Abstract
Here we report the radiosynthesis of an endogenous redox pair, [11C]ascorbic acid ([11C]VitC) and [11C]dehydroascorbic acid ([11C]DHA), the reduced and oxidized forms of vitamin C, and their application to ROS sensing. These results provide the basis for in vivo detection of ROS using positron emission tomography (PET).
Reactive oxygen species (ROS) are generated as a normal product of oxidative metabolism and are required signalling molecules in a diverse array of biological processes.1 Dysregulation of ROS in common disease states including cancer,2 neurodegeneration,3 chronic inflammation,4 and diabetes5 provides a powerful motivation to develop non-invasive biomarkers of oxidative stress. Current ROS sensing techniques in living systems are largely limited to in vitro study. Advances toward in vivo ROS detection include approaches using electron spin trapping (ESR),6 near-IR optical,7 bioluminescent,8 [13C] magnetic resonance imaging (MRI),9,21 chemiluminescent probes,10 fluorescent probes11 and positron emission tomography (PET).12 Due to its high sensitivity, good spatial resolution and low toxicity,13 PET has potential for detecting ROS in a clinical setting.
VitC is transported into cells via the sodium dependent vitamin C transporter (SVCT1–2).14 In the presence of ROS, VitC undergoes a two-electron oxidation to DHA, which in aqueous solution exists predominantly in bicyclic hemiketal form and is a substrate for glucose transport (GLUT 1, 3, 4).15 We hypothesized that by taking advantage of rapid GLUT transport,16 this mechanism could be employed to detect extracellular ROS (Figure 1). Previously Yamamoto17 and Kothari18 have investigated 6-[18F]-fluoro-6-deoxy-L-ascorbic acid as a PET analogue of VitC; however this probe cannot enter cells via GLUT, as the 18F label in the 6 position prevents formation of the bicyclic species of DHA.17b,17e,f We have therefore developed a new pair of endogenous PET radiotracers [11C] ascorbic acid ([11C]VitC) and its oxidized partner, [11C] dehydroascorbic acid ([11C]DHA) and have used this redox pair to sense ROS in vitro.
