Abstract
Fine-needle aspiration (FNA) is a reliable method for preoperative diagnosis of thyroid nodules; however, about 10%–40% nodules are classified as indeterminate. The BRAFV600E mutation is the most promising marker for thyroid FNA. This meta-analysis was conducted to investigate the diagnostic value of BRAFV600E analysis in thyroid FNA, especially the indeterminate cases. Systematic searches were performed in PubMed, Web of Science, Scopus, Ovid, Elsevier, and the Cochrane Library databases for relevant studies prior to June 2015, and a total of 88 studies were ultimately included in this meta-analysis. Compared with FNA cytology, the synergism of BRAFV600E testing increased the diagnostic sensitivity from 81.4% to 87.4% and decreased the false-negative rate from 8% to 5.2%. In the indeterminate group, the mutation rate of BRAFV600E was 23% and varied in different subcategories (43.2% in suspicious for malignant cells [SMC], 13.77% in atypia of undetermined significance/follicular lesion of undetermined significance [AUS/FLUS], and 4.43% in follicular neoplasm/suspicious for follicular neoplasm [FN/SFN]). The sensitivity of BRAFV600E analysis was higher in SMC than that in AUS/FLUS and FN/SFN cases (59.4% vs 40.1% vs 19.5% respectively), while specificity was opposite (86.1% vs 99.5% vs 99.7% respectively). The areas under the summary receiver-operating characteristic curve also confirmed the diagnostic value of BRAFV600E testing in SMC and AUS/FLUS rather than FN/SFN cases. Therefore, BRAFV600E analysis can improve the diagnostic accuracy of thyroid FNA, especially indeterminate cases classified as SMC, and select malignancy to guide the extent of surgery.
Keywords: thyroid cancer, fine-needle aspiration, BRAFV600E mutation, meta-analysis
Introduction
Thyroid cancer is the most common endocrine malignancy, with favorable outcome after early detection and treatment.1,2 Fine-needle aspiration (FNA) guided by ultrasound is a routine and reliable approach for preoperative evaluation of thyroid nodules. Approximately 10%–40% of FNA specimens yield indeterminate results, and the majority of them turn out to be benign after diagnostic surgery, and thus a sizable portion of indeterminate specimens lead to unnecessary thyroidectomy.3–7 The Bethesda System for Reporting Thyroid Cytopathology divides indeterminate nodules into three subgroups: atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS), follicular neoplasm/suspicious for follicular neoplasm (FN/SFN), and suspicious for malignant cells (SMC).8 The indeterminate thyroid nodule is the most intractable problem in clinical management, which highlights the urgency to develop effective ancillary testing to identify cancerous nodules for timely and appropriate management.
Great progress has been achieved in the understanding of molecular mechanisms of thyroid cancer, and various mutations have been identified in the early stage of thyroid cancer, such as BRAF, RAS, PI3K, and PTEN.9 These genetic alterations are excellent candidates for disease hallmarks, since 60%–70% of thyroid cancers harbor at least one genetic mutation.9 The BRAFV600E mutation appears to be the most promising biomarker specific for papillary thyroid cancer (PTC),9 which aberrantly activates the tumor-initiating MAPK pathway and drives the carcinogenesis and progression of thyroid cancer.9,10
Whether BRAFV600E analysis could be routinely used in clinical practice is still controversial. Numerous researchers have proved that BRAFV600E-mutation testing is an effective diagnostic approach for thyroid FNA,11 while others believe that its utility is limited by low prevalence of BRAFV600E mutation in indeterminate nodules.12 Therefore, we conducted a structured meta-analysis to estimate the additional diagnostic yield of BRAFV600E-mutation analysis in thyroid FNA, and further evaluated the malignancy rate, BRAFV600E-mutation frequency, and diagnostic value of BRAFV600E testing in different categories of indeterminate nodule.
Materials and methods
Search strategy and selection criteria
Systematic searches were performed in the PubMed, Web of Science, Scopus, Ovid, Elsevier, and Cochrane Library databases for relevant articles prior to June 2015. The search terms were: ([thyroid cancer] or [thyroid neoplasm] or [thyroid tumor]), (BRAF), and ([FNA] or [fine needle aspiration]). The references of available articles were also reviewed. Study selection consisted of initial screening of titles or abstracts and second screening of full texts. Studies were included if they met the following criteria: 1) research article rather than review, system review, case report, editorial, or comments; 2) the material for BRAFV600E-mutation analysis was obtained by FNA; 3) the final diagnosis was based on a definite gold standard, such as surgical histology, unequivocal histocytopathology, or reliable clinical follow-up; 4) the data were available to construct 2×2 tables or analyze malignancy rate or BRAFV600E-mutation prevalence.
Data extraction and quality assessment
The following items were extracted: study by author name(s), country, number of centers, enrollment period, study design, mean age of patients, mean diameter of nodules, reference standard of final diagnosis, and genotyping method. Most research classified cytological results according to the Bethesda system8 or the British Thyroid Association,13,14 as shown in Table 1. In this meta-analysis, FNA cases classified as AUS/FLUS (Thy3a) and FN/SFN (Thy3f) were regarded as cytologically negative and lesions diagnosed as SMC (Thy4) were cytologically positive. Final diagnosis was based on histopathologic examination after surgery or a combination of cytological examination and clinical follow-up. Then, patient numbers for true-positive, false-positive, false-negative, and true-negative results were extracted to construct the 2×2 tables.
Table 1.
Comparison between the British and Bethesda systems for classification of thyroid cytopathology
| Bethesda | British |
|---|---|
| Nondiagnostic or unsatisfactory | Thy1 (nondiagnostic) |
| Benign | Thy2 (nonneoplastic) |
| AUS/FLUS (atypia of undetermined significance/follicular lesion of undetermined significance) | Thy3a (neoplasm possible, atypia/nondiagnostic) |
| FN/SFN (follicular neoplasm/suspicious for follicular neoplasm) | Thy3f (neoplasm possible, suggesting follicular neoplasm) |
| SMC (suspicious for malignancy) | Thy4 (suspicious of malignancy) |
| Malignant | Thy5 (malignant) |
The methodological quality of studies eligible for diagnostic analysis of FNA cytology and/or BRAFV600E testing was assessed according to the Quality Assessment of Diagnostic Studies 2, which comprises four domains: patient selection, index test, reference standard, and flow and timing.15 A series of questions was used to judge the risk of bias and applicability concerns as low, high, or unclear risk.
Statistical analysis
The threshold effect was calculated by the Spearman correlation coefficient, and P<0.05 indicated the existence of a threshold effect. Nonthreshold heterogeneity was assessed by the Cochran Q test and inconsistency index (I2). I2>50% suggested significant heterogeneity, and a random-effect model (DerSimonian–Laird method) was chosen.16,17 Metaregression analysis was used to identify the possible sources of nonthreshold heterogeneity. The following covariates were considered in the metaregression analysis: country, number of centers (single or multiple), sample size (<100, 100–500, 500–1,000, or >1,000), study design (prospective or retrospective), reference standard (histology or cytology plus clinical follow-up), and genotyping method. If P<0.05, the covariate was to be regarded as the source of nonthreshold heterogeneity.
The pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) with 95% confidence interval (CI) were computed to estimate diagnostic accuracy. DOR combined the data of sensitivity and specificity into a single indicator ranging from 0 to infinity, reflecting the discriminatory performance of testing. The summary receiver-operating characteristic (SROC) curve was a mathematical model for the plot of sensitivity (1 – specificity). The Q index indicated the point at which sensitivity was equal to specificity. The areas under the SROC curve (AUCs) calculated the inherent capacity of the diagnostic test. If the AUC closed to 1, the diagnostic method was thought to be perfect.
The threshold effect, pooled diagnostic features, and metaregression were calculated by Meta-Disc (version 1.4; Ramony Cajal Hospital, Madrid, Spain). Pooled rates of malignancy and BRAFV600E mutation were calculated by R statistical software (version 3.2.1; R Foundation for Statistical Computing, Vienna, Austria). Quality assessment was conducted using Review Manager (version 5.2; Cochrane Collaboration). P<0.05 was considered statistically significant.
Results
Search results and quality assessment
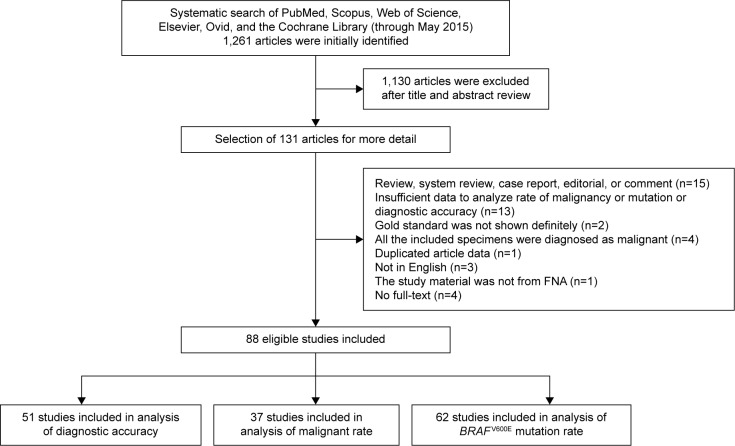
The search process is shown in Figure 1. A total of 1,261 articles were initially identified, and 1,130 of these were excluded after reviewing titles and abstracts. The remaining 131 articles were investigated in detail. In accordance with the selection criteria mentioned in the Materials and methods section, 43 articles were excluded after reading the full texts. Finally, 88 studies published from 2004 to 2015 were included in this meta-analysis. Among these, 51 studies were included in the analysis of diagnostic accuracy, and at the same time 37 studies and 62 studies were available for analysis of malignancy rate and BRAFV600E-mutation rate, respectively.
Figure 1.
Flowchart of study-selection process.
Abbreviation: FNA, fine-needle aspiration.
