Abstract
Idiopathic pulmonary fibrosis (IPF) is a progressive scarring disorder characterized by the proliferation of interstitial fibroblasts and the deposition of extracellular matrix causing impaired gas exchange. Spiruchostatin A (SpA) is a histone deacetylase inhibitor (HDI) with selectivity toward Class I enzymes, which distinguishes it from other nonspecific HDIs that are reported to inhibit (myo)fibroblast proliferation and differentiation. Because the selectivity of HDIs may be important clinically, we postulated that SpA inhibits the proliferation and differentiation of IPF fibroblasts. Primary fibroblasts were grown from lung biopsy explants obtained from patients with IPF or from normal control subjects, using two-dimensional or three-dimensional culture models. The effect of SpA on fibroproliferation in serum-containing medium ± transforming growth factor (TGF)–β1 was quantified by methylene blue binding. The acetylation of histone H3, the expression of the cell-cycle inhibitor p21waf1, and the myofibroblast markers α–smooth muscle actin (α-SMA) and collagens I and III were determined by Western blotting, quantitative RT-PCR, immunofluorescent staining, or colorimetry. SpA inhibited the proliferation of IPF or normal fibroblasts in a time-dependent and concentration-dependent manner (concentration required to achieve 50% inhibition = 3.8 ± 0.4 nM versus 7.8 ± 0.2 nM, respectively; P < 0.05), with little cytotoxicity. Western blot analyses revealed that SpA caused a concentration-dependent increase in histone H3 acetylation, paralleling its antiproliferative effect. SpA also increased p21waf1 expression, suggesting that direct cell-cycle regulation was the mechanism of inhibiting proliferation. Although treatment with TGF-β1 induced myofibroblast differentiation associated with increased expression of α-SMA, collagen I and collagen III and soluble collagen release, these responses were potently inhibited by SpA. These data support the concept that bicyclic tetrapeptide HDIs merit further investigation as potential treatments for IPF.
Keywords: pulmonary fibrosis, histone deacetylase inhibitor, spiruchostatin A, myofibroblast, proliferation
Clinical Relevance
Spiruchostatin A (SpA) is a histone deacetylase inhibitor (HDI) with selectivity toward Class I enzymes, which distinguishes it from other nonspecific HDIs that are reported to inhibit (myo)fibroblast proliferation and differentiation. We demonstrate the ability of spiruchostatin A to potently (in the nanomolar range) reduce the uncontrolled proliferation of idiopathic pulmonary fibrosis (IPF) fibroblasts and prevent their biosynthetic activity. Our data suggest that spiruchostatin A or similar compounds, in view of their selectivity, should be further investigated for their potential as a novel class of therapeutic agents for IPF.
Interstitial lung diseases (ILDs) are among the most difficult respiratory diseases to manage and treat effectively. One of the most common and important ILDs is idiopathic pulmonary fibrosis (IPF) (1), with an estimated incidence of 6.8–14 cases per 100,000, and a frequency that is slightly higher in the male population (2). It is a progressive disease that causes significant morbidity and mortality (3). The etiology of IPF is unknown, and its prognosis is as dismal as that of lung cancer (a median 2–4 years of survival from time of diagnosis) (4). Currently no effective treatment exists and, to make matters worse, available therapies that include corticosteroids and other immunosuppressive agents frequently cause unpleasant and sometimes dangerous side effects. The histologic hallmark of IPF is a heterogeneous appearance with areas of normal lung alternating with fibrosis, and honeycomb changes. The fibrotic zones are composed mainly of dense collagen with scattered foci of proliferating fibroblasts (so-called fibroblastic foci), typically found at the edges of dense scars. Because outcomes are poorer for individuals with more fibroblastic foci (5), these areas of fibroblastic activity may make an important pathological contribution to the disease process.
Fibroblasts have the capacity to differentiate into myofibroblasts, which are specialized contractile cells that synthesize large amounts of extracellular matrix (ECM; primarily interstitial collagens) in response to profibrotic agents such as transforming growth factor–β (TGF-β) (6). Myofibroblasts play a vital role in normal wound healing. However, in fibrotic diseases, they fail to undergo apoptosis as part of the normal resolution process. This leads to an excess accumulation of ECM, and results in tissue dysfunction as normal tissue is replaced by scar tissue. The role of myofibroblasts in orchestrating the active scarring process in IPF has come to be further appreciated (7, 8). Their involvement in a dysregulated wound-healing response appears to lead to the development of dysfunctional scar tissue in the lung, with resultant architectural distortion and disruption of gas exchange. Myofibroblast accumulation in the lung corresponds clinically with decreasing lung volumes and the development of more severe symptoms (9).
Persistent aberrations in fibrogenic pathways were detected previously in lung tissue and fibrotic cells derived from patients with IPF (10, 11). However, the reasons for these changes have not been investigated in detail. One potential mechanism lies at the level of reversible acetylation and deacetylation of histones and other nonhistone proteins by histone deacetylases (HDACs) and histone acetyltransferases (HATs) (12, 13). The reversible acetylation and deacetylation of histones comprise important mechanisms for regulating gene expression. Histone hyperacetylation is generally associated with euchromatin and increased transcription, whereas hypoacetylation leads to transcriptional repression. HATs can also acetylate nonhistone proteins, such as transcription factors and nuclear receptors, to facilitate gene expression. Imbalances in HAT/HDAC activity have been associated with several diseases, most notably the dysregulation of cell-cycle control linked to uncontrolled proliferation in several cancers (14, 15). A number of small-molecule HDAC inhibitors (HDIs) have been developed to investigate the role of HDACs in normal and disease processes. HDIs can increase or decrease the expression of specific genes through their effects on the acetylation of histones and nonhistone proteins. Thus, via their effects on chromatin structure and transcription factor/cofactor binding, HDIs modulate the expression of approximately 2–10% of cellular genes. The ability of HDIs to modify the acetylation status of a large number of cellular proteins involved in oncogenic processes has led to the development of this class of drugs as anticancer agents (16).
