Abstract
Background
The prevalence of hepatitis C virus (HCV) in United States prisoners is high; however, HCV testing and treatment is rare. Infected inmates released back into society contribute to the spread of HCV in the general population. Routine hepatitis screening of inmates followed by treatment with new therapies offers hope to reduce ongoing HCV transmission.
Objective
To evaluate the health and economic impact of HCV screening and treatment in prisons on the HCV epidemic in the society.
Design
An agent-based microsimulation model of transmission and progression of HCV.
Data Sources
Published literature.
Target population
Population in US prisons and general community.
Time horizon
30 years.
Perspective
Societal.
Interventions
Risk-based and universal opt-out hepatitis C screening in prisons followed by treatment in a portion of patients.
Outcome measures
Prevention of HCV transmission and associated-disease in prisons and society, costs, quality-adjusted life years (QALYs), incremental cost-effectiveness ratio (ICER), and total prison budget.
Results of Base-Case Analysis
Implementing risk-based and opt-out screening could diagnose 41 900–122 700 new HCV cases in the next 30 years in prisons. Compared with no screening, these scenarios could prevent 5500–12 700 new HCV infections caused by releasees, where about 90% of averted infections would have occurred outside of prisons. HCV screening could also prevent 4200–11 700 liver-related deaths. The ICERs of screening scenarios were between $19 600–$29 200/QALY, and the respective 1st year prison budget were between $900 and $1150 million. Prisons would require an additional 12.4% of their current healthcare budget to implement such interventions.
Results of Sensitivity Analysis
Results were sensitive to the time horizon; and ICERs otherwise remained below $50 000 per QALY.
Limitations
Data on transmission network, re-infection rate and opt-out HCV screening rate are lacking.
Conclusions
Universal opt-out HCV screening in prisons is highly cost-effective and would reduce HCV transmission and HCV-associated diseases primarily in the outside community. Investing in US prisons to manage hepatitis C is a strategic approach to address the current epidemic.
INTRODUCTION
In the United States (US), the prevalence of antibodies to hepatitis C in the non-institutionalized population is estimated at 1.0% (1). In contrast, the corresponding prevalence in US prisons is 17.3% (2). Liver disease is a frequent cause of death in inmates, as well as in society, and it has recently surpassed that from HIV (3-5). Hepatitis C virus (HCV) infection is also the leading cause of hepatocellular carcinoma and the most common indication for liver transplant (6).
Approximately 30% of all HCV infected persons in the US spend at least part of the year in correctional facilities (2). Most of the movement in and out of confinement is through jails, which are short-stay facilities. However, on a given day, twice as many Americans dwell in prisons, which are long-term facilities for felons (3, 7). Injection drug use (IDU) is the most common mode of HCV transmission, and 20%–55% of inmates have a history of IDU (8, 9). If infected releasees take up IDU upon return, they could contribute to HCV spread in society (10, 11). The US Preventive Services Task Force recommends that a history of incarceration should trigger HCV testing (12).
New hope to reduce the HCV burden in correctional settings has emerged with the recent availability of highly-effective oral direct-acting antivirals agents (DAAs) (13), for which treatment duration is 8–24 weeks, contraindications are few, and more than 90% of patients achieved cure. However, the high price of DAAs has drawn criticism from multiple stakeholders (14, 15). Despite their high price, HCV treatment in prisons is feasible and meets the standard criteria for cost-effectiveness (16, 17).
However, treatment will reduce disease burden only if HCV patients are identified in the first place. Three-quarters of the state prisons either offer no screening or targeted testing of inmates reporting high-risk behavior, which will miss many potential patients (2, 18). According to a US Supreme Court ruling (19), prisons cannot have deliberate indifference to medical needs, if they are known. Therefore, once a diagnosis is made, a provider may find it difficult to justify not treating those diagnosed. Because treatment is expensive and prison budgets are often limited, there could be an incentive not to test for HCV.
Routine hepatitis case finding and treatment in US state and Federal prisons in the era of DAAs could substantially reduce HCV disease in prisons as well as in the general population. However, the effect of such interventions on prevention of HCV transmission and disease burden has not been evaluated (10). In addition, the economic benefits to both prisons and the outside community of such programs, and the burden on state and Federal budgets, are not known (10, 20). Therefore, the objective of our study was to evaluate the health and economic benefits of multiple HCV screening strategies in US prisons on HCV prevention in the general population.
METHODS
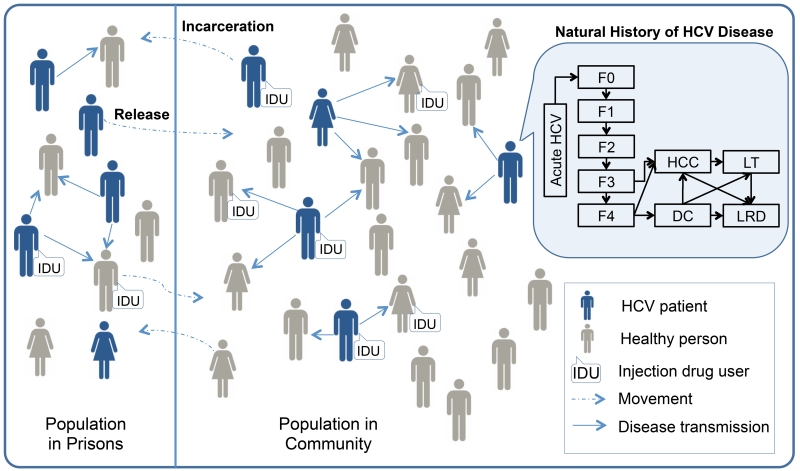
We developed the TapHCV (treatment as prevention of hepatitis C virus) model, an agent-based microsimulation model that projects the long-term benefits and costs of different HCV screening and treatment scenarios in US prisons. Our model includes HCV disease transmission, the natural history of HCV based on a previously validated Markov model (21), HCV screening in inmates, treatment of HCV infection with DAAs, and movement of people in and out of prisons. We simulated the disease and its progression both in prisons and in the general population to understand the complex dynamics between prison-related interventions and disease burden in society as a whole (Figure 1). The model was developed in the Java programming language from a societal perspective with a 30-year time horizon using monthly cycles (see Appendix for details).