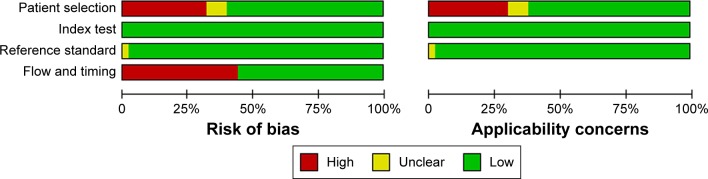
The characteristics of studies eligible for diagnostic analysis of FNA cytology and BRAFV600E testing are summarized in Table 2. As shown in Figure 2, about a third of studies had a high risk of bias in patient selection, because 14 of them did not enroll the samples consecutively or at random and eleven excluded a number of patients inappropriately. Twelve studies did not receive the same reference standard, since some patients were diagnosed by histopathology and others by FNA cytology plus clinical follow-up. Also, 17 studies did not include all patients, due to the unsatisfactory FNA or failure of BRAFV600E testing. As a result, nearly half of the studies harbored a high risk of bias in flow and timing. Fortunately, the risk of bias in the index test and reference standard was relatively low.
Table 2.
Characteristics of studies eligible for the diagnostic analysis of FNA cytology and BRAFV600E testing
| Study | Country | Centers, n | Enrollment period | Design | Mean age, years | Mean diameter, cm | Final diagnosis | Genotyping method |
|---|---|---|---|---|---|---|---|---|
| Cohen et al18 | USA | 1 | Jan 2001–Jan 2003 | Retroa | – | – | A | Direct sequencing + mutector assay |
| Xing et al19 | USA | 1 | – | Prob | – | – | B | Direct sequencing + colorimetric method |
| Domingues et al20 | Portugal | 1 | – | Retro | – | – | A | PCR-RFLP |
| Pizzolanti et al21 | Italy | 1 | Sep 2005–Jun 2006 | Pro | – | – | A | Real-time AS-PCR |
| Sapio et al22 | Italy | 2 | – | Retro | – | – | B | Direct sequencing |
| Sapio et al23 | Italy | 2 | – | Retro | – | – | B | MASA |
| Kim et al24 | South Korea | 1 | Aug 2005–Jul 2006 | Retro | – | – | A | Pyrosequencing |
| Bentz et al25 | USA | 1 | 1994–2004 | Retro | 40.9 | – | A | LCPCR + FMCA |
| Jo et al26 | South Korea | 1 | June 2006–Dec 2006 | Pro | – | 1 | A | Pyrosequencing |
| Marchetti et al27 | Italy | 1 | 1996–2008 | Retro | – | – | A | Direct sequencing |
| Nikiforov et al28 | USA | 2 | – | Pro | – | – | B | LCPCR + FMCA |
| Zatelli et al29 | Italy | 1 | Oct 2008–Dec 2009 | Pro | 50.7 | 1.1 | A | Direct sequencing |
| Cantara et al30 | Italy | 1 | – | Pro | 51.2 | – | A | DHPLC + direct sequencing |
| Girlando et al31 | Italy | 1 | – | Pro | – | – | A | Direct sequencing |
| Kim et al32 | South Korea | 1 | – | Pro | 50.6 | 1.29 | A | DPO-based multiplex PCR + direct sequencing |
| Kwak et al33 | South Korea | 1 | Mar 2008–Jun 2008 | Retro | 45.6 | 1.17 | A | DPO-based multiplex PCR |
| Moses et al34 | USA | 1 | Jun 2006–Jul 2008 | Pro | 51 | – | B | Direct sequencing |
| Musholt et al35 | Germany | 6 | Jan 2008–Jul 2009 | Pro | – | – | A | Direct sequencing |
| Adeniran et al36 | USA | 1 | Sep 2009–Nov 2010 | Pro | 52.6 | – | A | SSCP analysis |
| Kim et al37 | South Korea | 1 | Mar 2007–Feb 2009 | Pro | – | – | A | Pyrosequencing |
| Lee et al38 | South Korea | 1 | July 2007–Dec 2009 | Pro | 50.3 | 1.46 | A | Pyrosequencing |
| Moon et al39 | South Korea | 1 | Sep 2008–May 2009 | Retro | 49.4 | 0.95 | B | Direct sequencing |
| Pelizzo et al40 | Italy | 1 | Oct 2008–Sep 2009 | Pro | 47.8 | – | A | Direct sequencing + MASA |
| Smith et al41 | USA | 1 | – | Retro | – | – | A | MCA |
| Yeo et al42 | South Korea | 1 | Jul 2009–Jan 2010 | Pro | 51.27 | 1.3 | B | Pyrosequencing |
| Cañadas-Garre et al43 | Spain | 1 | Jun 2006–Dec 2009 | Pro | 49.8 | – | A | PCR-RFLP |
| Kang et al44 | South Korea | 1 | Apr 2008–Jul 2009 | Pro | – | – | A | AS-PCR + direct sequencing |
| Kwak et al45 | South Korea | 1 | Jun 2009–Oct 2010 | Retro | 48 | 0.92 | A | DPO-PCR + real-time PCR |
| Lee et al46 | South Korea | 1 | Aug 2008–Mar 2011 | Pro | 49.5 | – | A | MEMO-PCR + direct sequencing |
| Mancini et al47 | Italy | 1 | – | Pro | 55.1 | 2.38 | A | High-resolution melting analysis |
| Rossi et al48 | Italy | 1 | – | Pro | 52 | – | B | Direct sequencing |
| Tomei et al49 | Italy | 1 | – | Retro | – | – | A | Pyrosequencing |
| Brahma et al50 | Indonesia | 3 | Aug 2010–Jun 2011 | Pro | 46 | .1 | A | PCR-RFLP |
| Di Benedetto et al51 | Italy | 1 | – | Pro | – | – | A | Direct sequencing |
| Koh et al52 | South Korea | 1 | Jan 2009–Oct 2010 | Pro | 48.6 | 1.05 | B | DPO-PCR |
| Park et al53 | South Korea | 1 | Jan 2011–May 2011 | Retro | – | – | B | Real-time PCR + pyrosequencing |
| Beaudenon-Huibregtse et al54 | USA | 5 | Jul 2010–Oct 2012 | Pro | – | – | A | Multiplex PCR |
| Crescenzi et al55 | Italy | 1 | – | Pro | – | – | A | Real-time sequencing |
| Eszlinger et al56 | Germany | 1 | 1995–2009 | Retro | – | – | A | High-resolution melting PCR + pyrosequencing |
| Guo et al57 | PRC | 1 | Nov 2010–Jul 2011 | Pro | – | – | A | Direct sequencing |
| Johnson et al58 | UK | 1 | Sep 2011–Oct 2012 | Retro | – | – | A | High-resolution MCA |
| Liu et al59 | PRC | 1 | Sep 2012–Dec 2013 | Pro | – | – | B | Pyrosequencing |
| Seo et al60 | South Korea | 1 | Dec 2010–Jan 2011 | Pro | 48.4 | 1.11 | A | Real-time PCR |
| Seo et al61 | South Korea | 1 | Dec 2010–Feb 2012 | Pro | 50.3 | 1.9 | B | Real-time PCR |
| Wan et al62 | PRC | 1 | Mar 2013–Sep 2013 | Pro | 49 | A | – | |
| Zeck et al63 | USA | 1 | Apr 2011–Jan 2013 | Pro | – | – | A | miRInform test |
| Eszlinger et al64 | Italy | 1 | 1995–2009 | Retro | – | – | A | High-resolution melting analysis + pyrosequencing |
| Krane et al65 | Germany | 1 | May 2011–Mar 2012 | Pro | – | – | A | High-resolution melting PCR + pyrosequencing |
| Park et al66 | South Korea | 1 | Jul 2011–Mar 2012 | Pro | – | – | A | Real-time PCR/AS-PCR + MEMO sequencing |
| Shi et al67 | USA | 1 | Jan 2011–Feb 2013 | Retro | – | – | A | Real-time PCR |
Notes:
Retrospective;
prospective; A, histopathologic examination after surgery; B, combination of cytological examination and clinical follow-up.
Abbreviations: PCR-RFLP, polymerase chain reaction–restriction fragment-length polymorphism; AS, allele-specific; MASA, mutant allele-specific amplification; LCPCR, LightCycler PCR; FMCA, fluorescent melting-curve analysis; SSCP, single-strand conformational polymorphism; DHPLC, denaturing high-performance liquid chromatography; DPO, dual-priming oligonucleotide; MEMO, 3′-modified oligonucleotide; PRC, People’s Republic of China; FNA, fine-needle aspiration; –, data not available.
Figure 2.
Methodological quality of studies included, assessed by the Quality Assessment of Diagnostic Studies 2 criteria.
Synthesis of analysis results
Diagnostic value of FNA cytology, BRAFV600E-mutation analysis, and combined strategy in all the thyroid FNA specimens
Spearman correlation coefficients for FNA cytology, BRAFV600E testing and combined strategy were 0.032 (P=0.826), 0.254 (P=0.078), and 0.064 (P=0.661), respectively; therefore, no threshold effect existed in the analysis. However, there was substantial nonthreshold heterogeneity (I2>50%, P<0.05), so the random-effect model was chosen to pool the diagnostic features. A total of 51 studies were included in this part of the analysis,18–68 but one was excluded because it had no false-positive or true-negative case to calculate the diagnostic index (Table 3).68
Table 3.