Like cancer, IPF is characterized by uncontrolled cell (in this case, fibroblast) proliferation, and the HDI suberoylanilide hydroxamic acid (SAHA) has been shown previously to block the stimulatory effects of TGF-β1 on myofibroblast differentiation, suggesting that HDIs may have therapeutic potential in pulmonary fibrosis (17). SAHA is a hydroxamic acid derivative that chelates zinc ions and belongs to the broadest class of HDI, affecting both Class I and II HDACs. More selective HDIs are of interest, not only as tools for probing the biological functions of specific HDAC isoforms, but also as candidate therapeutic agents with fewer side effects. Here we test the hypothesis that spiruchostatin A (SpA) is a potent inhibitor of the proliferation and differentiation of primary fibroblasts derived from patients with IPF, compared with fibroblasts from normal control lungs. SpA is a cyclic, cysteine-containing depsipeptide natural product, and has a distinct selectivity profile with key pharmacodynamic differences compared with nonspecific HDIs such as SAHA or trichostatin A (TSA) (18, 19). It is more Class I–selective, and in particular these compounds differ in their effects on HDAC6. Furthermore, the effects of SpA on histone acetylation are slower but more prolonged, compared with the hydroxamic acid–based inhibitors, but this does not compromise efficacy (18). Moreover, histone acetylation persists after removal of the drug, which may be important clinically. This work was presented previously in abstract form (20).
Materials and Methods
Compounds
SpA was synthesized as previously described (21), and was derived from a 1 mM stock in dimethyl sulfoxide (DMSO; Sigma, Gillingham, UK). A vehicle control was included in each experiment.
Primary Fibroblast Culture
Fibroblast cultures were established from explant cultures of surgical lung biopsies from three patients with a confirmed diagnosis of IPF (i.e., pathology consistent with the usual interstitial pneumonia) or from macroscopically normal lung tissue from (1) a wedge resection for a solitary pulmonary nodule (a histologically demonstrated hamartoma), and (2) a lobectomy for a T1 non–small cell lung cancer. In addition, a commercially available normal lung fibroblast cell line (CCD-19Lu; American Type Culture Collection, Manassas, VA) was used.
Methylene Blue Proliferation Assay
Fibroblasts were treated in the absence or presence of 5 ng/ml TGF-β1 (Peprotech, London, UK) for 24 hours before treatment with SpA. The cells were retreated with SpA after 24 hours. Cells were fixed in formal saline at 48, 72, and 144 hours before staining with methylene blue (22). The cell number, as determined by direct cell counting, was proportional to absorbance at 630 nm.
Western Blot Analyses
Western blots were performed for acetyl histone H3 (Millipore, Watford, UK) and α–smooth muscle actin (α-SMA; Sigma), using enhanced chemiluminescence detection (ECL plus; GE Healthcare, Buckinghamshire, UK), and with pan histone H3 (Millipore) as loading control.
Quantitative RT-PCR
RNA was isolated using Trizol (Invitrogen, Paisley, UK), and was DNase-treated (Ambion, Warrington, UK) before cDNA synthesis. Changes in the mRNA expression of p21waf1, collagen I, collagen III, and α-SMA were analyzed by quantitative PCR and normalized to the housekeeping genes ubiquitin C and phospholipase A2 (UBC/A2), using the ΔΔCT method (23). All PCR reagents were purchased from PrimerDesign, Ltd. (Southampton, UK).
Indirect Immunofluorescent Staining
Fibroblasts (1 × 103/well in chamber slides) were treated in the absence or presence of TGF-β1 and SpA as for Western blot analysis. At 48 hours, they were fixed in 4% paraformaldehyde and permeabilized before immunostaining for α-SMA (1:150), using a secondary FITC-conjugated antibody (1:50). Nuclei were counterstained with 7-amino actinomycin D.
Three-Dimensional Fibroblast Culture
To model fibroblastic foci more closely, fibroblasts were grown in three-dimensional micromass pellet cultures similar to those used for mesenchymal stem cells (24). Fibroblasts were harvested by centrifugation in 20-ml universal containers. The resulting pellet (1.0–1.5 × 106 cells) was placed in 2 ml DMEM/FBS in the absence or presence of 5 ng/ml TGF-β1 and without or with 62.5 nM SpA. The media were changed on alternate days, and spent media were clarified by centrifugation before soluble collagen analysis. The pellets were fixed and paraffin-embedded, and sections were analyzed using Masson trichrome stain.
Collagen Assay
Culture media were treated with 4 M NaCl, and the collagen precipitate was collected by centrifugation. The pellet was solubilized in 0.5 M acetic acid and collagen quantified by reference to a standard curve using Sirius red dye, according to the manufacturer's instructions (Sircol collagen assay kit; Biocolor, Ltd., Carrickfergus, UK).
Statistical Analysis
Differences between two means were assessed using the Student t test. For multiple comparisons, one-way ANOVA was used. When mean values were significantly different, pairwise comparisons were performed using the Student t test. P < 0.05 was accepted as statistically significant.