Figure 1. Model Schematic of HCV Disease Transmission and Progression in Prisons and in the General Population.
The schematic of our agent-based model shows the prisons population and general population, and the dynamic movement between two groups (dashed blue arrows). Each person is defined by: demographics, liver stage, and injection drug use. As time progresses, the following characteristics are updated: age, injection drug use status, HCV infection status (infected individuals shown in blue), stage of liver disease, and location (inside or outside prison). HCV infected persons can transmit disease to others in their immediate network (solid blue arrows). The natural history of chronic HCV disease is represented by Markov states (top right insert). Chronic HCV stages are defined by the following METAVIR scores: no fibrosis (F0), portal fibrosis without septa (F1), portal fibrosis with few septa (F2), numerous septa without cirrhosis (F3), and compensated cirrhosis (F4), advanced liver diseases stages are decompensated cirrhosis (DC), hepatocellular carcinoma (HCC), liver transplants (LT) and liver-related deaths (LRD).
Baseline population
We simulated 2 million heterogeneous individuals representing the US population inside and outside of prisons in 2015. Patient characteristics included age, gender, drug use behavior (active or former IDU versus non-IDU), and imprisonment status (incarcerated in state or federal prisons versus general population). Prisoners constituted 0.5% of the total US population, and 91% were male (eTables 1-2) (22). We stratified HCV prevalence by individuals’ characteristics. We also defined the baseline distribution of common US HCV genotypes (GT1, 2, 3 and 4); chronic HCV stage using METAVIR (Meta-Analysis of Histologic Data in Viral Hepatitis) fibrosis scores (no fibrosis [F0], portal fibrosis without septa [F1], portal fibrosis with few septa [F2], numerous septa without cirrhosis [F3], or compensated cirrhosis [F4]); and treatment history (previously treated or treatment-naïve) using published studies (eTables 3-4). In the base case, we assumed that 25% of HCV patients in prisons and 50% outside were aware of their disease status, either through prior community or prison screening. More detailed description of the baseline population is provided in Appendix S1.2.
HCV Transmission and Progression
During each month, infected individuals could transmit the virus to uninfected individuals. We modeled two kinds of transmissions: 1) IDU-related, and 2) everything else, separately in prisons and community. The probability of transmission was dependent on the following factors: awareness status, active injection drug use, history of injection drug use, and prior HCV treatment (eTable 5, Appendix S1.3). We calibrated transmission-related parameters that were not directly available by choosing parameters that produced incidence results observed and published by the Center for Disease Control and Prevention (CDC) (Appendix S1.3). We assumed that the likelihood of HCV transmission was 50% lower for those who were aware of their infection status.
All newly infected individuals started with acute phase of HCV, which could develop into a chronic phase. We defined the natural history of HCV using Markov health states, which was based on our previously published models (21, 23). The chronic disease progressed to different stages of fibrosis, as defined by METAVIR fibrosis scores, from F0 to F4 (Figure 1). Patients at METAVIR score F3 and F4 could develop decompensated cirrhosis, hepatocellular carcinoma, or both. Patients with decompensated cirrhosis or hepatocellular carcinoma might receive a liver transplant, or experience a liver-related death (eTable 6). We also applied a background mortality adjusted by age, sex and IDU (Appendix S1.4).
HCV Screening
Unaware patients could be diagnosed through HCV testing. We evaluated 5 screening scenarios in prisons starting from the year 2015: no screening, 1-time risk-based screening of currently incarcerated and entrants who were active or former IDUs for 1 year (1Yr-Risk), 1-time opt-out universal HCV screening of currently incarcerated inmates followed by opt-out screening of all incoming inmates for up to 1 year (1Yr-All), 5 years (5Yr-All) and 10 years (10Yr-All). We assumed that the uptake rate of risk-based screening was 75%, based on the Arrestee Drug Abuse Monitoring (ADAM) jail study; and opt-out HCV screening, was 90%, similar to that of HIV opt-out screening in prisons (24, 25). In the general population, we implemented a combination of birth-cohort and risk-based screening, the current standard-of-care (eTable 7).
HCV Treatment
Those inmates diagnosed with HCV were eligible for treatment with recently approved DAAs. In the base case, we assumed that only those with METAVIR fibrosis scores F3 and F4 would receive treatment and others would wait because of limited resources. We assumed that those in F0–F2 stages would undergo annual APRI test and become eligible for treatment if advanced to F3 stage (26). Patients were assigned treatment with oral DAAs, as defined by the clinical guidelines (27). Because the SVR rates of the available oral drugs are similar to each other, we used the SVR rates of sofosbuvir-based therapies as a reference. Treatment regimen was dependent on HCV genotype, prior treatment outcomes, and presence of cirrhosis (eTable 8). We assumed that in 2030, generic drugs would become available and that all patients would be eligible for treatment with low cost drugs regardless of their fibrosis stage (28).