Diagnostic analysis of FNA cytological examination and BRAFV600E-mutation analysis in all the FNA specimens
| Study | Year | FNA
|
BRAF
|
FNA + BRAF
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | FP | FN | TN | TP | FP | FN | TN | TP | FP | FN | TN | ||
| Cohen et al18 | 2004 | 25 | 0 | 34 | 32 | 23 | 0 | 36 | 32 | 30 | 0 | 29 | 32 |
| Xing et al19 | 2004 | 10 | 0 | 19 | 12 | 8 | 0 | 22 | 14 | 12 | 0 | 17 | 12 |
| Domingues et al20 | 2005 | 10 | 0 | 3 | 11 | 3 | 0 | 10 | 11 | 10 | 0 | 3 | 11 |
| Pizzolanti et al21 | 2007 | 13 | 0 | 4 | 32 | 11 | 0 | 6 | 32 | 15 | 0 | 2 | 32 |
| Sapio et al22 | 2007 | 24 | 23 | 2 | 95 | 10 | 0 | 16 | 118 | 25 | 23 | 1 | 95 |
| Sapio et al23 | 2007 | 6 | 0 | 2 | 67 | 4 | 0 | 4 | 123 | 6 | 0 | 2 | 67 |
| Kim et al24 | 2008 | 60 | 0 | 21 | 22 | 63 | 0 | 18 | 22 | 73 | 0 | 8 | 22 |
| Bentz et al25 | 2009 | 22 | 0 | 18 | 5 | 17 | 0 | 20 | 5 | 24 | 0 | 16 | 5 |
| Jo et al26 | 2009 | 30 | 0 | 9 | 58 | 30 | 0 | 10 | 58 | 38 | 0 | 2 | 58 |
| Marchetti et al27 | 2009 | 88 | 2 | 4 | 17 | 59 | 0 | 32 | 19 | 88 | 2 | 4 | 17 |
| Nikiforov et al28 | 2009 | 27 | 2 | 21 | 36 | 18 | 0 | 30 | 38 | 33 | 2 | 15 | 36 |
| Zatelli et al29 | 2009 | 66 | 5 | 24 | 373 | 48 | 0 | 42 | 378 | 73 | 5 | 17 | 373 |
| Cantara et al30 | 2010 | 46 | 8 | 16 | 112 | 33 | 0 | 45 | 157 | 50 | 8 | 12 | 112 |
| Girlando et al31 | 2010 | 38 | 0 | 22 | 2 | 41 | 0 | 19 | 2 | 51 | 0 | 9 | 2 |
| Kim et al32 | 2010 | 251 | 2 | 6 | 690 | 221 | 5 | 47 | 688 | 253 | 6 | 4 | 686 |
| Kwak et al33 | 2010 | 108 | 10 | 1 | 10 | 87 | 0 | 22 | 20 | 109 | 10 | 0 | 10 |
| Moses et al34 | 2010 | 71 | 13 | 30 | 337 | 23 | 0 | 78 | 95 | 75 | 13 | 27 | 336 |
| Musholt et al35 | 2010 | 19 | 13 | 11 | 50 | 9 | 0 | 21 | 63 | 23 | 13 | 7 | 50 |
| Adeniran et al36 | 2011 | 47 | 0 | 13 | 12 | 40 | 0 | 20 | 12 | 55 | 0 | 5 | 12 |
| Kim et al37 | 2011 | 146 | 0 | 27 | 21 | 154 | 1 | 19 | 20 | 167 | 0 | 6 | 21 |
| Lee et al38 | 2011 | 127 | 0 | 70 | 29 | 174 | 1 | 24 | 28 | 183 | 0 | 15 | 29 |
| Moon et al39 | 2011 | 98 | 0 | 10 | 191 | 57 | 0 | 51 | 191 | 105 | 0 | 3 | 191 |
| Pelizzo et al40 | 2011 | 133 | 5 | 6 | 117 | 98 | 0 | 59 | 113 | 138 | 5 | 3 | 124 |
| Smith et al41 | 2011 | 10 | 0 | 5 | 5 | 10 | 0 | 5 | 5 | 11 | 0 | 4 | 5 |
| Yeo et al42 | 2011 | 183 | 1 | 9 | 709 | 99 | 0 | 93 | 710 | 185 | 1 | 7 | 709 |
| Cañadas-Garre et al43 | 2012 | 12 | 0 | 31 | 132 | 17 | 0 | 31 | 160 | 23 | 0 | 25 | 162 |
| Kang et al44 | 2012 | 289 | 1 | 15 | 8 | 226 | 2 | 78 | 7 | 291 | 3 | 13 | 6 |
| Kwak et al45 | 2012 | 318 | 0 | 33 | 86 | – | – | – | – | 192 | 85 | 1 | 169 |
| Lee et al46 | 2012 | 382 | 1 | 47 | 33 | 342 | 0 | 87 | 34 | 398 | 1 | 31 | 33 |
| Mancini et al47 | 2012 | 13 | 1 | 10 | 32 | 12 | 0 | 11 | 33 | 16 | 1 | 7 | 32 |
| Marchetti et al68 | 2012 | 85 | 0 | 5 | 0 | 63 | 0 | 22 | 0 | 32 | 0 | 15 | 0 |
| Rossi et al48 | 2012 | 159 | 3 | 73 | 1,621 | 114 | 0 | 172 | 93 | 193 | 4 | 42 | 1,672 |
| Tomei et al49 | 2012 | 44 | 0 | 5 | 38 | 28 | 0 | 21 | 38 | 44 | 0 | 5 | 38 |
| Brahma et al50 | 2013 | 23 | 0 | 26 | 21 | 17 | 0 | 32 | 21 | 25 | 0 | 24 | 21 |
| Di Benedetto et al51 | 2013 | 15 | 1 | 3 | 239 | 13 | 0 | 5 | 240 | 17 | 1 | 1 | 239 |
| Koh et al52 | 2013 | 277 | 0 | 27 | 194 | 176 | 3 | 141 | 198 | 287 | 3 | 30 | 198 |
| Park et al53 | 2013 | 71 | 5 | 8 | 31 | 44 | 1 | 37 | 35 | 76 | 5 | 3 | 31 |
| Beaudenon-Huibregtse et al54 | 2014 | 36 | 4 | 18 | 49 | 21 | 0 | 35 | 53 | 37 | 4 | 19 | 49 |
| Crescenzi et al55 | 2014 | 20 | 0 | 1 | 9 | 8 | 0 | 13 | 9 | 20 | 0 | 1 | 9 |
| Eszlinger et al56 | 2014 | 57 | 0 | 28 | 225 | 22 | 0 | 43 | 188 | 57 | 0 | 28 | 225 |
| Guo et al57 | 2014 | 55 | 1 | 8 | 19 | 41 | 0 | 22 | 20 | 57 | 1 | 6 | 19 |
| Johnson et al58 | 2014 | 31 | 3 | 19 | 44 | 16 | 0 | 28 | 42 | 29 | 3 | 17 | 44 |
| Liu et al59 | 2014 | 109 | 8 | 11 | 171 | 88 | 0 | 32 | 179 | 113 | 8 | 7 | 171 |
| Seo et al60 | 2014 | 115 | 0 | 17 | 7 | 98 | 0 | 34 | 7 | 121 | 0 | 11 | 7 |
| Seo et al61 | 2014 | 42 | 4 | 18 | 36 | 32 | 0 | 28 | 36 | 45 | 4 | 15 | 36 |
| Wan et al62 | 2014 | 18 | 0 | 23 | 7 | 25 | 0 | 16 | 7 | 30 | 0 | 11 | 7 |
| Zeck et al63 | 2014 | 7 | 2 | 6 | 6 | 5 | 0 | 8 | 8 | 7 | 2 | 6 | 6 |
| Eszlinger et al64 | 2015 | 69 | 1 | 68 | 201 | 57 | 0 | 80 | 201 | 80 | 1 | 57 | 201 |
| Krane et al65 | 2015 | 54 | 2 | 19 | 77 | 32 | 0 | 41 | 79 | 60 | 2 | 13 | 77 |
| Park et al66 | 2015 | 111 | 0 | 13 | 34 | 101 | 1 | 23 | 33 | 116 | 1 | 8 | 32 |
| Shi et al67 | 2015 | 20 | 0 | 3 | 7 | 11 | 0 | 12 | 7 | 20 | 0 | 3 | 7 |
Abbreviations: FNA, fine-needle aspiration; TP, true positive; FP, false positive; FN, false negative; TN, true negative; –, data not available.
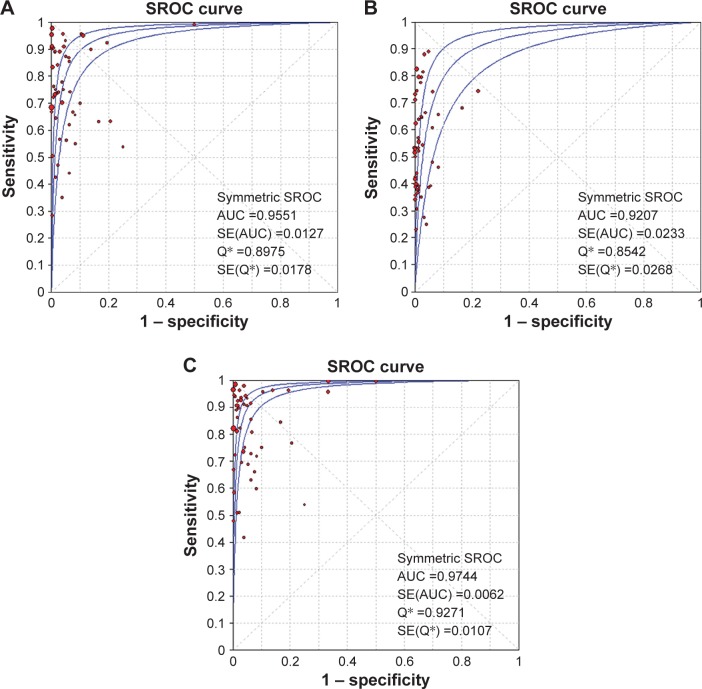
Based on the feasible FNA cytology results from 50 studies, pooled sensitivity, specificity, PLR, NLR, and DOR were 0.814 (95% CI 0.803–0.824), 0.981 (95% CI 0.978–0.985), 23.868 (95% CI 14.139–40.293), 0.216 (95% CI 0.172–0.273), and 127.73 (95% CI 75.082–217.28) (Table 4). The AUC of the SROC curve was 0.9551 (standard error [SE] 0.0127), with a Q-value of 0.8975 (SE 0.0178) (Figure 3A). Data for the BRAFV600E-mutation test were unavailable in one study,45 and 49 studies with 9,361 patients were finally analyzed. Pooled sensitivity, specificity, PLR, NLR, and DOR were 0.619 (95% CI 0.605–0.633), 0.997 (95% CI 0.995–0.998), 34.982 (95% CI 23.801–51.415), 0.433 (95% CI 0.384–0.489), and 96.570 (95% CI 63.932–145.87) (Table 4). The AUC of the SROC was 0.9207 (SE 0.0233), with a Q-value of 0.8542 (SE 0.0268) (Figure 3B). Also, the positive predictive value of BRAFV600E testing was 99.5% (2,886 of 2,900). After BRAFV600E analysis was combined with FNA cytology, sensitivity increased to 0.874 (95% CI 0.865–0.884), the DOR and AUC improved to 187.92 (95% CI 110.24–320.35) and 0.9744 (SE 0.0062), respectively, with a Q-value of 0.9271 (SE 0.0107) (Table 4, Figure 3C). The synergism between FNA cytology and BRAFV600E testing also decreased the false-negative rate from 8% in FNA cytology to 5.2%, but increased the false-positive rate from 3% to 5% at the same time.