Results
SpA Inhibits Fibroblast Proliferation
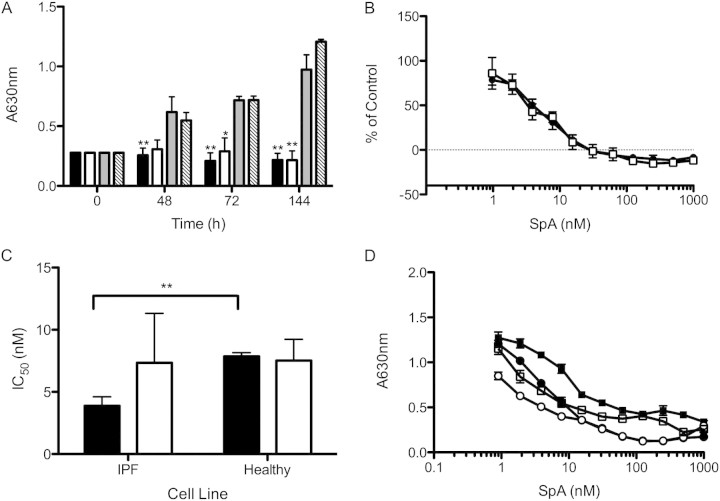
Initial experiments examined the effects of SpA on the proliferation of fibroblasts cultured from a 78-year-old patient with IPF. Cells were cultured in DMEM/FBS to favor maximal proliferation, and were tested in the absence or presence of TGF-β1 to mimic a profibrotic environment. Under these conditions, the cells doubled every 48 hours, and TGF-β1 exerted no effect on the proliferation of fibroblasts (Figure 1A). In the presence of SpA (10 nM), an inhibitory effect on fibroblast proliferation was evident, which was particularly marked after 72 and 144 hours, when significant inhibition occurred, irrespective of the presence of TGF-β1 (Figure 1A). At 144 hours, the concentration required to achieve 50% inhibition (IC50) of proliferation was 4.1 nM in serum-containing medium alone, or 3.8 nM in the presence of TGF-β1 (Figure 1B). Similar experiments were performed using fibroblasts from two further IPF donors and three normal control subjects. The results showed that the fibroblasts displayed significantly different proliferative potentials, with the IPF fibroblasts achieving a higher cell density at 144 hours (Figure E1 in the online supplement). Dose–response curves were performed for each fibroblast line (Figure E2), and the combined data revealed that the mean IC50 for SpA was significantly lower for IPF fibroblasts compared with normal control samples when the cells were grown in the absence of TGF-β1 (3.8 ± 0.4 nM versus 7.8 ± 0.2 nM, respectively; P < 0.05). However, this selectivity was lost when the cells were grown in the presence of TGF-β (7.3 ± 3.2 nM versus 7.5 ± 1.6 nM, respectively) (Figures 1C, and E2A, and E2B).
Figure 1.
Inhibition of fibroblast proliferation by spiruchostatin A (SpA). Idiopathic pulmonary fibrosis (IPF) fibroblasts were cultured for up to 144 hours in DMEM/FBS and SpA. (A) Time-course data for fibroblasts from a single IPF donor, assessing the antiproliferative effect of 10 nM SpA in the absence (solid bar) or presence (open bar) of transforming growth factor (TGF)–β1 compared with vehicle control alone (grey bar) or vehicle in the presence of TGF-β1 (hatched bar). (B) Growth inhibition plots for cells from the same donor, grown in the absence (solid circles) or presence (open squares) of TGF-β1 for 144 hours and treated with a range of SpA concentrations. The cell number was expressed as a percentage of the vehicle control at 144 hours, after subtraction of the cell number at t = 0. (C) The concentrations required to achieve 50% inhibition (IC50 values) for the antiproliferative effects of SpA, determined from dose–response curves for fibroblasts from three IPF donors and three normal control subjects (as shown in Figure E2). (D) The persistence of the effect of SpA (48 hours of exposure) on fibroblast proliferation in the absence (solid circles) or presence (open squares) of TGF-β1, measured 96 hours after the removal of SpA. For comparison, fibroblasts were continuously exposed to SpA in the absence (solid squares) or presence (open circles) of TGF-β1. Data are expressed as in B. Data represent means ± SD (n = 3 individual experiments). *P < 0.05, versus corresponding control sample. **P < 0.01, versus corresponding control sample (i.e., vehicle without or with TGF-β1).
Whereas SpA was observed to inhibit proliferation, little cytotoxicity was evident either morphologically (Figure E3) or by measurement of lactate dehydrogenase (LDH) release, which showed only an approximate doubling of basal LDH release even at 100 or 1,000 nM SpA (Figure E4). To assess the longevity of the inhibitory effect of SpA on IPF fibroblast proliferation, the inhibitor was removed after 48 hours of treatment and replaced with DMEM/FBS in the absence or presence of TGF-β1. Under these conditions, a significant dose-dependent inhibition of proliferation (ANOVA, P < 0.0001) was still evident after 96 hours (Figure 1D), suggesting a prolonged and potent antiproliferative effect. Under these conditions, the IC50 values were still in the nanomolar range (4.7 and 7.3 nM in the presence or absence of TGF-β1, respectively).
SpA Induces Histone H3 Acetylation
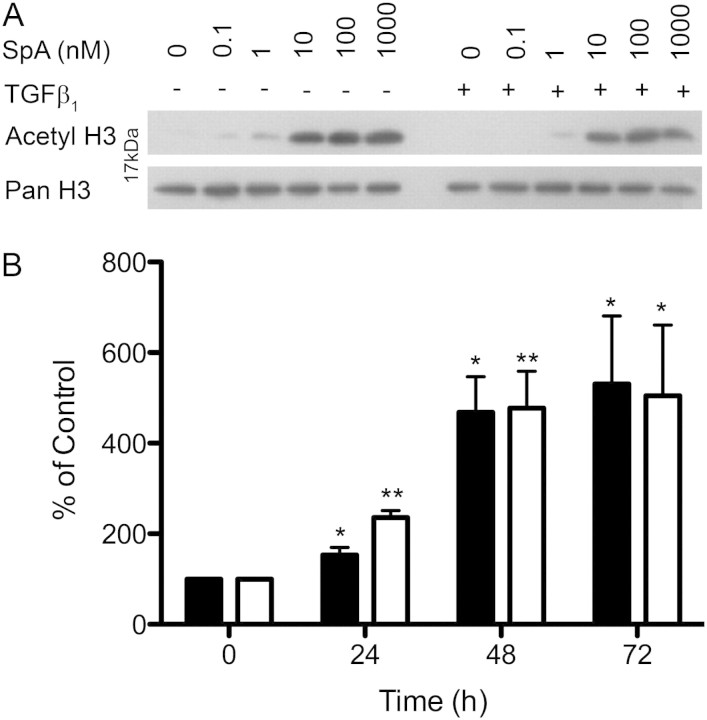
Because SpA inhibits histone deacetylase activity, we examined the effects of SpA on histone H3 acetylation. Previously, Crabb and colleagues (18) showed that SpA induced histone acetylation for up to 72 hours, compared with other HDIs such as TSA, where acetylation was limited to 24 hours. Therefore, cells were pretreated with or without TGF-β1 in DMEM/FBS for 24 hours, before treatment with SpA ± TGF-β1 for 24, 48, or 72 hours. Protein lysates were then extracted, and histone H3 acetylation was examined via Western blotting. This revealed that SpA caused a dose-dependent increase in histone H3 acetylation, which was evident at 1 nM SpA, and was markedly increased at greater than 10 nM SpA (Figure 2A). Using 10 nM SpA, the increase in histone H3 acetylation was detectable at 24 hours. It peaked at 48 hours, and remained elevated at 72 hours (Figure 2B) (ANOVA, P < 0.0003). Thus, the data suggest that the inhibitory effect of SpA is linked to its ability to inhibit histone deacetylation.