Admission and release of prisoners
We simulated movement of people from the community to prisons and vice versa (Figure 1). The probability of being incarcerated and lengths of sentence at admission were estimated from pooled data from the Bureau of Justice Statistics and published reports (Appendix S1.6, eTables 9-10).
Costs and Utilities
Our model included the cost of HCV testing, antiviral treatment, and management of chronic HCV disease (eTable 11). Screening costs included the cost of HCV antibody, HCV RNA, HCV genotype tests, and FibroSure test. Treatment costs included the wholesale acquisition cost of sofosbuvir ($7000 per week), ledipasvir ($1125 per week), and ribavirin ($309 per week) (29), and we considered drug discounts in sensitivity analysis (30). HCV disease management costs included the cost associated with chronic HCV infection, decompensated cirrhosis, hepatocellular carcinoma and liver transplant. All costs were converted to 2014 US dollars.
To each individual in our model, we assigned health-related quality-of-life (QOL) weights, with 0 denoting death and 1 denoting perfect health, and adjusted them by IDU, age and sex (eTable 12). We assumed that the QOL of patients who achieved SVR were equivalent to that of uninfected people if they had METAVIR scores F0–F1 and worse than uninfected people, otherwise.
Model outcomes
For each screening scenario, we projected the number of HCV cases diagnosed; the number of new HCV infections resulting from the release of untreated, HCV-infected inmates; quality-adjusted life years (QALYs); total cost to prisons and society, including the cost of HCV screening, antiviral treatment and the cost associated with HCV disease and its sequelae, which included the cases of decompensated cirrhosis, hepatocellular carcinoma, liver transplants and liver-related deaths. We further estimated the benefits gained in the community due to screening in prisons. Finally, we estimated the incremental cost-effectiveness ratios (ICERs) of all screening scenarios. We applied a standard 3% annual discount rate to all future costs and QALYs, and ran our model 40 times (Monte Carlo runs) to estimate confidence intervals of outcomes. Our analysis followed the recommendations of the US Panel on Cost-Effectiveness in Health and Medicine (31).
We also performed a subgroup cost-effectiveness analysis by excluding any health benefits gained while incarcerated, and only considered the benefits gained while in the community due to screening in prisons.
To evaluate the robustness of the model results to uncertainty in input parameters, we performed sensitivity analyses on baseline population characteristics, transition probabilities, behavior inputs, QOL utilities, SVR rates, all costs including the cost of treatment, and patent expiration year. We also conducted scenario analyses on the time of HCV treatment in prisons (eTables 20–21).
Validation
We performed external validation of TapHCV model by comparing intermediate model outcomes with known data. Specifically, we validated the natural history of HCV in the model by comparing the projected incidence rates of hepatitis C sequelae to the reported range of a large clinical study (Appendix S2.1, eTable 13). We also validated the projected number of admissions to US prisons with the Bureau of Justice Statistics data (Appendix S2.2, eTable 14).
Role of the Funding Source
The funding source had no role in the design, conduct, or analysis of the study or the decision to submit the manuscript for publication.
RESULTS
HCV Diagnosis in Prisons
Implementing risk-based screening of entering and currently incarcerated active or former IDUs for one year (1Yr-Risk) could diagnose 41 900 (95% CI, 40 700–43 200) new HCV cases among prisoners in the next 30 years. In comparison, 1Yr-All, 5Yr-All and 10Yr-All scenarios could diagnose 81 100 (95% CI, 79 600–82 700), 106 600 (95% CI, 104 700–108 500) and 122 700 (95% CI, 120 800–124 600) new HCV cases, respectively, where 70 700 (95% CI, 69 000–72 300) of those would be diagnosed among inmates currently incarcerated. The screening cost of per case identified under 1Yr-Risk, 1Yr-All, 5Yr-All and 10Yr-All would be $880, $1300, $1680 and $2030, respectively.
Prevention of HCV Disease
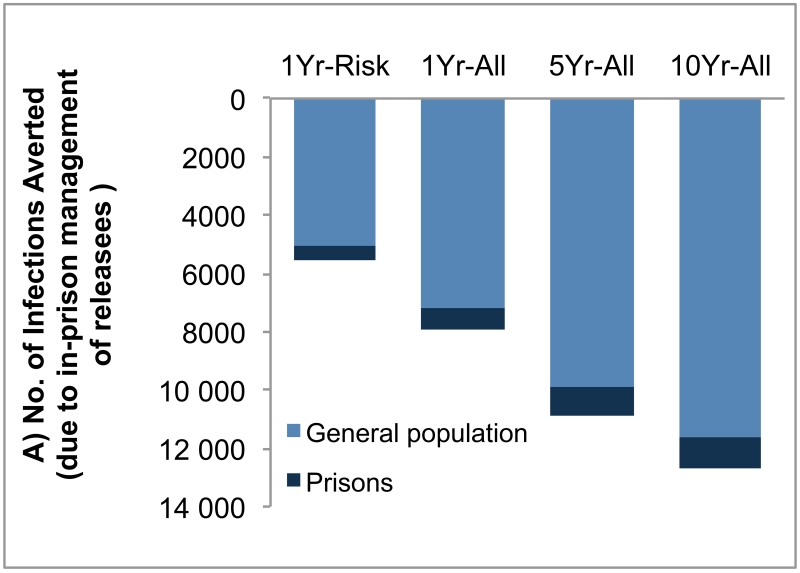
Compared with no screening scenario, 1Yr-Risk, 1Yr-All, 5Yr-All and 10Yr-All scenarios would prevent 5500 (95% CI, 4400–6600), 8000 (95% CI, 6800–9000), 10 900 (95% CI, 9700–12 000) and 12 700 (95% CI, 11 500–13 900) new HCV infections, respectively, in the next 30 years. Of these cases prevented by prison screening programs, 89%–92% of averted infections would have occurred in the general population (Figure 2A). For all scenarios, infections averted would peak between years 2020 and 2024 and decline afterwards (eFigure 1). Interventions in prisons would reduce the number of HCV-infected people in prisons over time, and the benefits of screening would peak around year 2035 and decline afterwards (eFigure 2). Furthermore, compared with no screening, HCV screening in prisons could prevent 4200–11 700 liver-related deaths, 300–900 liver transplants, 3000–8600 cases of hepatocellular carcinoma and 2600–7300 cases of decompensated cirrhosis in the next 30 years. Of note, among liver-related deaths averted, 80% would have occurred in the outside community.