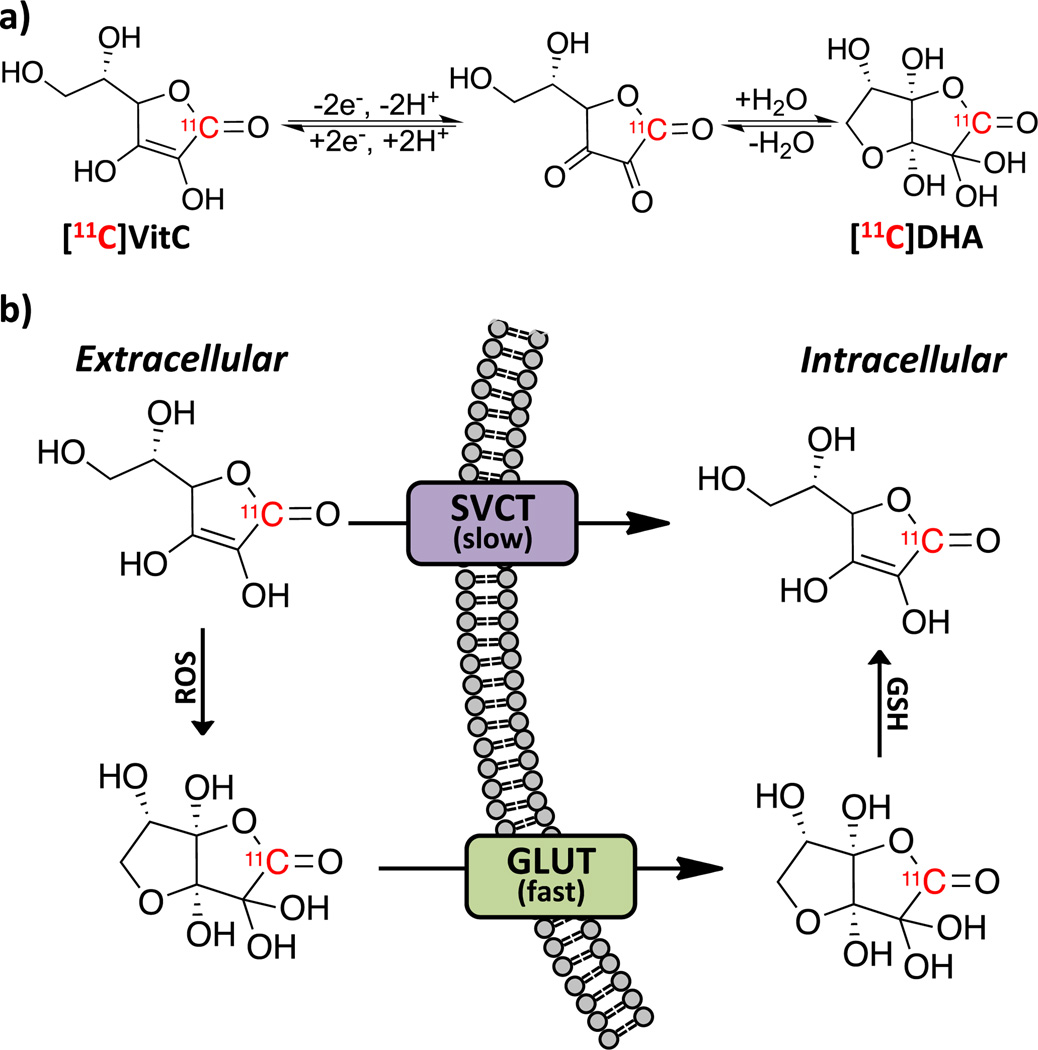
Figure 1.
(a) Oxidation of and hydration of VitC forming bicyclic DHA hydrate. (b) Transport mechanisms of VitC and DHA.
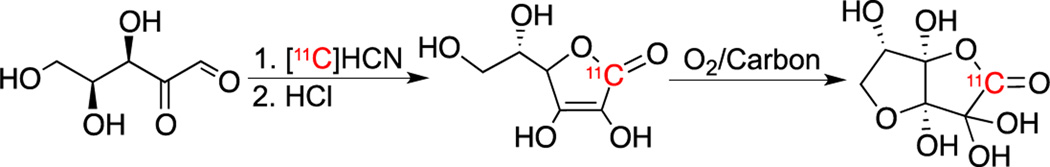
[11C]VitC was synthesized from L-xylosone based on a modification of the previously reported [13/14C] enriched techniques (Scheme 1).19 The methods employed and relevant analytical data are reported in full in the Supporting Information (Figures S1–S3). Table 1 summarizes radiochemical yields for synthesis with varying amounts of added KCN carrier. With no carrier added [11C]VitC in situ oxidation to [11C]DHA was observed at pH 7 (Figure S4) possibly related to generation of ROS by radiolysis. This phenomenon has been previously observed for 6-[18F]-Fluoro-6-deoxy-L-ascorbic acid.18 Thus, we used non-radioactive carrier VitC to protect against in situ oxidation. This was achieved by adding carrier KCN during the radiochemical preparation. With the presence of 0.6 – 1.0 mM (specific activity ≅ 3.0–10.0 mCi/µmol; 110–370 MBq) carrier in the final isolated product [11C]VitC is stable at all time points tested (Figure S5). As sampling of our institution’s clinical 2-deoxy-2-[18F]fluoroglucose ([18F]FDG) doses revealed that the administered [18F]FDG solution contained 1.2 ± 0.1 mM (n = 3) non-radioactive glucose, we do not anticipate that addition of carrier at this level will significantly diminish the ability to image [11C]DHA transport via GLUT. Other antioxidants were also considered to prevent [11C]VitC in situ oxidation, but given that these also react with ROS they would likely confound interpretation of in vitro and in vivo data.
Scheme 1.
Radiochemical syntheses of [11C]VitC and [11C]DHA.