Figure 2.
SpA increases histone H3 acetylation in primary fibroblasts. IPF fibroblasts were cultured in DMEM/FBS ± TGF-β1, with the indicated concentrations of SpA at 24, 48, or 72 hours before harvesting. Cells were analyzed by Western blotting, using a primary antibody against acetyl histone H3, with a pan histone H3 antibody as loading control. (A) Western blots obtained after 48 hours of treatment with different doses of SpA. (B) Semiquantitative analysis of the time-course analysis of histone acetylation in the presence of 10 nM SpA and in the absence (solid bar) or presence (open bar) of TGF-β1, determined using densitometry. The data represent means ± SD (n = 3 separate experiments). *P < 0.05, versus corresponding control sample. **P < 0.01, versus corresponding control sample, denoting an increase in histone acetylation.
SpA Induces the Expression of the Cell-Cycle Regulator p21waf1
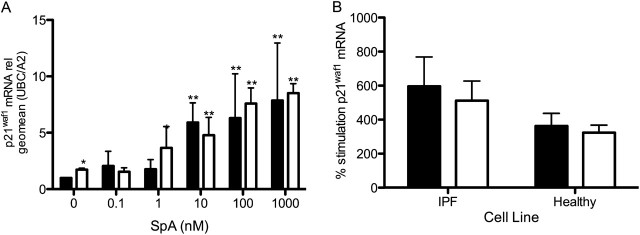
Other HDIs are known to induce the expression of cell-cycle regulators to inhibit the proliferation of cancer cells (15, 18). To determine whether the antiproliferative mechanism was similar in IPF fibroblasts, we investigated p21waf1 mRNA concentrations. As shown in Figure 3A, SpA caused a significant dose-dependent increase (ANOVA, P < 0.0001) in p21waf1 mRNA expression in fibroblasts at 48 hours, the time point beyond which an inhibition of proliferation was evident. In cells treated with SpA alone, a sixfold increase in p21waf1 mRNA was seen at 10 nM SpA, and at 1,000 nM, an eightfold increase was evident. After treatment with TGF-β1, the expression of p21waf1 was significantly increased in the absence of SpA (P < 0.05), and this almost doubled at 1 nM SpA, reaching its maximum at 1,000 nM (Figure 3A). Comparing the effects of 10 nM SpA on fibroblasts from three IPF donors and three normal control subjects revealed that SpA showed a trend toward greater induction of p21waf in IPF fibroblasts (Figure 3B), but this trend failed to reach statistical significance. These data suggest that the suppression of cell proliferation by SpA is attributable to its ability to induce the expression of cell-cycle inhibitory proteins.
Figure 3.
SpA increases the fibroblast expression of the cell-cycle regulator p21waf1. RNA was isolated from IPF (A and B) or normal (B) fibroblasts treated with the indicated concentrations of SpA (A), or with 10 nM SpA (B), without TGF-β1 (solid bars) or with TGF-β1 (open bars) for 48 hours. cDNA was synthesized for quantitative PCR analysis. p21waf1 mRNA expression was calculated relative to the geometric mean of the housekeeping genes UBC/A2, using the ΔΔCT method. The data in A are expressed as fold stimulation relative to the untreated control sample (minus TGF-β1), and represent means ± SD (n = 3 separate experiments), using one IPF fibroblast cell line. The data in B were calculated as percent stimulation by SpA, compared with expression in the absence or presence of TGF-β alone, and are shown as means ± SD from three IPF and three normal fibroblast lines (n = 3 per subject). *P < 0.05, versus corresponding control sample. **P < 0.01, versus corresponding control sample (i.e., vehicle with or without TGF-β).
SpA Inhibits Myofibroblast Differentiation Induced by TGF-β1
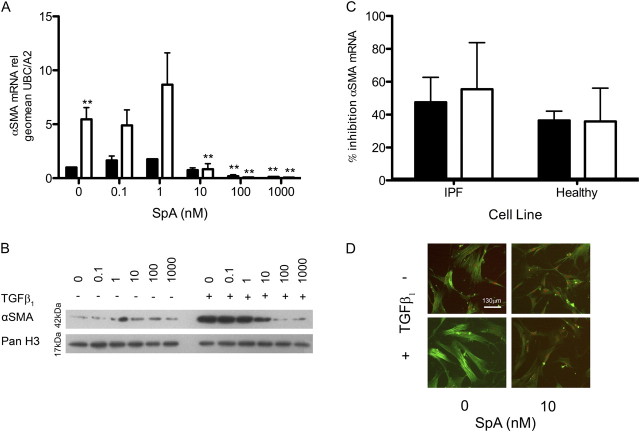
In fibrotic diseases, the ability of TGF-β1 to induce myofibroblast differentiation is deemed a key event in fibrogenesis. Therefore, we also examined the effects of SpA on the basal and TGF-β1–induced expression of α-SMA, a marker of myofibroblast differentiation. As expected, TGF-β1 caused a fivefold increase in α-SMA mRNA expression at 48 hours (Figure 4A), and this was paralleled by an increase in α-SMA protein expression at 72 hours after treatment (Figure 4B). When the cells were cotreated with TGF-β1 and SpA, the induction of α-SMA mRNA expression was dose-dependently inhibited by SpA (ANOVA, P < 0.0001), with the suppression of α-SMA gene expression evident at 10 nM SpA. The expression of the α-SMA gene was completely abolished between 100 and 1,000 nM SpA (Figure 4A). SpA also dose-dependently inhibited induction of α-SMA protein expression by TGF-β1 (Figure 4B). Comparing the effects of 10 nM SpA on fibroblasts from three IPF donors and three normal control subjects confirmed that SpA inhibited αSMA mRNA expression both at baseline and in the presence of TGF-β, with no significant difference between groups (Figure 4C). We further assessed the assembly of contractile actin filaments according to immunofluorescent staining. Figure 4D shows that TGF-β1 induced myofibroblast differentiation, as demonstrated by the formation of prominent actin filaments, and this differentiation was blocked by SpA.