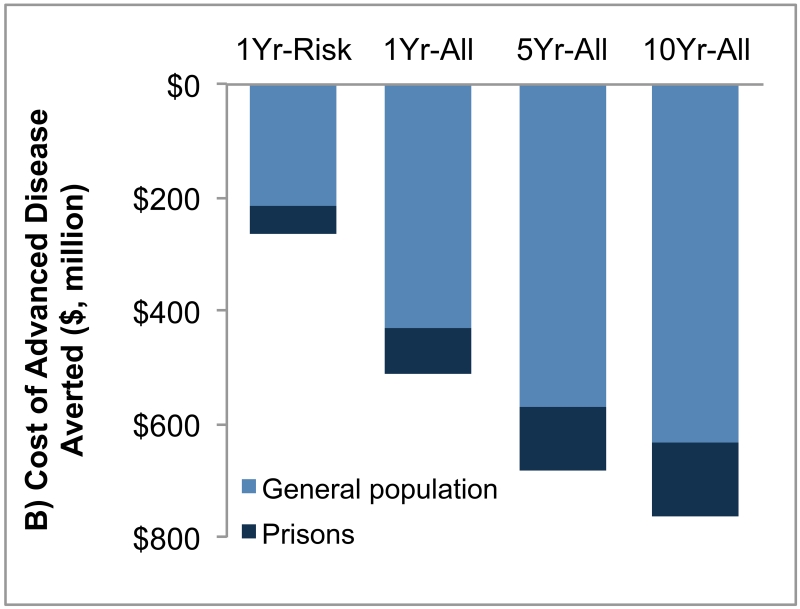
Figure 2. Projected Reduction of Hepatitis C Virus (HCV) Transmission and Hepatitis C Virus (HCV)-Associated Costs.
(A) Projected reduction of new infections over 30 years owing to HCV screening of inmates compared with no screening. Screening of inmates reduces new infections both inside the prisons and in the general US population (owing to the spread of infection via released inmates), and 89%–92% of total reductions occur in the general population. (B) Cost of advanced HCV diseases averted due to screening of inmates in the next 30 years in prisons and in the general population. Compared with no screening, screening of inmates reduced costs of HCV disease by averting advanced stages of disease. Note that future cost savings from adverted disease were discounted at 3% annually.
Reduction in HCV Disease Cost
Compared with no-screening scenario, 1Yr-Risk, 1Yr-All, 5Yr-All and 10Yr-All scenarios would reduce HCV disease cost due to in-prison management of infected releases by $260 (95% CI, 220–300) million, $510 (95% CI, 470–560) million, $680 (95% CI, 620–740) and $760 (95% CI, 700–820) million, respectively (Figure 2B). Reductions in HCV disease cost that were realized in the general population constituted 82–84% of the total savings stemming from screening in prisons. Of the total cost savings, 42–44% were due to fewer cases of decompensated cirrhosis, 39% due to fewer cases of hepatocellular carcinoma, and 17–19% due to fewer liver transplants.
Cost-effectiveness analysis
Compared with no screening scenario, 1Yr-Risk, 1Yr-All, 5Yr-All and 10Yr-All would increase the total QALYs by 41 900 (95% CI, 37 700–46 100), 75 600 (95% CI, 69 600–81 600), 95 800 (95% CI, 89 000–102 700) and 105 700 (95% CI, 99 200–112 200), respectively. The corresponding increase in total costs would be $820 (95% CI, 760–890) million, $1520 (95% CI, 1440–1590) million, $2000 (95% CI, 1920–2090) million and $2290 (95% CI, 2220–2370) million, respectively. The incremental cost-effectiveness ratios (ICERs) of 1Yr-Risk, 1Yr-All, 5Yr-All and 10Yr-All were $19 600, $20 600, $24 000 and $29 200 per additional QALY, respectively (Table 1).
Table 1. 30-year cumulative incidences of infection, advanced diseases, and results of cost-effectiveness analysis.
Compared with no screening, HCV screening and treatment in prison could reduce the cumulative incidence of new infections and cases of advance diseases. The screening costs and treatment costs increased as more intensive screening strategies were applied. However, HCV disease management costs decreased due to decreased number of HCV-related complications. Note that for the cost-effectiveness analysis, we presented incremental QALYs and total costs, and each scenario was compared to its less intensive scenario.