Table 1.
Summary of radiochemical yields and specific activities for [11C]VitCradiosyntheses with varying amounts of carrier added.
| KCN carrier added |
% Radiochemical Yield | Specific Activity (mCi/µmol) |
number of trials |
|---|---|---|---|
| 1 mg/mL | 45.4 ± 9.8 | 4.0 ± 1.2 | n = 6 |
| 0.75 mg/mL | 46.1 ± 9.31 | 6.4 ± 0.66 | n = 3 |
| 0.5 mg/mL | 29.7 ± 8.8 | 9.0 ± 3.7 | n = 5 |
| 0 mg/mL | 14.3 ± 10.4 | 267 ± 148 | n = 5 |
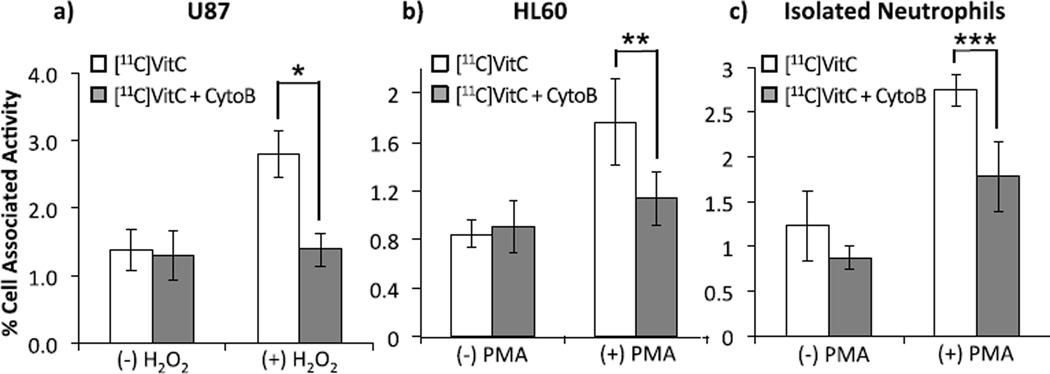
We first evaluated the transport of both [11C]VitC and [11C]DHA in U87 human glioblastoma cells using [18F]FDG as a standard radiotracer for GLUT transport. GLUT blocking studies were carried out with application of 10µg/ml cytochalasin B, a potent inhibitor of GLUT transport.15a,23 SVCT transport was interrogated by modulating (+)/(−) co-transport of Na+ as per Vera et al.15b–d While uptake of [11C]VitC is not affected by blocking of the GLUT receptor, uptake is notably decreased in the absence of Na+ (Figure 2a). However, uptake of [11C]DHA is not affected by availability of Na+ for co-transport, and is effectively blocked by application of cytochalasin B (Figure 2b) mirroring the trend observed for [18F]FDG (Figure 2c). This data confirms previously reported trends for uptake of the non-radioactive compounds.15 Since the uptake of [11C]DHA via GLUT is 10 fold higher than uptake of [11C]VitC we anticipated that intracellular accumulation of [11C]VitC would be primarily via an oxidation-dependent process. Indeed, transport of [11C]VitC via SVCT occurs more slowly than transport of the oxidized species, [11C]DHA via GLUT in many tissues.
Figure 2.