Figure 4.
TGF-β1 induces α–smooth muscle actin (α-SMA) mRNA and protein expression, which can be inhibited by SpA. IPF (A–D) or normal (C) fibroblasts were cultured without (solid bars) or with (open bars) TGF-β1, and with the indicated concentrations of SpA (A and B) or 10 nM SpA (C) before harvesting for RNA or protein extraction at the appropriate time point. (A and C) α-SMA mRNA expression was analyzed by quantitative RT-PCR and calculated relative to the geometric mean of the housekeeping genes UBC/A2, using the ΔΔCT method. The data in A are expressed as fold stimulation relative to the untreated control sample, and represent means ± SD (n = 3 separate experiments), using one IPF fibroblast cell line. The data in C were calculated as percent inhibition of gene expression by SpA compared with that seen in the absence or presence of TGF-β1 alone, and are shown as means ± SD from three IPF and three normal fibroblast lines (n = 3 per subject). (B) α-SMA protein expression at 72 hours was analyzed by Western blotting, with histone H3 as a loading control. (D) Immunofluorescent staining of α-SMA in fibroblasts, treated in the absence or presence of TGF-β1 ± SpA (10 nM) for 48 hours, as indicated. Nuclei were counterstained with 7-amino actinomycin D (red). *P < 0.05, versus corresponding control sample. **P < 0.01, versus corresponding control sample.
SpA Diminishes the Expression of Interstitial Collagens
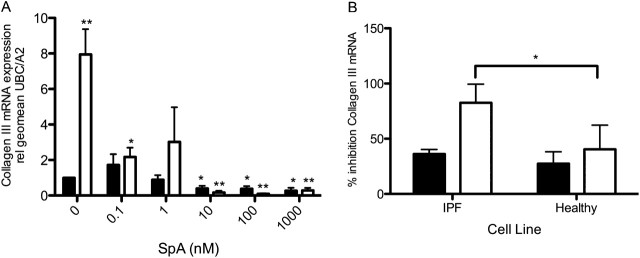
To confirm the inhibitory effect of SpA on myofibroblast activity, quantitative RT-PCR was used to investigate the mRNA expression of interstitial collagens I and III by (myo)fibroblast monolayers. This investigation revealed that SpA dose-dependently blocked the TGF-β1–induced expression of collagen I (ANOVA, P < 0.02) and collagen III (ANOVA, P < 0.0001) in fibroblasts at 48 hours (Figures E5A and 5A, respectively). The most marked inhibition was of collagen III mRNA expression: TGF-β1 caused an 8-fold induction of collagen III expression, which was reduced to below baseline levels by SpA. The induction of collagen I by TGF-β1 was also inhibited by SpA. However, in contrast with collagen III, no effect on basal collagen I mRNA expression was evident. Comparing the effects of 10 nM SpA on fibroblasts from three IPF donors and three normal control subjects revealed that SpA was significantly more potent in reducing TGF-β1–induced collagen III mRNA expression in IPF fibroblasts (Figure 5B), although its effects on collagen I gene expression were similar (Figure E5B).
Figure 5.
Effects of SpA on interstitial collagen expression. RNA was isolated from IPF fibroblasts treated without (solid bars) or with (open bars) TGF-β1, and with the indicated doses of SpA (A) or 10 nM SpA (B) for 48 hours. Collagen III mRNA expression was measured by quantitative RT-PCR and calculated relative to the housekeeping genes UBC/A2, using the ΔΔCT method. Data in A are expressed as fold stimulation relative to the untreated control sample, and represent means ± SD (n = 3 separate experiments), using one IPF fibroblast cell line. Data in B were calculated as percent inhibition of gene expression by SpA compared with that seen in the absence or presence of TGF-β1 alone, and are shown as means ± SD from three IPF and three normal fibroblast lines (n = 3 per subject). *P < 0.05, versus corresponding control sample. **P < 0.01, versus corresponding control sample.
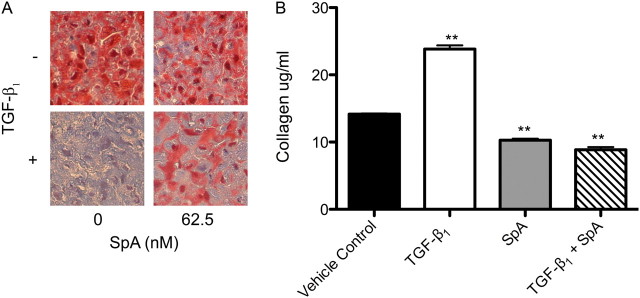
Because fibroblast cultures grown in two dimensions do not take into account the dense fibrotic regions of IPF lung, we modeled fibroblastic foci using three-dimensional micromass pellet cultures, similar to those used for mesenchymal stem cells (24). This tissue engineering strategy involves the trypsinization of monolayer cultures and centrifugation to form a pellet that can be cultured in situ under a variety of conditions, and then fixed for analysis by histochemistry. Under these conditions, an increase in fibroblast number occurred over a 7-day period of culture in DMEM/FBS ± TGF-β1. After 7 days, the pellets were fixed and paraffin-embedded for histochemical analysis, using Masson trichrome stain, which stains collagen blue due to the binding of aniline blue. Figure 6A demonstrates that when treated with TGF-β1, collagen deposition is dramatically increased within the pellets, compared with the medium-only control. This increase was significantly reduced when the cultures were cotreated with SpA. To assess further the profibrogenic activity in the cultures, soluble collagen was measured using the Sircol assay. Consistent with the responses seen during histochemical analysis of the pellets, the TGF-β1–treated pellets released significantly more soluble collagen than the control samples, and this was significantly (P < 0.01) suppressed by SpA (Figure 6B).
Figure 6.