| No screening | 1Yr-Risk vs. No Screening |
1Yr-All vs. No Screening |
5Yr-All vs. No Screening |
10Yr-All vs. No Screening |
|
|---|---|---|---|---|---|
| 30-year Cumulative Incidences | |||||
| Total HCV Infections | 166 084 | −5508 | −8041 | − 11 005 | − 12 607 |
| Decompensated Cirrhosis | 633 235 | −2476 | −4532 | −6218 | −6908 |
| Hepatocellular Carcinoma | 730 683 | −2930 | −5665 | −7519 | −8602 |
| Liver Transplants | 92 596 | −187 | −595 | −702 | −809 |
| Liver-related Deaths | 780 803 | −4310 | −7942 | − 10 368 | − 11 730 |
| 30-year Total Cost ($, million) | |||||
| Screening Cost | $0.0 | +$37 | +$107 | +$178 | +$249 |
| Treatment Cost | $59 035 | +$816 | +$1480 | +$1951 | +$2190 |
| Advanced HCV Complications Cost |
$ 94 326 | −$30 | −$71 | −$128 | −$148 |
| Cost-Effectiveness Analysis | No screening | 1Yr-Risk vs. No screening |
1Yr-All vs. 1Yr-Risk |
5Yr-All vs. 1Yr-All |
10Yr-All vs. 5Yr-All |
|---|---|---|---|---|---|
| QALY | 5 677 199 951 | +41 905 | +33 696 | +20 201 | +9898 |
| Total Cost ($, million) | $ 153 361 | +$823 | +$693 | +$486 | +$289 |
| ICER ($ per QALY) | ---- | $19 635 | $20 571 | $24 046 | $29 234 |
Scenarios:
“No screening”: No screening inside prisons;
“1Yr-Risk”: 1-time risk-based screening of currently incarcerated and entrants who were active or former IDUs for 1-year.
“1 Yr-All”: 1-time opt-out screening of currently incarcerated inmates and entrants for 1 year.
“5Yr-All”: 1-time opt-out screening of currently incarcerated inmates and entrants for 5 year.
“10Yr-All”: 1-time opt-out screening of currently incarcerated inmates and entrants for 10 years.
Abbreviations: QALY, quality-adjusted life-years; ICER, incremental cost-effectiveness ratio.
Note that any discrepancies in ICERs may be due to rounding.
Next, we estimated the benefit of interventions in prison on the society exclusively by excluding the QALYs gained inside prisons and only considering QALYs gained in the community. The estimated increase in QALYs because of 1Yr-Risk, 1Yr-All, 5Yr-All and 10Yr-All screening were 35 600 (95% CI, 31 500–39 600), 64 900 (95% CI, 59 300–70 500), 82 100 (95% CI, 75 900–88 400), and 90 300 (95% CI, 84 300–96 300), respectively. The corresponding ICERs were $23 100, $23 600, $28 200 and $35 400 per additional QALY, respectively.
Budget Impact on Prison System
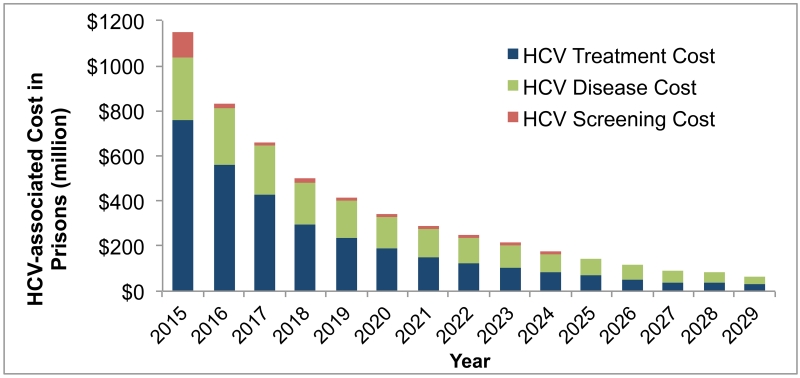
The first year cost of HCV screening, treatment and chronic HCV management in 2015 under risk-based screening and opt-out screening would be $900 million and $1146 million, respectively, spread over the prison systems in all states. The corresponding costs would decrease below 66 million after 15 years (Figure 3, eFigure 3). The first-year budget needed to implement universal opt-out screening followed by treatment with DAAs would require an additional 12.4% over the current healthcare budget of state and Federal prisons (32, 33). However, after 15 years, prisons would need only an additional 0.7% of the current healthcare budget because of decrease in HCV prevalence in both prisons and society.
Figure 3. Total cost of HCV screening and treatment in prisons from 2015 to 2029.
The budget needed to screen, treat HCV infection, and manage chronic hepatitis C in prisons under 1-time opt-out universal screening of currently incarcerated inmates and entrants for 10 years (10Yr-All scenario). The annual budget decreased substantially from 2015 to 2029 due to decrease in prevalence and discounting of future expenditures at 3% annually.
Sensitivity Analysis
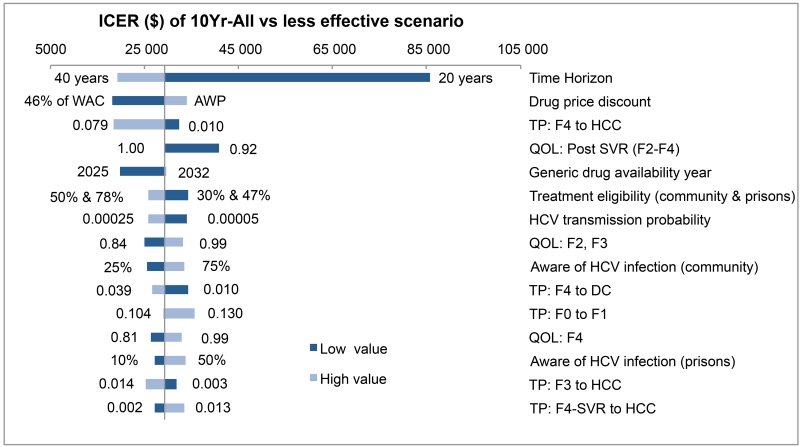
We performed 1-way sensitivity analysis on all key model parameters (eTables 15–19). Time-horizon was the most sensitive parameter and could influence the cost-effectiveness of opt-out HCV testing (Figure 4). For all other parameters, the ICERs always remained below $50 000 per QALY, indicating that uncertainty in model parameters did not influence the cost-effectiveness results. Of note, discounts on antiviral drugs would make HCV screening highly cost-effective. We also found that treatment of HCV in early stages and immediately after diagnosis also resulted in ICERs below $50 000 per QALY (eTables 20–21).