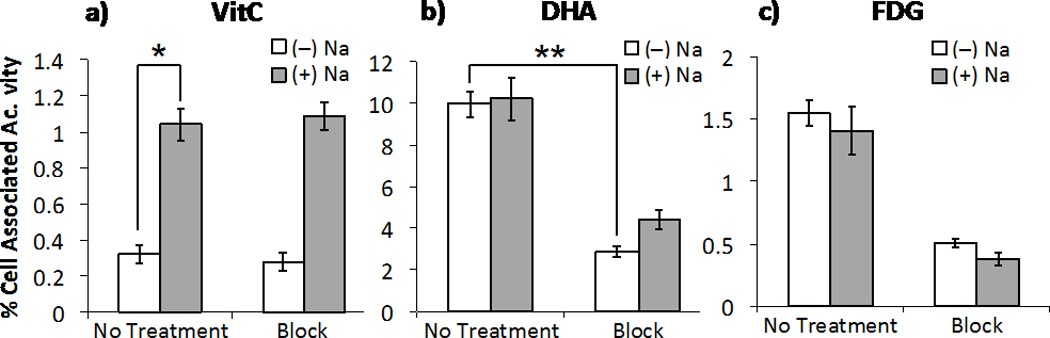
Uptake of (a) [11C]VitC (*p = 0.0002), (b) [11C]DHA (**p < 0.0001) and (c) [18F]FDG (+)/(−) availability of Na+ in media for co-transport via SVCT, (+)/(−) 10 µg/mL cytochalasin B blockade of GLUT in U87 human glioblastoma cancer cells.
Having shown the expected behavior of [11C]VitC and [11C]DHA in vitro, we performed a proof of concept in vivo experiment to demonstrate the differential transport of the two tracers. It has been well-established that DHA (but not VitC) crosses the blood-brain barrier transported primarily by GLUT1.23 Approximately 200 µCi of [11C]VitC (n = 3) and [11C]DHA (n = 3) each were administered to normal rats via tail vein injection and a 40 min dynamic scan was obtained using a microPET/CT scanner. As anticipated the brain accumulation of [11C]DHA was markedly higher than that of [11C]VitC (Figure 3), confirming our hypothesis that changes in uptake based on oxidized vs. reduced forms of ascorbic acid can be detected using PET.
Figure 3.
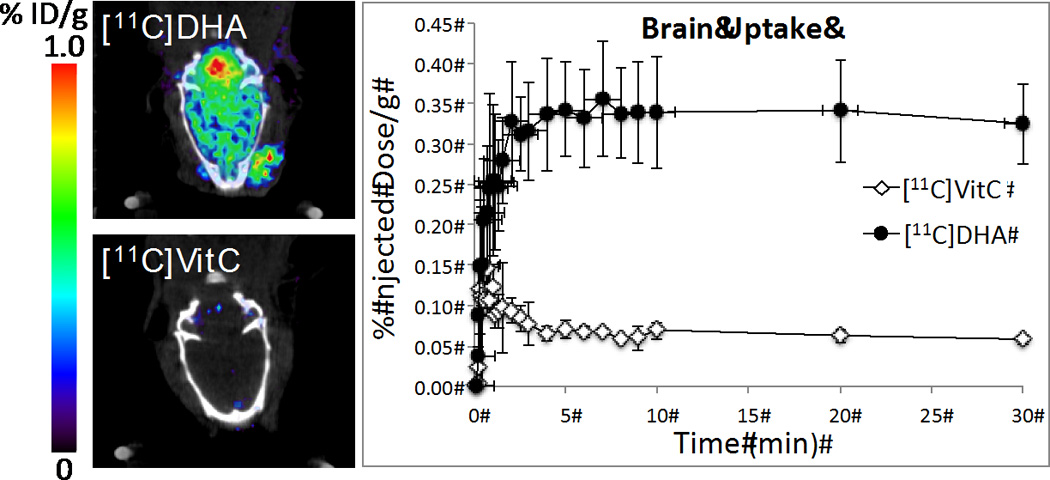
Representative in vivo microPET images of [11C]VitC and [11C]DHA in a normal rat brain (t = 0 – 30 min) and brain ROI data (n = 3) for dynamic scans.