SpA diminishes TGF-β1–induced collagen production. Three-dimensional pellet cultures were treated without or with TGF-β1 and 62.5 nM SpA before fixing, paraffin-embedding, and trichrome staining for collagen expression. (A) Histochemical analysis of pellets cultured under indicated conditions, where the binding of aniline blue in Masson trichrome stain denotes the presence of collagen (magnification, ×40). (B) Release of soluble collagen by cultured fibroblasts under the conditions indicated was assessed at 72 hours, using Sirius red dye. Data represent means ± SD (n = 3 individual experiments). **P < 0.01, versus corresponding control sample.
Discussion
Pulmonary fibrosis is characterized by an increase in fibroblast proliferation and the deposition of ECM proteins by myofibroblasts under the control of profibrogenic stimuli such as TGF-β1. As excess ECM accumulates, healthy tissue is replaced by scar tissue that disrupts the delicate alveolar architecture, leading to a severe disruption of gas exchange, ultimately leading to respiratory failure. We have shown that SpA, with its distinct activity profile toward Class I HDACs, exerts multiple biological effects on primary fibroblasts derived from patients with IPF. It potently inhibits the proliferation of fibroblasts in association with increased expression of the cell-cycle inhibitor, p21waf1, and reduces the profibrotic response driven by TGF-β1, as demonstrated by diminished α-SMA and interstitial collagen expression. Its effects were paralleled by a long-lived increase in the acetylation of histone H3, suggesting that its mode of action, at least in part, involves effects on chromatin structure. These effects are consistent with characterization studies by Crabb and colleagues (18), who reported that SpA inhibited the proliferation of breast cancer cells with an IC50 of 6 nM.
SpA is a cyclic, cysteine-containing depsipeptide natural product that was originally isolated from Pseudomonas sp. in a search for anticancer compounds that could mimic the antiproliferative effects of TGF-β1 by the induction of genes that caused cell-cycle arrest (25). This search was based on the rationale that although the TGF-β1 treatment of some cancer cells can result in an increased expression of p21waf1, a known cyclin-dependent kinase inhibitor (26, 27), other cancers escape this growth inhibition by down-regulating TGF-β receptors. Masuoka and colleagues (25) hypothesized that substances with the ability to induce the expression of growth-inhibitory genes independent of TGF-β1 would be potential chemotherapeutic agents. Consistent with this hypothesis, SpA was shown to inhibit the proliferation of several types of cancer cells by an induction of cell-cycle regulators (15, 18). The results of the present study show that although SpA also induces the expression of p21waf1 in pulmonary fibroblasts to cause similar antiproliferative effects, it does not simply induce all TGF-β–regulated genes, insofar as the other effects of TGF-β, such as myofibroblast differentiation and collagen gene expression, were suppressed by SpA. This finding probably reflects the complexity of TGF-β signaling that lies upstream of the epigenetic histone regulatory steps, and the involvement of distinct HDACs to control the pattern of gene expression. In addition, HDACs can influence the degree of acetylation of nonhistone proteins such as transcription factors, thus modulating their activity with consequent effects on cellular phenotype. For example, the transcription factor SP1 is known to be associated with Class I HDACs (28), and recent studies have shown that its acetylation is associated with an up-regulation of p21waf in colonic epithelial cells (29). One of the factors linked to the uncontrolled proliferation of IPF fibroblasts involves reduced levels of the antifibrotic lipid mediator prostaglandin E2 as a consequence of diminished cyclooxygenase-2 (COX-2) expression (30). Recent work suggests that histone hypoacetylation around the COX-2 promoter is responsible for low COX-2 expression in IPF fibroblasts, and that this low expression can be reversed by treatment with HDIs (31). Although the effect of SpA on fibroblast proliferation may be attributable to the induction of COX-2, in previous studies, the induction of COX-2 by HDIs was only observed when IPF fibroblasts were also treated with exogenous IL-1β or TGF-β. This contrasts with our work, which showed that SpA induced p21waf1 expression in the absence of exogenously added active TGF-β, suggesting that the antiproliferative effect of SpA is unlikely to be a consequence of increased COX-2 expression. However, because serum contains latent TGF-β, it would be of interest to further explore the effects of SpA on COX-2 expression under the conditions of our experiments, and the relationship of SpA to p21waf1 expression.
SpA is potent inhibitor of Class I HDACs (21). The effects of SpA on histone acetylation are slower but more prolonged compared with the hydroxamic acid–based inhibitors, but this does not compromise efficacy in terms of cell-cycle arrest or the inhibition of cell proliferation (18). Washout experiments also indicate that histone acetylation persists after the removal of drug, which may by important in governing the potency (or toxicity) of such compounds clinically. Consistent with this result, we found a relatively slow onset of histone acetylation, but histone acetylation persisted over several days, and the antiproliferative effects of SpA were still evident after 1 week, even after removal of the inhibitor. Previous studies looked at the effects of two broad-spectrum Class I and II HDIs, TSA and SAHA, on fibroblast proliferation. Guo and colleagues (32) examined the effect of TSA on normal human dermal fibroblasts. They found that TSA alone blocked the basal transcription of collagen I and α-SMA, and it also markedly suppressed induction of these genes by TGF-β1. However, the concentration of TSA necessary to produce these inhibitory effects was 200 nM, which is 20–50 times greater than the IC50 of SpA. Wang and colleagues (17) demonstrated that SAHA had both antifibrotic and anti-inflammatory properties in normal and IPF lung fibroblasts, where it abrogated TGF-β1–induced effects, including differentiation into myofibroblasts expressing α-SMA and collagen I. As with TSA, the concentration of SAHA used (5 μM) was very high (i.e., 1,000 times higher than the dose of SpA required to produce similar responses). Of interest, we found that SpA exhibited some selectivity toward IPF fibroblasts, in terms of its antiproliferative effect and suppression of collagen III expression. However, it was overall a potent inhibitor of both IPF and normal fibroblast responses. The results of the present study and two previous studies demonstrate that HDI can successfully inhibit the profibrotic effects of TGF-β1 on fibroblasts. In addition, the antiproliferative effect of SpA is consistent with the effect of SAHA (17). Thus, in both cases, this appears to occur without inducing apoptosis or cytotoxicity, and we have extended these studies by showing the induction of p21waf1 as the likely growth-inhibitory mechanism. The dose of SpA required to induce these responses is significantly lower than that of either TSA or SAHA, and the duration of action is markedly longer.