Figure 4. One-way sensitivity analysis showing top 15 most sensitive parameters in cost-effectiveness analysis comparing 10Yr-All scenario to less effective scenario.
The dark bars show the change in ICER from the base case when the lower value of an input was used while all other input parameters were kept constant, and the light bars show the change when the higher value was used. Abbreviations: ICER, incremental cost-effectiveness ratio; WAC, wholesale acquisition cost; AWP, average wholesale price; TP, transition probability; HCC, hepatocellular carcinoma; QOL, quality-of-life; SVR, sustained viral response; DC, decompensated cirrhosis; LT, liver transplant; F0-4, METAVIR fibrosis score; METAVIR, meta-analysis of histologic data in viral hepatitis.
DISCUSSION
Our results suggest that the universal opt-out screening of inmates for HCV is highly cost-effective for at least 10 years and would reduce ongoing HCV transmission, the incidence of advanced liver diseases and liver-related deaths. The majority of the benefits of interventions in prisons would accrue in the community, as a larger proportion of releasees to the community would have been cured of the disease. However, to achieve these benefits, the government needs to provide additional resources to prison healthcare, which will be a good investment for society.
Earlier research found that HCV treatment with DAAs is cost-effective in prison settings (16). However, the benefits of treatment would only be incurred if an effective screening policy is in place and HCV cases are diagnosed in a timely manner. To our knowledge, our study is the first to evaluate the society-wide benefits and cost-effectiveness of opt-out hepatitis C screening in prisons. We developed a comprehensive mathematical model to simulate HCV transmission and interactions between prisons and community, thereby evaluated complex emergent dynamics that otherwise could not be predicted (34).
The US Preventive Services Task Force and the CDC in 2012 updated their HCV screening policy to include persons born between 1945 and 1965 (12, 35). Modeling studies have shown that HCV screening in the birth-cohort is cost-effective for general population (36). In contrast, the majority of HCV-infected individuals in prisons may no longer be among this birth-cohort, as new cases of HCV are rising among youth who inject drugs (37, 38). For this reason, we did not confine screening of hepatitis C in prisons to members of the baby boom generation.
Our findings suggest that interventions in prisons provide an even better value for money when compared with the current screening recommendations for the general US population. The cost of the screening per case identified with opt-out screening in prisons ($900–$2000) was lower than that identified with the birth-cohort screening in the general population ($2900) (36). Additionally, the ICER of opt-out screening in prisons was lower than that of the birth-cohort screening in the general population, which was in the range of $35 700–$65 700 per QALY (36, 39-41). Even when excluding all health benefits gained while in prison, the opt-out HCV screening still remained highly cost-effective. Our conclusions are based on wholesale acquisition costs of HCV treatment, however, in practice, several prisons would get drug discounts that would make HCV testing even more cost-effective.
Because US correctional systems cannot display deliberate indifference to apparent health care needs (19, 42), without adequate healthcare budgets, health administrators in prisons may be reluctant to uncover all HCV cases (42). Our study emphasizes the benefits of state or Federal government allocating additional resources to prisons to screen and treat HCV. Such investment in prisons is beneficial from the societal perspective because early detection and treatment in correctional settings could prevent future need for treatment, which would occur predominantly in the society among releasees. Furthermore, these interventions would prevent ongoing viral transmission in the general community.
Our study has several limitations. First, not all parameters were available or up-to-date, including the incidence of HCV infection inside prisons, and arrest rates among people who inject drugs. Therefore, we indirectly estimated these parameters through calibration and tested them in the sensitivity analysis. Secondly, we made simplifying assumptions about future trends in incarceration, risk-behavior, and birth and death rates, which could affect our results. Third, the effectiveness of new therapies in prison settings is unknown. However studies using earlier treatments suggest that in-prison outcomes would be similar to that in clinical trials (17, 43). Opt-out HCV screening rate in prisons is not known, therefore, we assumed the rate to be similar to that of HIV testing in prisons. In addition, we ended our simulation at the end of 30 years with no additional benefit, which tend to underestimate the benefits of screening.
Because of lack of data on HCV transmission, we made simplifying assumptions about the modes of HCV transmission and types of social network structure. We did not model advanced social networks of IDUs or sexual contacts. We modeled IDU transmission explicitly and all other transmissions grouped separately (see appendix S1.3). We used a simplified temporal network of IDUs, and assumed that age and sex have no impact on IDU mixing. Finally, we did not consider the possibility of future new antiviral therapies, which could be even more effective and less expensive.
Conclusion
Screening the incarcerated population would play an important role in reducing HCV burden society-wide. Therefore, HCV prevention efforts should increase its focus on prison inmates. From a societal perspective, investing in US prison healthcare to manage hepatitis C would be money well spent.
Supplementary Material
Acknowledgment
Funding/Support: This research was partly supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number KL2TR000146. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Tianhua He’s effort was supported by the China Scholarship Council.