Table 4.
Results of meta-analysis for diagnostic value of FNA cytology, BRAFV600E-mutation analysis, and the combined strategy in all FNA specimens
| Parameter | FNA
|
BRAF
|
FNA + BRAF
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Result | 95% CI | Heterogeneity, I2 | Result | 95% CI | Heterogeneity, I2 | Result | 95% CI | Heterogeneity, I2 | |
| Pooled sensitivity | 0.814 | 0.803–0.824 | 93.5% | 0.619 | 0.605–0.633 | 93% | 0.874 | 0.865–0.884 | 92.5% |
| Pooled specificity | 0.981 | 0.978–0.985 | 86.4% | 0.997 | 0.995–0.998 | 14.1% | 0.968 | 0.963–0.972 | 92.5% |
| Pooled LR, + | 23.868 | 14.139–40.293 | 87.7% | 34.982 | 23.801–51.415 | 19.5% | 22.353 | 13.027–38.355 | 93.1% |
| Pooled LR, − | 0.216 | 0.172–0.273 | 94.2% | 0.433 | 0.384–0.489 | 91.8% | 0.146 | 0.111–0.192 | 93% |
| Pooled DOR SROC | 127.73 | 75.082–217.28 | 76.1% | 96.570 | 63.932–145.87 | 21.4% | 187.92 | 110.24–320.35 | 76.4% |
| AUC | 0.9551 | 0.9207 | 0.9744 | ||||||
| Q* | 0.8975 | 0.8542 | 0.9271 | ||||||
Note:
The Q index indicates the point at which sensitivity is equal to specificity.
Abbreviations: FNA, fine-needle aspiration; CI, confidence interval; LR, likelihood ratio; DOR, diagnostic odds ratio; SROC, summary receiver-operating characteristic; AUC, area under the curve.
Figure 3.
Summary receiver-operating characteristic (SROC) curve and area under the curve (AUC).
Notes: FNA cytology (A), BRAFV600E-mutation analysis (B), and combination of BRAFV600E mutation and FNA cytology (C). *The Q index indicates the point at which sensitivity is equal to specificity.
Abbreviations: FNA, fine-needle aspiration; SE, standard error.
Diagnostic value of BRAFV600E-mutation analysis in indeterminate cases (Bethesda categories III–V)
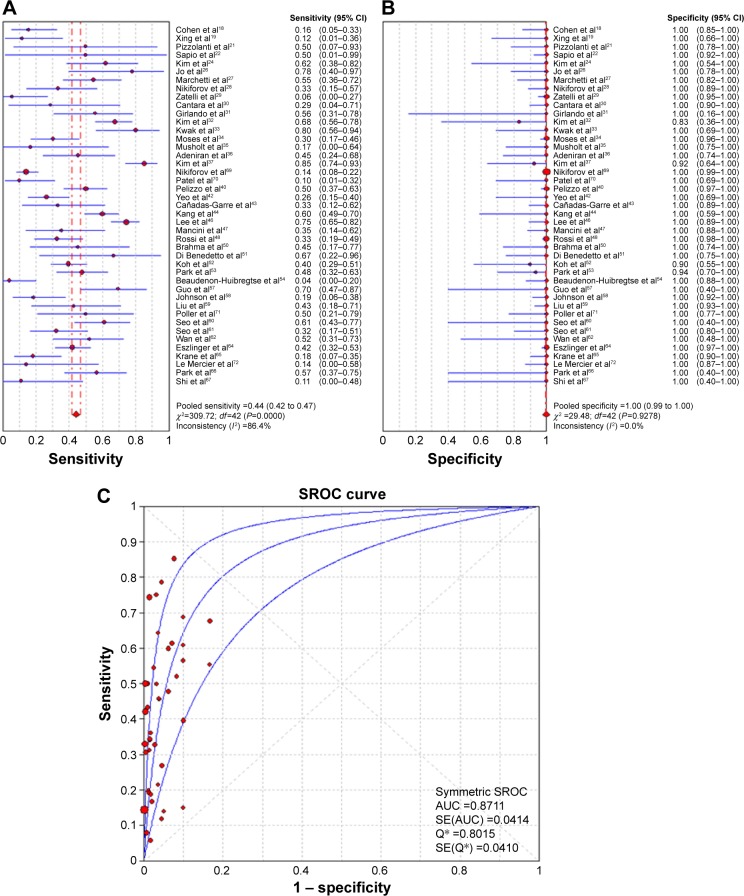
There were 43 studies included in the diagnostic analysis of BRAFV600E testing in the indeterminate thyroid nodules (Table 5).18,19,21,22,24,26–37,40,42–44,46–48,50–54,57–62,64–67,69–72 Our data showed that 23% of indeterminate nodules harbored the BRAFV600E mutation. No threshold effect was detected, so the random-effect model was chosen to pool the diagnostic features: sensitivity 0.442 (95% CI 0.416–0.468), specificity 0.997 (95% CI 0.994–0.999), PLR 12.267 (95% CI 8.175–18.406), NLR 0.613 (95% CI 0.551–0.683), and DOR 23.939 (95% CI 15.388–37.242) (Table 6; Figure 4A and B). The AUC of the SROC was 0.8711 (SE 0.0414), with a Q-value of 0.8015 (SE 0.0410) (Figure 4C).
Table 5.
Diagnostic analysis of BRAFV600E-mutation analysis for indeterminate cases
| Study | Year |
BRAF
|
|||
|---|---|---|---|---|---|
| TP | FP | FN | TN | ||
| Cohen et al18 | 2004 | 5 | 0 | 27 | 23 |
| Xing et al19 | 2004 | 2 | 0 | 15 | 9 |
| Pizzolanti et al21 | 2007 | 2 | 0 | 2 | 15 |
| Sapio et al22 | 2007 | 1 | 0 | 1 | 45 |
| Kim et al24 | 2008 | 13 | 0 | 8 | 6 |
| Jo et al26 | 2009 | 7 | 0 | 2 | 15 |
| Marchetti et al27 | 2009 | 18 | 0 | 15 | 19 |
| Nikiforov et al28 | 2009 | 7 | 0 | 14 | 31 |
| Zatelli et al29 | 2009 | 1 | 0 | 17 | 71 |
| Cantara et al30 | 2010 | 2 | 0 | 5 | 34 |
| Girlando et al31 | 2010 | 10 | 0 | 8 | 2 |
| Kim et al32 | 2010 | 50 | 1 | 24 | 5 |
| Kwak et al33 | 2010 | 16 | 0 | 4 | 10 |
| Moses et al34 | 2010 | 13 | 0 | 30 | 94 |
| Musholt et al35 | 2010 | 1 | 0 | 5 | 13 |
| Adeniran et al36 | 2011 | 10 | 0 | 12 | 12 |
| Kim et al37 | 2011 | 52 | 1 | 9 | 12 |
| Nikiforov et al69 | 2011 | 17 | 0 | 104 | 392 |
| Patel et al70 | 2011 | 2 | 0 | 18 | 10 |
| Pelizzo et al40 | 2011 | 30 | 0 | 30 | 104 |
| Yeo et al42 | 2011 | 14 | 0 | 39 | 10 |
| Cañadas-Garre et al43 | 2012 | 5 | 0 | 10 | 32 |
| Kang et al44 | 2012 | 57 | 0 | 38 | 7 |
| Lee et al46 | 2012 | 79 | 0 | 27 | 33 |
| Mancini et al47 | 2012 | 6 | 0 | 11 | 30 |
| Rossi et al48 | 2012 | 14 | 0 | 29 | 157 |
| Brahma et al50 | 2013 | 5 | 0 | 6 | 12 |
| Di Benedetto et al51 | 2013 | 4 | 0 | 2 | 13 |
| Koh et al52 | 2013 | 32 | 1 | 49 | 9 |
| Park et al53 | 2013 | 21 | 1 | 23 | 15 |
| Beaudenon-Huibregtse et al54 | 2014 | 1 | 0 | 24 | 28 |
| Guo et al57 | 2014 | 16 | 0 | 7 | 4 |
| Johnson et al58 | 2014 | 5 | 0 | 22 | 42 |
| Liu et al59 | 2014 | 6 | 0 | 8 | 49 |
| Poller et al71 | 2014 | 6 | 0 | 6 | 14 |
| Seo et al60 | 2014 | 22 | 0 | 14 | 4 |
| Seo et al61 | 2014 | 10 | 0 | 21 | 17 |
| Wan et al62 | 2014 | 12 | 0 | 11 | 5 |
| Eszlinger et al64 | 2015 | 37 | 0 | 51 | 119 |
| Krane et al65 | 2015 | 6 | 0 | 27 | 35 |
| Le Mercier et al72 | 2015 | 1 | 0 | 6 | 27 |
| Park et al66 | 2015 | 17 | 0 | 13 | 4 |
| Shi et al67 | 2015 | 1 | 0 | 8 | 4 |
Abbreviations: TP, true positive; FP, false positive; FN, false negative; TN, true negative.
Table 6.