The “classic” HDACs (Classes I and II) are zinc metalloenzymes that catalyze the hydrolysis of acetylated lysine residues and are characteristically inhibited by TSA. HDACs 1, 2, 3, and 8 belong to Class I, which contain a nuclear localization signal and exert their function in the nucleus. HDACs 4, 5, 6, and 7 are members of Class II, and shuttle between the nucleus and the cytoplasm. Several studies have implicated Class II HDACs in the control of myofibroblast and smooth muscle function. In adult cardiac myocytes, Class II HDACs appear to play a key role in the suppression of hypertrophy by binding to and suppressing the activity of the transcription factor, myocyte enhancer factor 2 (33). In contrast, Class I HDACs are considered to play a prohypertrophic role, although their relevant gene targets are less well described (34). These studies suggest that Class I and II HDACs play opposing roles in the control of hypertrophy, highlighting the potential benefit of Class I HDAC-specific inhibitors as therapeutic agents. In studies of dermal myofibroblast differentiation, the silencing of HDAC8, HDAC4, or HDAC6 impaired TGF-β1–induced α-SMA expression. Although the effect of HDAC8 silencing was not investigated further, the down-regulation of HDAC4 was shown to suppress TGF-β signaling, suggesting that HDAC4 is an essential epigenetic regulator of myofibroblast differentiation. Because HDAC4 is a member of Class II, this observation appears to be inconsistent with our finding that SpA is a potent inhibitor of myofibroblast differentiation. However, HDAC4 has been shown to be enzymatically inactive when it is not associated with Class I HDAC3 (35), which is a target for SpA.
In many cancers, an overexpression of different HDACs has been reported (36). For example, HDAC1 is overexpressed in prostate cancer cells (37), and HDAC2 is commonly overexpressed in colorectal and gastric carcinomas (38). HDIs such as SAHA (e.g., vorinostat and zolinza) and FK228 (e.g., romidepsin, a depsipeptide HDI structurally similar to SpA) are already clinically approved as treatments for cutaneous T-cell lymphoma and other cancers. The key features of their clinical significance include their selective cytotoxicity against tumor cells, while being relatively innocuous to normal cells, and their effective activation of several molecular pathways to mediate their antitumor effects (39). The data generated in the present study support the potential for HDIs as novel treatments for IPF. Like cancer, IPF is characterized by uncontrolled cellular proliferation, and IPF involves a prognosis as poor as that for many forms of lung cancer. The potency of SpA toward fibrotic fibroblasts and its improved selectivity and duration of action compared with SAHA suggest that it (or similar compounds such as romidepsin) may have therapeutic potential. Although we found that SpA also affected the proliferation of normal lung fibroblasts, its effects appeared to be cytostatic rather than cytotoxic, suggesting that quiescent fibroblasts in areas of normal lung would be relatively unaffected by it. The ability of SpA to reduce the uncontrolled proliferation of IPF fibroblasts and prevent their biosynthetic activity leads us to conclude that SpA or similar compounds should be further investigated for their potential use as a novel class of therapeutic agents for IPF.
Acknowledgments
The authors thank Sandy Pink, Malcolm North, and the nursing staff from the NIHR Respiratory Biomedical Research Unit at Southampton General Hospital for their assistance, and the Histochemistry Research Unit at the University of Southampton for assistance with the processing and tinctorial staining of three-dimensional fibroblast cultures.
Footnotes
This work was funded by the National Institute for Health Research (NIHR) Respiratory Biomedical Research Unit at Southampton General Hospital, by the University of Southampton, and by National Institutes of Health grant HL075432 (P.J.S. and T.H.T.). H.M.H. is supported by an MRC Clinician Scientist Fellowship.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0040OC on January 12, 2012
References
- 1.Valeyre D, Freynet O, Dion G, Bouvry D, Annesi-Maesano I, Nunes H. Epidemiology of interstitial lung diseases. Presse Med 2010;39:53–59. [DOI] [PubMed] [Google Scholar]
- 2.Meltzer EB, Noble PW. Idiopathic pulmonary fibrosis. Orphanet J Rare Dis 2008;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2002;165:277–304. [DOI] [PubMed] [Google Scholar]
- 4.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2006;174:810–816. [DOI] [PubMed] [Google Scholar]
- 5.King TE, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JA, Flint A, Thurlbeck W, Cherniack RM. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med 2001;164:1025–1032. [DOI] [PubMed] [Google Scholar]
- 6.Coward WR, Saini G, Jenkins G. The pathogenesis of idiopathic pulmonary fibrosis. Ther Adv Respir Dis. 2010;4:367–388. [DOI] [PubMed] [Google Scholar]
- 7.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001;134:136–151. [DOI] [PubMed] [Google Scholar]
- 8.Sime PJ, O’Reilly KM. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin Immunol 2001;99:308–219. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson AG, Fulford LG, Colby TV, du Bois RM, Hansell DM, Wells AU. The relationship between individual histologic features and disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2002;166:173–177. [DOI] [PubMed] [Google Scholar]
- 10.Golec M, Lambers C, Hofbauer E, Geleff S, Bankier A, Czerny M, Ziesche R. Assessment of gene transcription demonstrates connection with the clinical course of idiopathic interstitial pneumonia. Respiration 2008;76:261–269. [DOI] [PubMed] [Google Scholar]
- 11.Ramos C, Montano M, Garcia-Alvarez J, Ruiz V, Uhal BD, Selman M, Pardo A. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol 2001;24:591–598. [DOI] [PubMed] [Google Scholar]
- 12.Zheng YG, Wu J, Chen Z, Goodman M. Chemical regulation of epigenetic modifications: opportunities for new cancer therapy. Med Res Rev 2008;28:645–687. [DOI] [PubMed] [Google Scholar]
- 13.de Ruijter AJM, van Gennip AH, Caron HN, Kemp S, van Kuilenburg ABP. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 2003;370:737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emanuele S, Lauricella M, Tesoriere G. Histone deacetylase inhibitors: apoptotic effects and clinical implications. Int J Oncol 2008;33:637–646. [PubMed] [Google Scholar]
- 15.Johnstone RW. Histone–deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov 2002;1:287–299. [DOI] [PubMed] [Google Scholar]
- 16.Marks PA, Xu WS. Histone deacetylase inhibitors: potential in cancer therapy. J Cell Biochem 2009;107:600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Chen C, Finger SN, Kwajah S, Jung M, Schwarz H, Swanson N, Lareu FF, Raghunath M. Suberoylanilide hydroxamic acid: a potential epigenetic therapeutic agent for lung fibrosis? Eur Respir J 2009;34:145–155. [DOI] [PubMed] [Google Scholar]
- 18.Crabb SJ, Howell M, Rogers H, Ishfaq M, Yurek-George A, Carey K, Pickering BM, East P, Mitter R, Maeda S, et al. Characterisation of the in vitro activity of the depsipeptide histone deacetylase inhibitor spiruchostatin A. Biochem Pharmacol 2008;76:463–475. [DOI] [PubMed] [Google Scholar]
- 19.Khan N, Jeffers M, Kumar S, Hackett C, Boldog F, Khramtsov N, Qian XZ, Mills E, Berghs SC, Carey N, et al. Determination of the class and isoform selectivity of small-molecule histone deacetylase inhibitors. Biochem J 2008;409:581–589. [DOI] [PubMed] [Google Scholar]
- 20.Davies ER, Haitchi HM, Ganesan A, Packham G, O'Reilly KMA, Davies DE, editors. Spiruchostatin A inhibits proliferation and differentiation of primary fibroblasts from patients with interstitial lung disease [abstract]. Am J Respir Crit Care Med 2010;181:A2994.