We thank Kenneth Sherman and Rich Feffer for constructive comments that improved the quality of the manuscript, and Jill Delsigne for editing the manuscript. Dr. Chhatwal had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Primary Funding Source: National Institutes of Health
Footnotes
Conflict of interest disclosure: Chhatwal received consulting fee from Gilead Sciences, Merck, and Complete HEOR Solutions outside the submitted work. Spaulding has received research grants through Emory University from Gilead Sciences and Bristol Myers Squibb. She has served on advisory committees for Gilead Sciences and Janssen. There are none to report for He, Li, Roberts, Ayer, and Grefenstette.
Author contribution:
Study concept and design: He, Li, Roberts, Spaulding, Chhatwal
Drafting of manuscript: He, Chhatwal
Critical revision of the manuscript for important intellectual content: He, Li, Roberts, Spaulding, Ayer, Grefenstette, Chhatwal
Statistical analysis: He, Li, Chhatwal
Interpretation of data: He, Li, Roberts, Spaulding, Ayer, Grefenstette, Chhatwal
Potential conflict of interest disclosures: Spaulding has received research grants through Emory University from Gilead Sciences and Bristol Myers Squibb outside the submitted work. She has served on advisory committees for Gilead Sciences and Janssen. Chhatwal received consulting fee from Gilead Sciences, Merck, and Complete HEOR Solutions outside the submitted work. There are none to report for He, Li, Roberts, Ayer, and Grefenstette.
References
- 1.Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varan AK, Mercer DW, Stein MS, Spaulding AC. Hepatitis C seroprevalence among prison inmates since 2001: still high but declining. Public Health Rep. 2014;129(2):187–95. doi: 10.1177/003335491412900213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spaulding AC, Seals RM, McCallum VA, Perez SD, Brzozowski AK, Steenland NK. Prisoner survival inside and outside of the institution: implications for health-care planning. Am J Epidemiol. 2011;173(5):479–87. doi: 10.1093/aje/kwq422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ginder S MN. Mortality in local jails and state prisons, 2000-2011 - Statistical tables. Bureau of Justice Statistics Statistical Tables. 2013 NCJ 242186. [Google Scholar]
- 5.Spaulding AC, Sharma A, Messina LC, Zlotorzynska M, Miller L, Binswanger IA. A Comparison of Liver Disease Mortality With HIV and Overdose Mortality Among Georgia Prisoners and Releasees: A 2-Decade Cohort Study of Prisoners Incarcerated in 1991. American journal of public health. 2015;105(5):e51–e7. doi: 10.2105/AJPH.2014.302546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosen HR. Chronic hepatitis C infection. N Engl J Med. 2011;364(25):2429–38. doi: 10.1056/NEJMcp1006613. [DOI] [PubMed] [Google Scholar]
- 7.Hughes TA, Wilson DJ. Reentry trends in the United States. US Department of Justice, Bureau of Justice Statistics; [Last accessed August 25, 2015]. 2003. Available at: http://www.bjs.gov/content/pub/pdf/reentry.pdf. [Google Scholar]
- 8.Spaulding AC, Weinbaum CM, Lau DTY, Sterling R, Seeff LB, Margolis HS, et al. A framework for management of hepatitis C in prisons. Ann Intern Med. 2006;144(10):762–9. doi: 10.7326/0003-4819-144-10-200605160-00010. [DOI] [PubMed] [Google Scholar]
- 9.Harrison PM, Beck AJ. Prisoners in 2002. US Department of Justice, Bureau of Justice Statistics; Washington, DC: [Last accessed August 25, 2015]. 2003. Available at: https://http://www.ncjrs.gov/App/abstractdb/AbstractDBDetails.aspx?id=200248. [Google Scholar]
- 10.Rich JD, Allen SA, Williams BA. Responding to hepatitis C through the criminal justice system. N Engl J Med. 2014;370(20):1871–4. doi: 10.1056/NEJMp1311941. [DOI] [PubMed] [Google Scholar]
- 11.Martin NK, Hickman M, Miners A, Hutchinson SJ, Taylor A, Vickerman P. Cost-effectiveness of HCV case-finding for people who inject drugs via dried blood spot testing in specialist addiction services and prisons. BMJ Open. 2013;3(8) doi: 10.1136/bmjopen-2013-003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moyer VA. Screening for hepatitis C virus Infection in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2013 doi: 10.7326/0003-4819-159-5-201309030-00672. [DOI] [PubMed] [Google Scholar]
- 13.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889–98. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. New England Journal of Medicine. 2011;364(25):2405–16. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 15.Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, et al. Telaprevir for retreatment of HCV infection. New England Journal of Medicine. 2011;364(25):2417–28. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Watcha D, Holodniy M, Goldhaber-Fiebert JD. Sofosbuvir-based treatment regimens for chronic, genotype 1 hepatitis C virus infection in U.S. incarcerated populations: a cost-effectiveness analysis. Ann Intern Med. 2014;161(8):546–53. doi: 10.7326/M14-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moorjani H, Koenigsmann C, Kim MJ, Spaulding AC. Prisoners Treated for Hepatitis C with Protease Inhibitor, New York, USA, 2012. Emerg Infect Dis. 2015;21(1):186–8. doi: 10.3201/eid2101.141025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beck AJ, Maruschak LM. Hepatitis testing and treatment in state prisons. US Department of Justice, Office of Justice Programs; [Last accessed August 25, 2015]. 2004. Available at: https://http://www.ncjrs.gov/App/abstractdb/AbstractDBDetails.aspx?id=199173. [Google Scholar]