Results of meta-analysis for diagnostic value of BRAFV600E mutation in indeterminate cases
| Parameter | Indeterminate
|
SMC
|
AUS/FLUS
|
FN/SFN
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Result | 95% CI | Heterogeneity, I2 | Result | 95% CI | Heterogeneity, I2 | Result | 95% CI | Heterogeneity, I2 | Result | 95% CI | Heterogeneity, I2 | |
| Pooled sensitivity | 0.442 | 0.416–0.468 | 86.4% | 0.594 | 0.556–0.631 | 76% | 0.401 | 0.328–0.477 | 77.4% | 0.195 | 0.128–0.278 | 73.1% |
| Pooled specificity | 0.997 | 0.994–0.999 | 0 | 0.861 | 0.784–0.918 | 70.8% | 0.995 | 0.982–0.999 | 17.9% | 0.997 | 0.983–1.000 | 11.8% |
| Pooled LR, + | 12.267 | 8.175–18.406 | 0 | 3.434 | 1.625–7.259 | 64.1% | 7.001 | 3.336–14.691 | 0 | 9.573 | 3.611–25.379 | 0 |
| Pooled LR, − | 0.613 | 0.551–0.683 | 84.8% | 0.542 | 0.462–0.637 | 29.8% | 0.694 | 0.576–0.835 | 56.1% | 0.733 | 0.522–1.030 | 85% |
| Pooled DOR SROC | 23.939 | 15.388–37.242 | 0 | 7.588 | 3.944–14.598 | 0 | 14.469 | 6.100–34.320 | 0 | 14.808 | 4.966–44.156 | 2.2% |
| AUC | 0.8711 | 0.7674 | 0.7999 | – | ||||||||
| Q* | 0.8015 | 0.7079 | 0.7358 | – | ||||||||
Note:
The Q index indicates the point at which sensitivity is equal to specificity. “–’’ indicates the AUC of the SROC was not significant in FN/SFN cases, since the lower limit of the AUC was less than 0.5.
Abbreviations: FNA, fine-needle aspiration; CI, confidence interval; LR, likelihood ratio; DOR, diagnostic odds ratio; SROC, summary receiver-operating characteristic; AUC, area under the curve.
Figure 4.
Forest plots.
Notes: Sensitivity (A), specificity (B), and summary receiver-operating characteristic (SROC) curve and area under the curve (AUC) (C) of BRAFV600E-mutation analysis in cases classified as indeterminate by FNA cytology. *The Q index indicates the point at which sensitivity is equal to specificity.
Abbreviations: FNA, fine-needle aspiration; CI, confidence interval; SE, standard error.
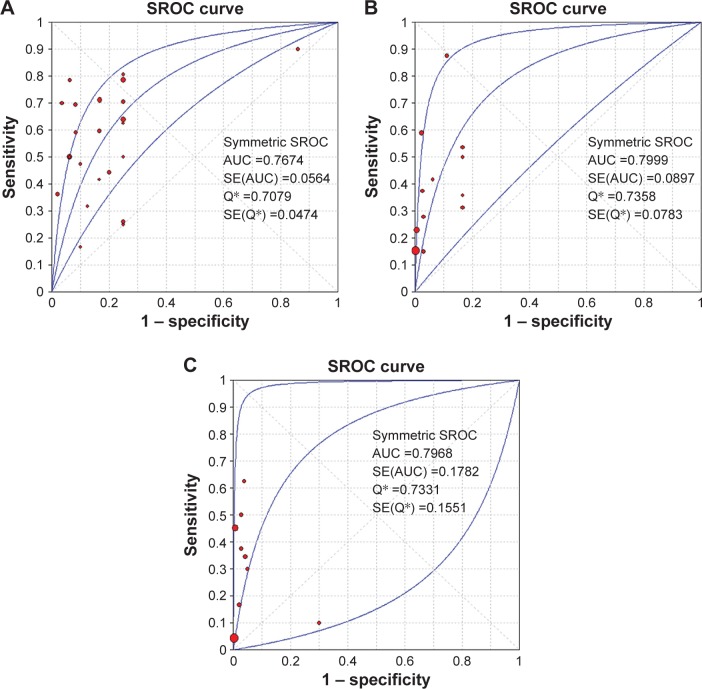
To evaluate the diagnostic value of BRAFV600E testing in different categories of indeterminate nodules, we separated the indeterminate cases into three different and more specific categories according to the Bethesda system. Studies with sample sizes fewer than ten were excluded to avoid potential bias. The malignancy rates of FN/SFN and AUS/FLUS were 30.55% and 34.99%, while 90.35% of SMC cases turned out to be malignant (Table 7). Besides that, the BRAFV600E-mutation rate varied among these groups: it existed in 43.2% of SMC cases, but only 13.77% in AUS/FLUS and 4.43% in FN/SFN patients (Table 7). Furthermore, the sensitivity of BRAFV600E testing was higher in SMC (0.594, 95% CI 0.556–0.631) than AUS/FLUS (0.401, 95% CI 0.328–0.477) and FN/SFN (0.195, 95% CI 0.128–0.278), while specificity was higher in the AUS/FLUS (0.995, 95% CI 0.982–0.999) and FN/SFN (0.997, 95% CI 0.983–1.000) groups than the SMC group (0.861, 95% CI 0.784–0.918) (Table 6). The AUC of the SROC was 0.7674 (SE 0.0564) with a Q-value of 0.7079 (SE 0.0474) in the SMC group, and 0.7999 (SE 0.0897) with a Q-value of 0.7358 (SE 0.0783) in the AUS/FLUS group, but was not significant in FN/SFN cases, since the lower limit of the AUC was less than 0.5 (Figure 5).
Table 7.
Malignancy rate and BRAFV600E-mutation prevalence in three categories of indeterminate cases
| Category | Malignancy rate
|
BRAFV600E-mutation rate
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Event | Pooled | 95% CI | Heterogeneity, I2 | n | Event | Pooled | 95% CI | Heterogeneity, I2 | |
| SMC | 1,214 | 1,067 | 0.9035 | 0.8769–0.9301 | 83.62% | 2,382 | 1,074 | 0.4320 | 0.3340–0.5299 | 98.22% |
| FN/SFN | 509 | 158 | 0.3055 | 0.2394–0.3715 | 54.6% | 1,758 | 101 | 0.0443 | 0.0292–0.0594 | 64.02% |
| AUS/FLUS | 594 | 198 | 0.3499 | 0.2956–0.4042 | 83.01% | 2,304 | 310 | 0.1377 | 0.0989–0.1765 | 95.93% |
Abbreviations: CI, confidence interval; SMC, suspicious for malignant cells; FN/SFN, follicular neoplasm/suspicious for FN; AUS/FLUS, atypia of undetermined significance/follicular lesion of undetermined significance.
Figure 5.
Summary receiver-operating characteristic (SROC) curve and area under the curve (AUC) of SMC cases (A), AUS/FLUS cases (B) and FN/SFN cases (C).
Note: *The Q index indicates the point at which sensitivity is equal to specificity.
Abbreviations: SMC, suspicious for malignant cells; AUS/FLUS, atypia of undetermined significance/follicular lesion of undetermined significance; FN/SFN, follicular neoplasm/suspicious for FN; SE, standard error.
Heterogeneity test
Heterogeneity was present in our meta-analysis, and Spearman correlation coefficients suggested no significant threshold effect. To explore sources of heterogeneity, we assessed multiple variables by metaregression, including country, number of centers, sample size, study design, reference standard, and genotyping method. The results indicated that country and sample size were possible sources of heterogeneity (data not shown). Other covariates that may have caused heterogeneity, such as enrollment period, age, sex, nodule diameter, size of needle, use of blinding method, and differences in operating protocol, were not analyzed here, due to the loss of partial data.
Discussion
Thyroid cancer is on a rapid increase these days, partially due to advancing diagnostic methods. The majority of cases have an excellent prognosis, with 30-year survival rate exceeding 90% after thyroidectomy and/or radioiodine ablation.2 Preoperative diagnosis is of indisputable value in distinguishing thyroid cancer from benign nodules. FNA biopsy is a conventional technique to identify malignant thyroid nodules preoperatively and effectively, which has also been demonstrated in our meta-analysis. However, the extensive use of this approach is influenced by its inherent limitations, such as size or location of nodule, quantity and quality of obtained material, technical skill of the cytopathologist, and the overlap of cytomorphological features between malignant and benign nodules. Therefore, a fraction of cases are classified as nondiagnostic or indeterminate, and about 15%–30% of them get malignant pathology after diagnostic surgery.8,73 Since the occurrence of malignancy is too high for just watchful waiting, numerous patients with indeterminate diagnosis accept unnecessary surgical intervention. BRAFV600E mutation is the most promising marker for thyroid nodules. A similar meta-analysis conducted by Jia et al of 16 studies suggested that BRAFV600E analysis had diagnostic value in indeterminate thyroid nodules,11 but another analysis of eight eligible studies found a low BRAFV600E-mutation rate within indeterminate cases, and thus the value of BRAFV600E-mutation testing remains controversial.12 However, the number of studies these two analyses included was limited, and did not systematically stratify the indeterminate categories. Therefore, we designed a more comprehensive meta-analysis to evaluate the diagnostic yield of BRAFV600E analysis in thyroid FNA, especially those specific categories of indeterminate cases.
Consistent with previous research, our meta-analysis showed that BRAFV600E analysis had high specificity and positive predictive value. As a rule-in test, a positive result of BRAFV600E analysis indicates a high probability of malignancy so that therapeutic surgery is recommended, but the negative result cannot exclude malignancy, and further evaluations, such as follow-up ultrasound or repeat FNA, are needed. When we combined BRAFV600E-mutation testing with FNA cytological examination, sensitivity increased by 6% and the false-negative rate decreased from 8% to 5.2%, while the false-positive rate increased from 3% to 5% at the same time. However, BRAFV600E testing had relatively low sensitivity of 44.2% in the indeterminate group. Also, the yield and usefulness of BRAFV600E analysis can be greatly varied with the prevalence of BRAFV600E mutation in different subcategories of indeterminate nodules. BRAFV600E mutation was present in 43.2% of SMC cases regarded as cytologically positive in our meta-analysis, but only 13.77% in AUS/FLUS and 4.43% in FN/SFN cases. Therefore, it was reasonable that BRAFV600E analysis did best in SMC lesions (sensitivity 59.4%, specificity 86.1%) and also had certain diagnostic value in AUS/FLUS nodules (sensitivity 40.1%, specificity 99.5%), but no significant benefit in the FN/SFN group, which needs other diagnostic approaches with high sensitivity.