- 21.Yurek-George A, Habens F, Brimmell M, Packham G, Ganesan A. Total synthesis of spiruchostatin A, a potent histone deacetylase inhibitor. J Am Chem Soc 2004;126:1030–1031. [DOI] [PubMed] [Google Scholar]
- 22.Oliver MH, Harrison NK, Bishop JE, Cole PJ, Laurent GJ. A rapid and convenient assay for counting cells cultured in microwell plates: application for assessment of growth factors. J Cell Sci 1989;92:513–518. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 24.Tare RS, Howard D, Pound JC, Roach HI, Oreffo RO. Tissue engineering strategies for cartilage generation: micromass and three dimensional cultures using human chondrocytes and a continuous cell line. Biochem Biophys Res Commun 2005;333:609–621. [DOI] [PubMed] [Google Scholar]
- 25.Masuoka YAN, Shin-ya K, Furihata K, Nagai K, Suzuki K, Hayakawa Y, Seto H. Spiruchostatins A and B, novel gene expression-enhancing substances produced by Pseudomonas sp. Tetrahedron Letter 2001;42:41–44. [Google Scholar]
- 26.Li CY, Suardet L, Little JB. Potential role of WAF1/Cip1/p21 as a mediator of TGF-beta cytoinhibitory effect. J Biol Chem 1995;270:4971–4974. [DOI] [PubMed] [Google Scholar]
- 27.Yoo YD, Choi JY, Lee SJ, Kim JS, Min BR, Lee YI, Kang YK. TGF-beta–induced cell-cycle arrest through the p21(WAF1/CIP1)-G1 cyclin/Cdks-p130 pathway in gastric-carcinoma cells. Int J Cancer 1999;83:512–517. [DOI] [PubMed] [Google Scholar]
- 28.Doetzlhofer A, Rotheneder H, Lagger G, Koranda M, Kurtev V, Brosch G, Wintersberger E, Seiser C. Histone deacetylase 1 can repress transcription by binding to Sp1. Mol Cell Biol 1999;19:5504–5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waby JS, Chirakkal H, Yu C, Griffiths GJ, Benson RS, Bingle CD, Corfe BM. Sp1 acetylation is associated with loss of DNA binding at promoters associated with cell cycle arrest and cell death in a colon cell line. Mol Cancer 2010;9:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilborn J, Crofford LJ, Burdick MD, Kunkel SL, Strieter RM, Peters-Golden M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J Clin Invest 1995;95:1861–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coward WR, Watts K, Feghali-Bostwick CA, Knox A, Pang L. Defective histone acetylation is responsible for the diminished expression of cyclooxygenase 2 in idiopathic pulmonary fibrosis. Mol Cell Biol 2009;29:4325–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo W, Shan B, Klingsberg RC, Qin X, Lasky JA. Abrogation of TGF-beta1–induced fibroblast–myofibroblast differentiation by histone deacetylase inhibition. Am J Physiol Lung Cell Mol Physiol 2009;297:L864–L870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, McKinsey TA. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol 2004;24:8374–8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKinsey TA, Olson EN. Cardiac histone acetylation: therapeutic opportunities abound. Trends Genet 2004;20:206–213. [DOI] [PubMed] [Google Scholar]
- 35.Fischle W, Dequiedt F, Hendzel MJ, Guenther MG, Lazar MA, Voelter W, Verdin E. Enzymatic activity associated with Class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol Cell 2002;9:45–57. [DOI] [PubMed] [Google Scholar]
- 36.Abbas A, Gupta S. The role of histone deacetylases in prostate cancer. Epigenetics 2008;3:300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halkidou K, Gaughan L, Cook S, Leung HY, Neal DE, Robson CN. Upregulation and nuclear recruitment of HDAC1 in hormone refractory prostate cancer. Prostate 2004;59:177–189. [DOI] [PubMed] [Google Scholar]
- 38.Song J, Noh JH, Lee JH, Eun JW, Ahn YM, Kim SY, Lee SH, Park WS, Yoo NJ, Lee JY, et al. Increased expression of histone deacetylase 2 is found in human gastric cancer. Acta Path Microbiol Immunol Scand 2005;113:264–118. [DOI] [PubMed] [Google Scholar]
- 39.Mottet D, Castronovo V. Histone deacetylases: target enzymes for cancer therapy. Clin Exp Metastasis 2008;25:183–189. [DOI] [PubMed] [Google Scholar]