:535–567. doi: 10.1146/annurev-neuro-061010-113717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sussel L. Marin O. Kimura S. Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- 6.Wonders CP. Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–695. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 7.Gelman DM. Martini FJ. Nobrega-Pereira S. Pierani A. Kessaris N. Marin O. The embryonic preoptic area is a novel source of cortical GABAergic interneurons. J Neurosci. 2009;29:9380–9389. doi: 10.1523/JNEUROSCI.0604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wichterle H. Garcia-Verdugo JM. Herrera DG. Alvarez-Buylla A. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat Neurosci. 1999;2:461–466. doi: 10.1038/8131. [DOI] [PubMed] [Google Scholar]
- 9.Pleasure SJ. Anderson S. Hevner R. Bagri A. Marin O. Lowenstein DH. Rubenstein JL. Cell migration from the ganglionic eminences is required for the development of hippocampal GABAergic interneurons. Neuron. 2000;28:727–740. doi: 10.1016/s0896-6273(00)00149-5. [DOI] [PubMed] [Google Scholar]
- 10.Butt SJ. Fuccillo M. Nery S. Noctor S. Kriegstein A. Corbin JG. Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 11.Corbin JG. Butt SJ. Developmental mechanisms for the generation of telencephalic interneurons. Dev Neurobiol. 2011;71:710–732. doi: 10.1002/dneu.20890. [DOI] [PubMed] [Google Scholar]
- 12.Liodis P. Denaxa M. Grigoriou M. Akufo-Addo C. Yanagawa Y. Pachnis V. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J Neurosci. 2007;27:3078–3089. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nobrega-Pereira S. Kessaris N. Du T. Kimura S. Anderson SA. Marin O. Postmitotic Nkx2-1 controls the migration of telencephalic interneurons by direct repression of guidance receptors. Neuron. 2008;59:733–745. doi: 10.1016/j.neuron.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nat R. Dechant G. Milestones of directed differentiation of mouse and human embryonic stem cells into telencephalic neurons based on neural development in vivo. Stem Cells Dev. 2011;20:947–958. doi: 10.1089/scd.2010.0417. [DOI] [PubMed] [Google Scholar]
- 15.Marin O. Baker J. Puelles L. Rubenstein JL. Patterning of the basal telencephalon and hypothalamus is essential for guidance of cortical projections. Development. 2002;129:761–773. doi: 10.1242/dev.129.3.761. [DOI] [PubMed] [Google Scholar]
- 16.Ericson J. Muhr J. Placzek M. Lints T. Jessell T. Edlund T. Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell. 1995;81:747–756. doi: 10.1016/0092-8674(95)90536-7. [DOI] [PubMed] [Google Scholar]
- 17.Yu W. Wang Y. McDonnell K. Stephen D. Bai CB. Patterning of ventral telencephalon requires positive function of Gli transcription factors. Dev Biol. 2009;334:264–275. doi: 10.1016/j.ydbio.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 18.Wonders CP. Taylor L. Welagen J. Mbata IC. Xiang JZ. Anderson SA. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev Biol. 2008;314:127–136. doi: 10.1016/j.ydbio.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Q. Wonders CP. Anderson SA. Sonic hedgehog maintains the identity of cortical interneuron progenitors in the ventral telencephalon. Development. 2005;132:4987–4998. doi: 10.1242/dev.02090. [DOI] [PubMed] [Google Scholar]