BRAFV600E mutation is specific to PTC or anaplastic thyroid cancer arising from PTC, and more common in conventional and tall-cell PTC than follicular-variant PTC (FVPTC), which results in the discrepancy of BRAFV600E test in different indeterminate subgroups. The FN/SFN category is mainly constituted of FVPTC, follicular thyroid cancer (FTC), adenomatoid hyperplasia, and follicular adenoma,74 which harbors low prevalence of BRAFV600E mutation and is hard for BRAFV600E testing to determine malignancy, so FVPTC and FTC may be the main source of false-negative results. The molecular profiles of FVPTC and FTC are similar, with frequent RAS and rare BRAF mutation.75,76 RAS mutation, mutually exclusive with BRAF mutation, is the most frequent genetic mutation in indeterminate nodules, and provides important diagnostic information for BRAFV600E-negative nodules.69,77 An et al reported that single RAS-mutation analysis had a sensitivity of 93.3% and specificity of 75.0% in indeterminate nodules, and the combination of RAS and BRAF mutation provided additional diagnosis value for 60%–70% indeterminate thyroid nodules.78 Other genetic alterations, such as RET/PTC and PAX8/PPARG, also contribute to the definite diagnosis of indeterminate nodules.69,79,80 Therefore, an expanded panel can be more effective, which is also recommended by the revised American Thyroid Association management guidelines.73 As some mutations also present in benign nodules, the accompanying increase in false-positive rate should not be neglected. For instance, RAS mutation and PAX8/PPARG translocation are also found in follicular adenoma.79,81 Additionally, some thyroid cancer does not have definitive molecular mutation, and other efficient rule-out testing with high negative predictive value should be explored.
The clinical management decision is directly based on the malignant risk, ranging from repeat FNA to diagnostic lobectomy to total thyroidectomy. Uncertain diagnosis may lead to delayed treatment or unnecessary intervention. Based on the Bethesda classification, malignancy rates for FN/SFN and SMC nodules are 15%–30% and 60%–75%, respectively, and are much more variable in AUS/FLUS cases (7%–48%).8 In our analysis, the malignancy rate of the SMC group was higher than that recorded in the Bethesda classification, and this discrepancy might have resulted from continuous improvement in FNA technique, since the data for the Bethesda system were collected several years ago. BRAFV600E mutation is a strong indicator for malignancy, and total thyroidectomy should be proposed as the first-line treatment for BRAFV600E-positive nodules to decrease the recurrence and avoid complications caused by standard two-stage surgery. Nevertheless, BRAFV600E testing is relatively insufficient for AUS/FLUS and even has no effect in FN/SFN patients, due to the low prevalence of BRAFV600E mutation, but their malignant occurrence (30.55% and 34.99%) was too high to perform clinical observation. Other approaches, such as core-needle biopsy and immunohistochemistry, are also required to confidently guide the management. Several multicenter studies have reported that BRAFV600E mutation is associated with aggressive clinicopathological characteristics and predicts recurrence and mortality for PTC patients.82–89 Therefore, more aggressive surgery, such as prophylactic central lymph-node dissection and closer follow-up, should be considered in the management of BRAFV600E-positive thyroid cancer.
Despite its achievements, our meta-analysis had several limitations. Firstly, there was significant nonthreshold heterogeneity, partly caused by country and sample size of different studies, but other possible covariates were unable to be analyzed due to the paucity of data. The heterogeneity from country may be due to the different BRAFV600E prevalence in worldwide populations, eg, it is up to 80% in South Korea, which is much higher than other regions.24 Secondly, about a third of the studies had a high risk of bias in patient selection, and nearly half had a high risk of bias in flow and timing, which may affect the reliability of our results.
Conclusion
This meta-analysis demonstrated that BRAFV600E analysis using residual material obtained from routine FNA could improve diagnostic accuracy and reduce false-negative rates. Besides, BRAFV600E analysis had certain diagnostic value in SMC and AUS/FLUS cases, especially the SMC group, selecting cases with high malignancy possibility and guiding intraoperative or postoperative management, though its value in FN/SFN cases was doubtful, and expanded panels containing other diagnostic markers are recommended. Therefore, more studies of high quality are needed to balance the advantages and disadvantages of BRAFV600E testing for patients and to select the most suitable population for this diagnostic method.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (81202141 and 81272676), Key Project of Scientific and Technological Innovation of Hangzhou (20131813A08), National Science and Technology Major Project of the Ministry of Science and Technology of China (2013ZX09506015), and Medical Science and Technology Project of Zhejiang Province (2011ZDA009).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Markovina S, Grigsby PW, Schwarz JK, et al. Treatment approach, surveillance, and outcome of well-differentiated thyroid cancer in childhood and adolescence. Thyroid. 2014;24(7):1121–1126. doi: 10.1089/thy.2013.0297. [DOI] [PubMed] [Google Scholar]
- 3.Bongiovanni M, Krane JF, Cibas ES, Faquin WC. The atypical thyroid fine-needle aspiration: past, present, and future. Cancer Cytopathol. 2012;120(2):73–86. doi: 10.1002/cncy.20178. [DOI] [PubMed] [Google Scholar]
- 4.Faquin WC, Bongiovanni M, Sadow PM. Update in thyroid fine needle aspiration. Endocr Pathol. 2011;22(4):178–183. doi: 10.1007/s12022-011-9182-7. [DOI] [PubMed] [Google Scholar]
- 5.Seningen JL, Nassar A, Henry MR. Correlation of thyroid nodule fine-needle aspiration cytology with corresponding histology at Mayo Clinic, 2001–2007: an institutional experience of 1,945 cases. Diagn Cytopathol. 2012;40(Suppl 1):E27–E32. doi: 10.1002/dc.21566. [DOI] [PubMed] [Google Scholar]
- 6.Puxeddu E, Filetti S. BRAF mutation assessment in papillary thyroid cancer: are we ready to use it in clinical practice? Endocrine. 2014;45(3):341–343. doi: 10.1007/s12020-013-0139-0. [DOI] [PubMed] [Google Scholar]
- 7.Piana S, Frasoldati A, Ferrari M, et al. Is a five-category reporting scheme for thyroid fine needle aspiration cytology accurate? Experience of over 18,000 FNAs reported at the same institution during 1998–2007. Cytopathology. 2011;22(3):164–173. doi: 10.1111/j.1365-2303.2010.00777.x. [DOI] [PubMed] [Google Scholar]
- 8.Cibas ES, Ali SZ. The Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2009;132(5):658–665. doi: 10.1309/AJCPPHLWMI3JV4LA. [DOI] [PubMed] [Google Scholar]
- 9.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13(3):184–199. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28(7):742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 11.Jia Y, Yu Y, Li X, et al. Diagnostic value of B-RAF(V600E) in difficult-to-diagnose thyroid nodules using fine-needle aspiration: systematic review and meta-analysis. Diagn Cytopathol. 2014;42(1):94–101. doi: 10.1002/dc.23044. [DOI] [PubMed] [Google Scholar]
- 12.Trimboli P, Treglia G, Condorelli E, et al. BRAF-mutated carcinomas among thyroid nodules with prior indeterminate FNA report: a systematic review and meta-analysis. Clin Endocrinol (Oxf) 2016;84(3):315–320. doi: 10.1111/cen.12806. [DOI] [PubMed] [Google Scholar]
- 13.Dottorini ME, Mansi LP. Perros (ed), British Thyroid Association, Royal College of Physicians. Guidelines for the management of thyroid cancer, 2nd edition. Report of the Thyroid Cancer Guidelines Update Group. Eur J Nucl Med Mol Imaging. 2008;35(6):1218–1219. [Google Scholar]
- 14.Cross P, Chandra A, Giles T, et al. Guidance on the reporting of thyroid cytology specimens. 2016. [Accessed February 19, 2016]. Available from: http://ukeps.com/docs/thyroidfna.pdf.