Finally we investigated ROS-dependent [11C]VitC accumulation in cells. This was first accomplished in U87 cells by addition of exogenous H2O2 to the media,12a resulting in a greater than 2-fold increase in [11C] accumulation (*p = 0.0006) as shown in Figure 4a. We next applied [11C]VitC to a model of endogenous ROS production, namely stimulated neutrophil-lineage cells undergoing oxidative burst. For this study we used the HL60 cell-line, a human leukemia neutrophilic precursor, and human neutrophils, which had been freshly isolated from whole blood. The mechanism of VitC uptake in human neutrophils has been well established in literature.24,15c During phagocytosis, neutrophils undergo Nox-mediated generation of ROS to destroy bacteria and simultaneously oxidize extracellular VitC.25 [11C]VitC was oxidized to [11C]DHA by the major ROS produced during the oxidative burst, H2O2, O2− and ClO− (Figure S6). To investigate tracer uptake via this mechanism, cells were incubated with 10 µCi (0.37 MBq) [11C]VitC +/− activation with 2 µM phorbol 12-myristate 13-acetate and +/− 20 µg/mL cytochalasin B blocking.24,15c For both HL60 cells and neutrophils a significant increase in cell-associated activity, approximately 2-fold, was noted with activation (**p = 0.0025, ***p = 0.00041) (Figure 4b,c). For HL60 cells administration of cytochalasin B decreased the uptake of [11C]VitC to approximately the amount observed for (−) PMA. For neutrophils partial blocking was observed. This partial blocking effect in activated neutrophils could be explained by nitric oxide mediated expression of SVCT, as previously described.26 As expected % cell associated activity of [11C]DHA with co-administration of cytochalasin B did not differ significantly between +/− PMA in human neutrophils due to blocking of GLUT transport (Figure S7). These results provide the basis for detection of endogenously produced ROS using [11C]VitC PET.
Figure 4.
Uptake of [11C]VitC in (a) in U87 glioma cells (+)/(−) 100 µm H2O2, b) HL60 human promeylocyctic leukemia cells (+)/(−) 2 µM PMA activation and (c) freshly isolated human neutrophils (+)/(−) 2 µM PMA and (+)/(−) 20 µg/mL cytochalasin B blockade.
In conclusion, we have developed a new PET radiotracer [11C]VitC, which exhibits ROS-dependent cellular accumulation. [11C]VitC and its redox partner [11C]DHA behaved as anticipated in vitro and in vivo, consistent with their markedly different transport mechanisms. [11C]VitC is capable of detecting endogenously produced ROS in activated neutrophil-lineage cells, suggesting potential clinical utility in studying inflammation and/or monitoring immunotherapy. We hypothesize that the ascorbate recycling mechanism may be used to image a myriad of ROS-driven disease states. Furthermore, as high-dose vitamin C has been studied as an anticancer therapy for decades,27 low toxicity in patients has already been well-established.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Health (R01 CA166766, R01 CA176671, R00 CA172695, P41 EB013598 and P50 CA114747) and the Ben and Catherine Ivy Foundation. M.J.E. was supported by the 2014 David H. Koch Young Investigator Award from the Prostate Cancer Foundation. The authors would like to thank Prof. Kayvan Keshari (Memorial Sloan-Kettering Cancer Center) and Dr. Shikai Zhao (Omicron Biochemicals) for helpful discussions.