- 19.Krahn M, Wong JB, Heathcote J, Scully L, Seeff L. Estimating the prognosis of hepatitis C patients infected by transfusion in Canada between 1986 and 1990. Medical Decision Making. 2004;24(1):20. doi: 10.1177/0272989X03261568. [DOI] [PubMed] [Google Scholar]
- 20.Graham CS. Hepatitis c and HIV co-infection: Closing the gaps. JAMA. 2015 doi: 10.1001/jama.2015.1111. [DOI] [PubMed] [Google Scholar]
- 21.Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Annals of internal medicine. 2015;162(6):397–406. doi: 10.7326/M14-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West HC. Prison inmates at midyear 2009: Statistical tables. Bureau of Justice Statistics Statistical Tables; [Last accessed August 26, 2015]. 2010. NCJ 230113. Available at: http://www.bjs.gov/content/pub/pdf/pim09st.pdf. [Google Scholar]
- 23.Kabiri M, Jazwinski AB, Roberts MS, Schaefer AJ, Chhatwal J. The changing burden of hepatitis C virus infection in the United States: model-based predictions. Ann Intern Med. 2014;161(3):170–80. doi: 10.7326/M14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sweeting MJ, De Angelis D, Neal KR, Ramsay ME, Irving WL, Wright M, et al. Estimated progression rates in three United Kingdom hepatitis C cohorts differed according to method of recruitment. Journal of Clinical Epidemiology. 2006;59(2):144–52. doi: 10.1016/j.jclinepi.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Bonabeau E. Agent-based modeling: Methods and techniques for simulating human systems. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(Suppl 3):7280. doi: 10.1073/pnas.082080899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macal CM, North MJ. Tutorial on agent-based modelling and simulation. Journal of Simulation. 2010;4(3):151–62. [Google Scholar]
- 27.IMS Brogan . Delta PA; Ontario: 2010. [Google Scholar]
- 28.Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries. Clin Infect Dis. 2014;58(7):928–36. doi: 10.1093/cid/ciu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krajden M, Kuo M, Zagorski B, Alvarez M, Yu A, Krahn M. Health care costs associated with hepatitis C: A longitudinal cohort study. Canadian journal of Gastroenterology. 2010;24(12):717. doi: 10.1155/2010/569692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverman E. [Accessed Feb 4, 2015];What the ‘Shocking’ Gilead Discounts on its Hepatitis C Drugs Will Mean. The Wall Street Journal. Retrieved from: http://blogs.wsj.com/pharmalot/2015/02/04/what-the-shocking-gilead-discounts-on-its-hepatitis-c-drugs-will-mean/
- 31.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. Jama. 1996;276(15):1253–8. [PubMed] [Google Scholar]
- 32.James N, The Bureau of Prisons (BOP) [Last accessed August 27, 2015. 2014];Operations and Budget. Congressional Research Service. 2014 Mar 4; Available at: http://www.fas.org/sgp/crs/misc/R42486.pdf.
- 33.Kyckelhahn T. State corrections expenditures, FY 1982-2010. Bureau of Justice Statistics; [Last accessed August 27, 2015]. Dec, 2012. NCJ 239672. Available at: http://www.bjs.gov/content/pub/pdf/scefy8210.pdf. [Google Scholar]
- 34.Chhatwal J, He T. Economic Evaluations with Agent-Based Modelling: An Introduction. PharmacoEconomics. 2015;33(5):423–33. doi: 10.1007/s40273-015-0254-2. [DOI] [PubMed] [Google Scholar]
- 35.Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR-4):1–32. [PubMed] [Google Scholar]
- 36.Rein DB, Smith BD, Wittenborn JS, Lesesne SB, Wagner LD, Roblin DW, et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med. 2012;156(4):263–70. doi: 10.7326/0003-4819-156-4-201202210-00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larney S, Mahowald MK, Scharff N, Flanigan TP, Beckwith CG, Zaller ND. Epidemiology of hepatitis C virus in Pennsylvania state prisons, 2004-2012: Limitations of 1945-1965 birth cohort screening in correctional settings. Am J Public Health. 2014;104(6):e69–e74. doi: 10.2105/AJPH.2014.301943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suryaprasad AG, White JZ, Xu F, Eichler BA, Hamilton J, Patel A, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006-2012. Clin Infect Dis. 2014 doi: 10.1093/cid/ciu643. [DOI] [PubMed] [Google Scholar]
- 39.Eckman MH, Talal AH, Gordon SC, Schiff E, Sherman KE. Cost-effectiveness of screening for chronic hepatitis C infection in the United States. Clin Infect Dis. 2013;56(10):1382–93. doi: 10.1093/cid/cit069. [DOI] [PubMed] [Google Scholar]
- 40.McGarry LJ, Pawar VS, Panchmatia HR, Rubin JL, Davis GL, Younossi ZM, et al. Economic model of a birth cohort screening program for hepatitis C virus. Hepatology. 2012;55(5):1344–55. doi: 10.1002/hep.25510. [DOI] [PubMed] [Google Scholar]
- 41.Liu S, Cipriano LE, Holodniy M, Goldhaber-Fiebert JD. Cost-effectiveness analysis of risk-factor guided and birth-cohort screening for chronic hepatitis C infection in the United States. PLoS One. 2013;8(3):e58975. doi: 10.1371/journal.pone.0058975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spaulding AS, Kim AY, Harzke AJ, Sullivan JC, Linas BP, Brewer A, et al. Impact of new therapeutics for hepatitis C virus infection in incarcerated populations. Top Antivir Med. 2013;21(1):27–35. [PMC free article] [PubMed] [Google Scholar]
- 43.Maru DS, Bruce RD, Basu S, Altice FL. Clinical outcomes of hepatitis C treatment in a prison setting: feasibility and effectiveness for challenging treatment populations. Clin Infect Dis. 2008;47(7):952–61. doi: 10.1086/591707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.