- 15.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 16.Vamvakas EC. Meta-analyses of studies of the diagnostic accuracy of laboratory tests: a review of the concepts and methods. Arch Pathol Lab Med. 1998;122(8):675–686. [PubMed] [Google Scholar]
- 17.Ferreira ML, Smeets RJ, Kamper SJ, Ferreira PH, Machado LA. Can we explain heterogeneity among randomized clinical trials of exercise for chronic back pain? A meta-regression analysis of randomized controlled trials. Phys Ther. 2010;90(10):1383–1403. doi: 10.2522/ptj.20090332. [DOI] [PubMed] [Google Scholar]
- 18.Cohen Y, Rosenbaum E, Clark DP, et al. Mutational analysis of BRAF in fine needle aspiration biopsies of the thyroid: a potential application for the preoperative assessment of thyroid nodules. Clin Cancer Res. 2004;10(8):2761–2765. doi: 10.1158/1078-0432.ccr-03-0273. [DOI] [PubMed] [Google Scholar]
- 19.Xing M, Tufano RP, Tufaro AP, et al. Detection of BRAF mutation on fine needle aspiration biopsy specimens: a new diagnostic tool for papillary thyroid cancer. J Clin Endocrinol Metab. 2004;89(6):2867–2872. doi: 10.1210/jc.2003-032050. [DOI] [PubMed] [Google Scholar]
- 20.Domingues R, Mendonca E, Sobrinho L, Bugalho MJ. Searching for RET/PTC rearrangements and BRAF V599E mutation in thyroid aspirates might contribute to establish a preoperative diagnosis of papillary thyroid carcinoma. Cytopathology. 2005;16(1):27–31. doi: 10.1111/j.1365-2303.2004.00223.x. [DOI] [PubMed] [Google Scholar]
- 21.Pizzolanti G, Russo L, Richiusa P, et al. Fine-needle aspiration molecular analysis for the diagnosis of papillary thyroid carcinoma through BRAF V600E mutation and RET/PTC rearrangement. Thyroid. 2007;17(11):1109–1115. doi: 10.1089/thy.2007.0008. [DOI] [PubMed] [Google Scholar]
- 22.Sapio MR, Guerra A, Posca D, et al. Combined analysis of galectin-3 and BRAFV600E improves the accuracy of fine-needle aspiration biopsy with cytological findings suspicious for papillary thyroid carcinoma. Endocr Relat Cancer. 2007;14(4):1089–1097. doi: 10.1677/ERC-07-0147. [DOI] [PubMed] [Google Scholar]
- 23.Sapio MR, Posca D, Raggioli A, et al. Detection of RET/PTC, TRK and BRAF mutations in preoperative diagnosis of thyroid nodules with indeterminate cytological findings. Clin Endocrinol (Oxf) 2007;66(5):678–683. doi: 10.1111/j.1365-2265.2007.02800.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim SK, Kim DL, Han HS, et al. Pyrosequencing analysis for detection of a BRAFV600E mutation in an FNAB specimen of thyroid nodules. Diagn Mol Pathol. 2008;17(2):118–125. doi: 10.1097/PDM.0b013e31815d059d. [DOI] [PubMed] [Google Scholar]
- 25.Bentz BG, Miller BT, Holden JA, Rowe LR, Bentz JS. B-RAF V600E mutational analysis of fine needle aspirates correlates with diagnosis of thyroid nodules. Otolaryngol Head Neck Surg. 2009;140(5):709–714. doi: 10.1016/j.otohns.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Jo YS, Huang S, Kim YJ, et al. Diagnostic value of pyrosequencing for the BRAF V600E mutation in ultrasound-guided fine-needle aspiration biopsy samples of thyroid incidentalomas. Clin Endocrinol (Oxf) 2009;70(1):139–144. doi: 10.1111/j.1365-2265.2008.03293.x. [DOI] [PubMed] [Google Scholar]
- 27.Marchetti I, Lessi F, Mazzanti CM, et al. A morpho-molecular diagnosis of papillary thyroid carcinoma: BRAF V600E detection as an important tool in preoperative evaluation of fine-needle aspirates. Thyroid. 2009;19(8):837–842. doi: 10.1089/thy.2009.0074. [DOI] [PubMed] [Google Scholar]
- 28.Nikiforov YE, Steward DL, Robinson-Smith TM, et al. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. 2009;94(6):2092–2098. doi: 10.1210/jc.2009-0247. [DOI] [PubMed] [Google Scholar]
- 29.Zatelli MC, Trasforini G, Leoni S, et al. BRAF V600E mutation analysis increases diagnostic accuracy for papillary thyroid carcinoma in fine-needle aspiration biopsies. Eur J Endocrinol. 2009;161(3):467–473. doi: 10.1530/EJE-09-0353. [DOI] [PubMed] [Google Scholar]
- 30.Cantara S, Capezzone M, Marchisotta S, et al. Impact of proto-oncogene mutation detection in cytological specimens from thyroid nodules improves the diagnostic accuracy of cytology. J Clin Endocrinol Metab. 2010;95(3):1365–1369. doi: 10.1210/jc.2009-2103. [DOI] [PubMed] [Google Scholar]
- 31.Girlando S, Cuorvo LV, Bonzanini M, et al. High prevalence of B-RAF mutation in papillary carcinoma of the thyroid in north-east Italy. Int J Surg Pathol. 2010;18(3):173–176. doi: 10.1177/1066896910363133. [DOI] [PubMed] [Google Scholar]
- 32.Kim SW, Lee JI, Kim JW, et al. BRAFV600E mutation analysis in fine-needle aspiration cytology specimens for evaluation of thyroid nodule: a large series in a BRAFV600E-prevalent population. J Clin Endocrinol Metab. 2010;95(8):3693–3700. doi: 10.1210/jc.2009-2795. [DOI] [PubMed] [Google Scholar]
- 33.Kwak JY, Kim EK, Kim JK, et al. Dual priming oligonucleotide-based multiplex PCR analysis for detection of BRAFV600E mutation in FNAB samples of thyroid nodules in BRAFV600E mutation-prevalent area. Head Neck. 2010;32(4):490–498. doi: 10.1002/hed.21210. [DOI] [PubMed] [Google Scholar]
- 34.Moses W, Weng J, Sansano I, et al. Molecular testing for somatic mutations improves the accuracy of thyroid fine-needle aspiration biopsy. World J Surg. 2010;34(11):2589–2594. doi: 10.1007/s00268-010-0720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Musholt TJ, Fottner C, Weber MM, et al. Detection of papillary thyroid carcinoma by analysis of BRAF and RET/PTC1 mutations in fine-needle aspiration biopsies of thyroid nodules. World J Surg. 2010;34(11):2595–2603. doi: 10.1007/s00268-010-0729-4. [DOI] [PubMed] [Google Scholar]
- 36.Adeniran AJ, Theoharis C, Hui P, et al. Reflex BRAF testing in thyroid fine-needle aspiration biopsy with equivocal and positive interpretation: a prospective study. Thyroid. 2011;21(7):717–723. doi: 10.1089/thy.2011.0021. [DOI] [PubMed] [Google Scholar]
- 37.Kim SK, Hwang TS, Yoo YB, et al. Surgical results of thyroid nodules according to a management guideline based on the BRAF(V600E) mutation status. J Clin Endocrinol Metab. 2011;96(3):658–664. doi: 10.1210/jc.2010-1082. [DOI] [PubMed] [Google Scholar]
- 38.Lee EJ, Song KH, Kim DL, Jang YM, Hwang TS, Kim SK. The BRAF(V600E) mutation is associated with malignant ultrasonographic features in thyroid nodules. Clin Endocrinol (Oxf) 2011;75(6):844–850. doi: 10.1111/j.1365-2265.2011.04154.x. [DOI] [PubMed] [Google Scholar]
- 39.Moon HJ, Kim EK, Chung WY, Choi JR, Yoon JH, Kwak JY. Diagnostic value of BRAF(V600E) mutation analysis of thyroid nodules according to ultrasonographic features and the time of aspiration. Ann Surg Oncol. 2011;18(3):792–799. doi: 10.1245/s10434-010-1354-z. [DOI] [PubMed] [Google Scholar]
- 40.Pelizzo MR, Boschin IM, Barollo S, et al. BRAF analysis by fine needle aspiration biopsy of thyroid nodules improves preoperative identification of papillary thyroid carcinoma and represents a prognostic factor: a mono-institutional experience. Clin Chem Lab Med. 2011;49(2):325–329. doi: 10.1515/CCLM.2011.031. [DOI] [PubMed] [Google Scholar]
- 41.Smith GD, Zhou L, Rowe LR, et al. Allele-specific PCR with competitive probe blocking for sensitive and specific detection of BRAF V600E in thyroid fine-needle aspiration specimens. Acta Cytol. 2011;55(6):576–583. doi: 10.1159/000333453. [DOI] [PubMed] [Google Scholar]
- 42.Yeo MK, Liang ZL, Oh T, et al. Pyrosequencing cut-off value identifying BRAFV600E mutation in fine needle aspiration samples of thyroid nodules. Clin Endocrinol (Oxf) 2011;75(4):555–560. doi: 10.1111/j.1365-2265.2011.04115.x. [DOI] [PubMed] [Google Scholar]
- 43.Cañadas-Garre M, Becerra-Massare P, Torre-Casares ML, et al. Reduction of false-negative papillary thyroid carcinomas by the routine analysis of BRAF(T1799A) mutation on fine-needle aspiration biopsy specimens: a prospective study of 814 thyroid FNAB patients. Ann Surg. 2012;255(5):986–992. doi: 10.1097/SLA.0b013e31824e8d70. [DOI] [PubMed] [Google Scholar]
- 44.Kang G, Cho EY, Shin JH, Chung JH, Kim JW, Oh YL. Role of BRAFV600E mutation analysis and second cytologic review of fine- needle aspiration for evaluating thyroid nodule. Cancer Cytopathol. 2012;120(1):44–51. doi: 10.1002/cncy.20179. [DOI] [PubMed] [Google Scholar]
- 45.Kwak JY, Han KH, Yoon JH, et al. BRAFV600E mutation testing in fine needle aspirates of thyroid nodules: potential value of real-time PCR. Ann Clin Lab Sci. 2012;42(3):258–265. [PubMed] [Google Scholar]
- 46.Lee ST, Kim SW, Ki CS, et al. Clinical implication of highly sensitive detection of the BRAF V600E mutation in fine-needle aspirations of thyroid nodules: a comparative analysis of three molecular assays in 4585 consecutive cases in a BRAF V600E mutation-prevalent area. J Clin Endocrinol Metab. 2012;97(7):2299–2306. doi: 10.1210/jc.2011-3135. [DOI] [PubMed] [Google Scholar]
- 47.Mancini I, Pinzani P, Pupilli C, et al. A high-resolution melting protocol for rapid and accurate differential diagnosis of thyroid nodules. J Mol Diagn. 2012;14(5):501–509. doi: 10.1016/j.jmoldx.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Rossi M, Buratto M, Bruni S, et al. Role of ultrasonographic/clinical profile, cytology, and BRAF V600E mutation evaluation in thyroid nodule screening for malignancy: a prospective study. J Clin Endocrinol Metab. 2012;97(7):2354–2361. doi: 10.1210/jc.2011-3494. [DOI] [PubMed] [Google Scholar]
- 49.Tomei S, Marchetti I, Zavaglia K, et al. A molecular computational model improves the preoperative diagnosis of thyroid nodules. BMC Cancer. 2012;12:396. doi: 10.1186/1471-2407-12-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brahma B, Yulian ED, Ramli M, et al. Surgical perspective of T1799A BRAF mutation diagnostic value in papillary thyroid carcinoma. Asian Pac J Cancer Prev. 2013;14(1):31–37. doi: 10.7314/apjcp.2013.14.1.31. [DOI] [PubMed] [Google Scholar]