- 20.Xu Q. Guo L. Moore H. Waclaw RR. Campbell K. Anderson SA. Sonic hedgehog signaling confers ventral telencephalic progenitors with distinct cortical interneuron fates. Neuron. 2010;65:328–340. doi: 10.1016/j.neuron.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stenman JM. Wang B. Campbell K. Tlx controls proliferation and patterning of lateral telencephalic progenitor domains. J Neurosci. 2003;23:10568–10576. doi: 10.1523/JNEUROSCI.23-33-10568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sousa VH. Miyoshi G. Hjerling-Leffler J. Karayannis T. Fishell G. Characterization of Nkx6-2-derived neocortical interneuron lineages. Cereb Cortex. 2009;19(Suppl 1):i1–10. doi: 10.1093/cercor/bhp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakovcevski I. Mayer N. Zecevic N. Multiple origins of human neocortical interneurons are supported by distinct expression of transcription factors. Cereb Cortex. 2011;21:1771–1782. doi: 10.1093/cercor/bhq245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rakic S. Zecevic N. Emerging complexity of layer I in human cerebral cortex. Cereb Cortex. 2003;13:1072–1083. doi: 10.1093/cercor/13.10.1072. [DOI] [PubMed] [Google Scholar]
- 25.Westmoreland JJ. Hancock CR. Condie BG. Neuronal development of embryonic stem cells: a model of GABAergic neuron differentiation. Biochem Biophys Res Commun. 2001;284:674–680. doi: 10.1006/bbrc.2001.5031. [DOI] [PubMed] [Google Scholar]
- 26.Barberi T. Klivenyi P. Calingasan NY. Lee H. Kawamata H. Loonam K. Perrier AL. Bruses J. Rubio ME, et al. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol. 2003;21:1200–1207. doi: 10.1038/nbt870. [DOI] [PubMed] [Google Scholar]
- 27.Danjo T. Eiraku M. Muguruma K. Watanabe K. Kawada M. Yanagawa Y. Rubenstein JL. Sasai Y. Subregional specification of embryonic stem cell-derived ventral telencephalic tissues by timed and combinatory treatment with extrinsic signals. J Neurosci. 2011;31:1919–1933. doi: 10.1523/JNEUROSCI.5128-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bissonnette CJ. Lyass L. Bhattacharyya BJ. Belmadani A. Miller RJ. Kessler JA. The controlled generation of functional basal forebrain cholinergic neurons from human embryonic stem cells. Stem Cells. 2011;29:802–811. doi: 10.1002/stem.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li XJ. Zhang X. Johnson MA. Wang ZB. Lavaute T. Zhang SC. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development. 2009;136:4055–4063. doi: 10.1242/dev.036624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma L. Hu B. Liu Y. Vermilyea SC. Liu H. Gao L. Sun Y. Zhang X. Zhang SC. Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell. 2012;10:455–464. doi: 10.1016/j.stem.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goulburn AL. Alden D. Davis RP. Micallef SJ. Ng ES. Yu QC. Lim SM. Soh CL. Elliott DA, et al. A targeted NKX2.1 human embryonic stem cell reporter line enables identification of human basal forebrain derivatives. Stem Cells. 2011;29:462–473. doi: 10.1002/stem.587. [DOI] [PubMed] [Google Scholar]
- 32.Gerrard L. Rodgers L. Cui W. Differentiation of human embryonic stem cells to neural lineages in adherent culture by blocking bone morphogenetic protein signaling. Stem Cells. 2005;23:1234–1241. doi: 10.1634/stemcells.2005-0110. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X. Huang CT. Chen J. Pankratz MT. Xi J. Li J. Yang Y. Lavaute TM. Li XJ, et al. Pax6 is a human neuroectoderm cell fate determinant. Cell Stem Cell. 2010;7:90–100. doi: 10.1016/j.stem.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marin O. Anderson SA. Rubenstein JL. Origin and molecular specification of striatal interneurons. J Neurosci. 2000;20:6063–6076. doi: 10.1523/JNEUROSCI.20-16-06063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohyama K. Ellis P. Kimura S. Placzek M. Directed differentiation of neural cells to hypothalamic dopaminergic neurons. Development. 2005;132:5185–5197. doi: 10.1242/dev.02094. [DOI] [PubMed] [Google Scholar]