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/x0xx00000x
Contributor Information
P. J. H. Scott, Email: PJHScott@umich.edu.
F. T. Chin, Email: ChinF@Stanford.edu.
D. M. Wilson, Email: David.M.Wilson@ucsf.edu.
Notes and references
- 1.(a) D'Autreaux B, Toledano MB. Nat. Rev. Mol. Cell Biol. 2007;8:813. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]; (b) Dickinson BC, Chang CJ. Nat. Chem. Biol. 2011;7:504. doi: 10.1038/nchembio.607. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Holmstrom KM, Finkel T. Nat. Rev. Mol. Cell Biol. 2014;15:411. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 2.(a) Chandel NS, Vander Heiden MG, Thompson CB, Schumacker PT. Oncogene. 2000;19:3840. doi: 10.1038/sj.onc.1203727. [DOI] [PubMed] [Google Scholar]; (b) Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, Iadecola C. Proc. Natl. Acad. Sci. 2008;105:1347. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi JI. Science. 2008;320:661. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 3.(a) Huang Y, Mucke L. Cell. 2012;148:1204. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lin MT, Beal MF. Nature. 2006;443:787. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]; (c) Mattson MP. Nature. 2004;430:631. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Barnham KJ, Masters CL, Bush AI. Nat. Rev. Drug Discov. 2004;3:205. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 4.(a) Salvemini D, Doyle TM, Cuzzocrea S. Biochem. Soc. Trans. 2006;34:965. doi: 10.1042/BST0340965. [DOI] [PubMed] [Google Scholar]; (b) Rubartelli A, Lotze MT. Trends in Immunology. 2007;28:429. doi: 10.1016/j.it.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 5.(a) Houstis N, Rosen ED, Lander ES. Nature. 2006;440:944. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]; (b) Pop-Busui R, Sima A, Stevens M. DiabetesMetab. Res. Rev. 2006;22:257. doi: 10.1002/dmrr.625. [DOI] [PubMed] [Google Scholar]; (c) Jay D, Hitomi H, Griendling KK. Free Radic. Biol. Med. 2006;40:183. doi: 10.1016/j.freeradbiomed.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Dikalov S, Jiang J, Mason RP. Free Radical Research. 2005;39:825. doi: 10.1080/10715760500155688. [DOI] [PubMed] [Google Scholar]
- 7.Karton-Lifshin N, Segal E, Omer L, Portnoy M, Satchi-Fainaro R, Shabat D. J. Am. Chem. Soc. 2011;133:10960. doi: 10.1021/ja203145v. [DOI] [PubMed] [Google Scholar]
- 8.(a) Van de Bittner GC, Dubikovskaya EA, Bertozzi CR, Chang DJ, J C. Proc. Natl. Acad. Sci. 2010;107:21316. doi: 10.1073/pnas.1012864107. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Van de Bittner GC, Bertozzi CR, Chang CJ. J. Am. Chem. Soc. 2013;135:1783. doi: 10.1021/ja309078t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Lippert AR, Keshari KR, Kurhanewicz J, Chang CJ. J. Am. Chem. Soc. 2011;133:3776. doi: 10.1021/ja111589a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Keshari KR, Kurhanewicz J, Bok R, Larson PEZ, Vigneron DB, Wilson DM. Proc. Natl. Acad. Sci. 2011;108:18606. doi: 10.1073/pnas.1106920108. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Keshari KR, Sai V, Wang ZJ, VanBrocklin HF, Kurhanewicz J, Wilson DM. J. Nucl. Med. 2013;54:1. doi: 10.2967/jnumed.112.115402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowan EA, Taylor JL, Oldham CD, Dasari M, Doyle D, Murthy N, May SW. Enzyme Microb. Technol. 2013;53:373. doi: 10.1016/j.enzmictec.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 11.(a) Albers AE, Dickinson BC, Miller EW, Chang CJ. Bioorg. Med. Chem. Lett. 2008;18:5948. doi: 10.1016/j.bmcl.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dickinson BC, Chang CJ. J. Am. Chem. Soc. 2008;130:9638. doi: 10.1021/ja802355u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Dickinson BC, Huynh C, Chang CJ. J. Am. Chem. Soc. 2010;132:5906. doi: 10.1021/ja1014103. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Dickinson BC, Srikun D, Chang CJ. Curr. Opin. Chem. Biol. 2010;14:50. doi: 10.1016/j.cbpa.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Carroll V, Michel BW, Blecha J, VanBrocklin HF, Keshari KR, Wilson DM, Chang CJ. J. Am. Chem. Soc. 2014;136:14742. doi: 10.1021/ja509198w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chu W, Chepetan A, Zhou D, Shoghi KI, Xu J, Dugan LL, Gropler RJ, Mintun MA, Mach RH. Org. Biomol. Chem. 2014;12:4421. doi: 10.1039/c3ob42379d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gambhir SS. Nat. Rev. Cancer. 2002;2:683. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 14.Bürzle M, Suzuki Y, Ackermann D, Miyazaki H, Maeda N, Clémençon B, Burrier R, Hediger MA MA. Mol. Aspects of Med. 2013;34:436. doi: 10.1016/j.mam.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 15.(a) Vera JC, Rivas CI, Fischbarg J, Golde DW. Nature. 1993;364:79. doi: 10.1038/364079a0. [DOI] [PubMed] [Google Scholar]; (b) Spielholz C, Golde DW, Houghton AN, Nualart F F, Vera JC. Cancer Res. 1997;57:2529. [PubMed] [Google Scholar]; (c) Vera JC, Rivas CI, Zhang RH, Golde DW. Blood. 1998;91:2536. [PubMed] [Google Scholar]; (d) Agus DB, Vera JC, Golde DW. Cancer Res. 1999;59:4555. [PubMed] [Google Scholar]; (e) Corpe CP, Lee JH, Kwon O, Eck P, Narayanan J, Kirk KL, Levine M. J. Biol. Chem. 2005;280:5211. doi: 10.1074/jbc.M412925200. [DOI] [PubMed] [Google Scholar]; (f) Rumsey SC, Welch RW, Garraffo HM, Ge P, Lu SF, Crossman AT, Kirk KL, Levine M. J. Biol. Chem. 1999;274:23215. doi: 10.1074/jbc.274.33.23215. [DOI] [PubMed] [Google Scholar]
- 16.(a) Warburg O. Science. 1956;123:309. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]; (b) Vander Heiden MG, Cantley LC, Thompson CB. Science. 2009;324:1029. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gambhir SS, Czernin J, Schwimmer J, Silverman DHS, Coleman RE, Phelps ME. J. Nucl. Med. 2001;42:1S. [PubMed] [Google Scholar]
- 17.(a) Yamamoto F, Sasaki S, Maeda M. Appl. Radiat. Isot. 1992;43:633. doi: 10.1016/0883-2889(92)90032-a. [DOI] [PubMed] [Google Scholar]; (b) Kim J, Yamamoto F, Kuwabara Y, Honda H, Mukai T, Maeda M. Radioisotopes. 2009;58:47. [Google Scholar]
- 18.Kothari PJ, Finn RD, Bornmann WG, Agus DB, Vera JC, Larson SM. Radiochim. Acta. 1997;77:87. [Google Scholar]
- 19.(a) Salomon LL, Burns JJ, King CG. J. Am. Chem. Soc. 1952;74:5161. [Google Scholar]; (b) Hamilton JK, Smith F. J. Am. Chem. Soc. 1952;74:5162. [Google Scholar]; (c) Drew KN, Church TJ, Basu B, Vuorinen T, Serianni AS. Carbohydr. Res. 1996;284:135. [Google Scholar]
- 20.(a) Hara T, Iio M. Appl. Radiat. Isot. 1987;38:1092. [Google Scholar]; (b) Drandarov K, Schubiger PA, Westera G. Appl. Radiat. Isot. 2006;64:1613. doi: 10.1016/j.apradiso.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Bohndiek SE, Kettunen MI, Hu DE, Kennedy BWC, Boren J, Gallagher FA, Brindle KM. J. Am. Chem. Soc. 2011;133(30):11795. doi: 10.1021/ja2045925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung CY, Rampal AL. J. Biol. Chem. Soc. 1977;252:5456. [PubMed] [Google Scholar]
- 23.Agus DB, Gambhir SS, Pardridge WM, Spielholz C, Baselga J, Vera JC, Golde DW. J. Clin. Invest. 1997;100:2842. doi: 10.1172/JCI119832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Washko PW, Wang Y, Levine M. J. Biol. Chem. 1993;268:15531. [PubMed] [Google Scholar]
- 25.Lee WL, Harrison RE, Grinstein S S. Microbes Infect. 2003;5:1299. doi: 10.1016/j.micinf.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Portugal CC, da Encarnacao TG, Socodato R. J. Biol. Chem. 2013;287(6):3860. doi: 10.1074/jbc.M111.260166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cameron E, Pauling L. Proc. Natl. Acad. Sci. 1976;73:3685. doi: 10.1073/pnas.73.10.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.