- 51.Di Benedetto G, Fabozzi A, Rinaldi C, Rinaldi CR. BRAF test and cytological diagnosis with a single fine needle cytology sample. Acta Cytol. 2013;57(4):337–340. doi: 10.1159/000350618. [DOI] [PubMed] [Google Scholar]
- 52.Koh J, Choi JR, Han KH, et al. Proper indication of BRAF(V600E) mutation testing in fine-needle aspirates of thyroid nodules. PLoS One. 2013;8(5):e64505. doi: 10.1371/journal.pone.0064505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park SJ, Sun JY, Hong K, et al. Application of BRAF, NRAS, KRAS mutations as markers for the detection of papillary thyroid cancer from FNAB specimens by pyrosequencing analysis. Clin Chem Lab Med. 2013;51(8):1673–1680. doi: 10.1515/cclm-2012-0375. [DOI] [PubMed] [Google Scholar]
- 54.Beaudenon-Huibregtse S, Alexander EK, Guttler RB, et al. Centralized molecular testing for oncogenic gene mutations complements the local cytopathologic diagnosis of thyroid nodules. Thyroid. 2014;24(10):1479–1487. doi: 10.1089/thy.2013.0640. [DOI] [PubMed] [Google Scholar]
- 55.Crescenzi A, Guidobaldi L, Nasrollah N, et al. Immunohistochemistry for BRAF(V600E) antibody VE1 performed in core needle biopsy samples identifies mutated papillary thyroid cancers. Horm Metab Res. 2014;46(5):370–374. doi: 10.1055/s-0034-1368700. [DOI] [PubMed] [Google Scholar]
- 56.Eszlinger M, Krogdahl A, Munz S, et al. Impact of molecular screening for point mutations and rearrangements in routine air-dried fine-needle aspiration samples of thyroid nodules. Thyroid. 2014;24(2):305–313. doi: 10.1089/thy.2013.0278. [DOI] [PubMed] [Google Scholar]
- 57.Guo HQ, Zhao H, Zhang ZH, Zhu YL, Xiao T, Pan QJ. Impact of molecular testing in the diagnosis of thyroid fine needle aspiration cytology: data from mainland China. Dis Markers. 2014;2014:912182. doi: 10.1155/2014/912182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson SJ, Hardy SA, Roberts C, Bourn D, Mallick U, Perros P. Pilot of BRAF mutation analysis in indeterminate, suspicious and malignant thyroid FNA cytology. Cytopathology. 2014;25(3):146–154. doi: 10.1111/cyt.12125. [DOI] [PubMed] [Google Scholar]
- 59.Liu S, Gao A, Zhang B, et al. Assessment of molecular testing in fine-needle aspiration biopsy samples: an experience in a Chinese population. Exp Mol Pathol. 2014;97(2):292–297. doi: 10.1016/j.yexmp.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 60.Seo JY, Kim EK, Baek JH, Shin JH, Han KH, Kwak JY. Can ultrasound be as a surrogate marker for diagnosing a papillary thyroid cancer? Comparison with BRAF mutation analysis. Yonsei Med J. 2014;55(4):871–878. doi: 10.3349/ymj.2014.55.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seo JY, Kim EK, Kwak JY. Additional BRAF mutation analysis may have additional diagnostic value in thyroid nodules with “suspicious for malignant” cytology alone even when the nodules do not show suspicious US features. Endocrine. 2014;47(1):283–289. doi: 10.1007/s12020-013-0150-5. [DOI] [PubMed] [Google Scholar]
- 62.Wan H, Zhang B, Wang Y, et al. Clinical role of BRAF V600E mutation testing in thyroid nodules. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014;49(6):468–472. Chinese. [PubMed] [Google Scholar]
- 63.Zeck J, Sidawy M, Busseniers A. Correlation of thyroid aspirate molecular profiles to respective cytology and histology results: a single aspirator’s experience. J Am Soc Cytopathol. 2014;3(5):S60. [Google Scholar]
- 64.Eszlinger M, Piana S, Moll A, et al. Molecular testing of thyroid fine-needle aspirations improves presurgical diagnosis and supports the histologic identification of minimally invasive follicular thyroid carcinomas. Thyroid. 2015;25(4):401–409. doi: 10.1089/thy.2014.0362. [DOI] [PubMed] [Google Scholar]
- 65.Krane JF, Cibas ES, Alexander EK, Paschke R, Eszlinger M. Molecular analysis of residual ThinPrep material from thyroid FNAs increases diagnostic sensitivity. Cancer Cytopathol. 2015;123(6):356–361. doi: 10.1002/cncy.21546. [DOI] [PubMed] [Google Scholar]
- 66.Park KS, Oh YL, Ki CS, Kim JW. Evaluation of the Real-Q BRAF V600E detection assay in fine-needle aspiration samples of thyroid nodules. J Mol Diagn. 2015;17(4):431–437. doi: 10.1016/j.jmoldx.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 67.Shi Q, Ibrahim A, Herbert K, et al. Detection of BRAF mutations on direct smears of thyroid fine-needle aspirates through cell transfer technique. Am J Clin Pathol. 2015;143(4):500–504. doi: 10.1309/AJCP5BG0KUEOJCVS. [DOI] [PubMed] [Google Scholar]
- 68.Marchetti I, Iervasi G, Mazzanti CM, et al. Detection of the BRAF(V600E) mutation in fine needle aspiration cytology of thyroid papillary microcarcinoma cells selected by manual macrodissection: an easy tool to improve the preoperative diagnosis. Thyroid. 2012;22(3):292–298. doi: 10.1089/thy.2011.0107. [DOI] [PubMed] [Google Scholar]
- 69.Nikiforov YE, Ohori NP, Hodak SP, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab. 2011;96(11):3390–3397. doi: 10.1210/jc.2011-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patel A, Klubo-Gwiezdzinska J, Hoperia V, et al. BRAF(V600E) mutation analysis from May-Grunwald Giemsa-stained cytological samples as an adjunct in identification of high-risk papillary thyroid carcinoma. Endocr Pathol. 2011;22(4):195–199. doi: 10.1007/s12022-011-9180-9. [DOI] [PubMed] [Google Scholar]
- 71.Poller DN, Glaysher S, Agrawal A, Caldera S, Kim D, Yiangou C. BRAF V600 co-testing in thyroid FNA cytology: short-term experience in a large cancer centre in the UK. J Clin Pathol. 2014;67(8):684–689. doi: 10.1136/jclinpath-2014-202348. [DOI] [PubMed] [Google Scholar]
- 72.Le Mercier M, D’Haene N, De Neve N, et al. Next-generation sequencing improves the diagnosis of thyroid FNA specimens with indeterminate cytology. Histopathology. 2015;66(2):215–224. doi: 10.1111/his.12461. [DOI] [PubMed] [Google Scholar]
- 73.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 74.Wang CC, Friedman L, Kennedy GC, et al. A large multicenter correlation study of thyroid nodule cytopathology and histopathology. Thyroid. 2011;21(3):243–251. doi: 10.1089/thy.2010.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7(10):569–580. doi: 10.1038/nrendo.2011.142. [DOI] [PubMed] [Google Scholar]
- 76.Park JY, Kim WY, Hwang TS, et al. BRAF and RAS mutations in follicular variants of papillary thyroid carcinoma. Endocr Pathol. 2013;24(2):69–76. doi: 10.1007/s12022-013-9244-0. [DOI] [PubMed] [Google Scholar]
- 77.Gupta N, Dasyam AK, Carty SE, et al. RAS mutations in thyroid FNA specimens are highly predictive of predominantly low-risk follicular-pattern cancers. J Clin Endocrinol Metab. 2013;98(5):E914–E922. doi: 10.1210/jc.2012-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.An JH, Song KH, Kim SK, et al. RAS mutations in indeterminate thyroid nodules are predictive of the follicular variant of papillary thyroid carcinoma. Clin Endocrinol. 2015;82(5):760–766. doi: 10.1111/cen.12579. [DOI] [PubMed] [Google Scholar]
- 79.Nikiforova MN, Nikiforov YE. Molecular diagnostics and predictors in thyroid cancer. Thyroid. 2009;19(12):1351–1361. doi: 10.1089/thy.2009.0240. [DOI] [PubMed] [Google Scholar]
- 80.Albarel F, Conte-Devolx B, Oliver C. From nodule to differentiated thyroid carcinoma: contributions of molecular analysis in 2012. Ann Endocrinol (Paris) 2012;73(3):155–164. doi: 10.1016/j.ando.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 81.Nikiforova MN, Lynch RA, Biddinger PW, et al. RAS point mutations and PAX8-PPARγ rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab. 2003;88(5):2318–2326. doi: 10.1210/jc.2002-021907. [DOI] [PubMed] [Google Scholar]
- 82.Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2015;33(1):42–50. doi: 10.1200/JCO.2014.56.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309(14):1493–1501. doi: 10.1001/jama.2013.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Howell GM, Nikiforova MN, Carty SE, et al. BRAF V600E mutation independently predicts central compartment lymph node metastasis in patients with papillary thyroid cancer. Ann Surg Oncol. 2013;20(1):47–52. doi: 10.1245/s10434-012-2611-0. [DOI] [PubMed] [Google Scholar]
- 85.Prescott JD, Sadow PM, Hodin RA, et al. BRAFV600E status adds incremental value to current risk classification systems in predicting papillary thyroid carcinoma recurrence. Surgery. 2012;152(6):984–990. doi: 10.1016/j.surg.2012.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Elisei R, Ugolini C, Viola D, et al. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93(10):3943–3949. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 87.Lee X, Gao M, Ji Y, et al. Analysis of differential BRAF(V600E) mutational status in high aggressive papillary thyroid microcarcinoma. Ann Surg Oncol. 2009;16(2):240–245. doi: 10.1245/s10434-008-0233-3. [DOI] [PubMed] [Google Scholar]
- 88.Elisei R, Viola D, Torregrossa L, et al. The BRAF(V600E) mutation is an independent, poor prognostic factor for the outcome of patients with low-risk intrathyroid papillary thyroid carcinoma: single-institution results from a large cohort study. J Clin Endocrinol Metab. 2012;97(12):4390–4398. doi: 10.1210/jc.2012-1775. [DOI] [PubMed] [Google Scholar]
- 89.Pak K, Suh S, Kim SJ, Kim IJ. Prognostic value of genetic mutations in thyroid cancer: a meta-analysis. Thyroid. 2015;25(1):63–70. doi: 10.1089/thy.2014.0241. [DOI] [PubMed] [Google Scholar]