- 36.Conti L. Pollard SM. Gorba T. Reitano E. Toselli M. Biella G. Sun Y. Sanzone S. Ying QL. Cattaneo E. Smith A. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005;3:e283. doi: 10.1371/journal.pbio.0030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song HJ. Stevens CF. Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002;5:438–445. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- 38.de Lanerolle NC. Kim JH. Robbins RJ. Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495:387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- 39.Magloczky Z. Wittner L. Borhegyi Z. Halasz P. Vajda J. Czirjak S. Freund T. Changes in the distribution and connectivity of interneurons in the epileptic human dentate gyrus. Neuroscience. 2000;96:7–25. doi: 10.1016/s0306-4522(99)00474-1. [DOI] [PubMed] [Google Scholar]
- 40.Morimoto K. Fahnestock M. Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Houser CR. Miyashiro JE. Swartz BE. Walsh GO. Rich JR. Delgado-Escueta AV. Altered patterns of dynorphin immunoreactivity suggest mossy fiber reorganization in human hippocampal epilepsy. J Neurosci. 1990;10:267–282. doi: 10.1523/JNEUROSCI.10-01-00267.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibley H. Smith BN. Pilocarpine-induced status epilepticus results in mossy fiber sprouting and spontaneous seizures in C57BL/6 and CD-1 mice. Epilepsy Res. 2002;49:109–120. doi: 10.1016/s0920-1211(02)00012-8. [DOI] [PubMed] [Google Scholar]
- 43.Obenaus A. Esclapez M. Houser CR. Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J Neurosci. 1993;13:4470–4485. doi: 10.1523/JNEUROSCI.13-10-04470.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thind KK. Yamawaki R. Phanwar I. Zhang G. Wen X. Buckmaster PS. Initial loss but later excess of GABAergic synapses with dentate granule cells in a rat model of temporal lobe epilepsy. J Comp Neurol. 2010;518:647–667. doi: 10.1002/cne.22235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baraban SC. Southwell DG. Estrada RC. Jones DL. Sebe JY. Alfaro-Cervello C. Garcia-Verdugo JM. Rubenstein JL. Alvarez-Buylla A. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Prot Natl Acad Sci U S A. 2009;106:15472–15477. doi: 10.1073/pnas.0900141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zipancic I. Calcagnotto ME. Piquer-Gil M. Mello LE. Alvarez-Dolado M. Transplant of GABAergic precursors restores hippocampal inhibitory function in a mouse model of seizure susceptibility. Cell Transplant. 2010;19:549–564. doi: 10.3727/096368910X491383. [DOI] [PubMed] [Google Scholar]
- 47.Calcagnotto ME. Ruiz LP. Blanco MM. Santos-Junior JG. Valente MF. Patti C. Frussa-Filho R. Santiago MF. Zipancic I, et al. Effect of neuronal precursor cells derived from medial ganglionic eminence in an acute epileptic seizure model. Epilepsia. 2010;51(Suppl 3):71–75. doi: 10.1111/j.1528-1167.2010.02614.x. [DOI] [PubMed] [Google Scholar]
- 48.Tagliatela S. Litvina E. Maisano X. Woods N. Vallo M. Royston S. Gupta J. Yanagawa Y. Naegele JR. Long-term functional integration and persistent seizure suppression by hilar but not entorhinal cortex grafts of fetal GABAergic interneuron progenitors in mice with temporal lobe epilepsy. American Epilepsy Society 65th Annual Meeting; Baltimore, MD. 2011. [Google Scholar]
- 49.Cai C. Thorne J. Grabel L. Hedgehog serves as a mitogen and survival factor during embryonic stem cell neurogenesis. Stem Cells. 2008;26:1097–1108. doi: 10.1634/stemcells.2007-0684. [DOI] [PubMed] [Google Scholar]
- 50.Maisano X. Litvina E. Tagliatela S. Aaron GB. Grabel LB. Naegele JR. Differentiation and functional incorporation of embryonic stem cell-derived GABAergic interneurons in the dentate gyrus of mice with temporal lobe epilepsy. J Neurosci. 2012;32:46–61. doi: 10.1523/JNEUROSCI.2683-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]