Abstract
Acute canine polyradiculoneuritis (ACP) is considered to be the canine equivalent of the human peripheral nerve disorder Guillain-Barré syndrome (GBS); an aetiological relationship, however, remains to be demonstrated. In GBS, anti-glycolipid antibodies (Abs) are considered as important disease mediators. To address the possibility of common Ab biomarkers, the sera of 25 ACP dogs, 19 non-neurological, and 15 epileptic control dogs were screened for IgG Abs to 10 glycolipids and their 1 : 1 heteromeric complexes using combinatorial glycoarrays. Anti-GM2 ganglioside Abs were detected in 14/25 ACP dogs, and anti-GA1 Abs in one further dog. All controls except for one were negative for anti-glycolipid Abs. In this cohort of cases and controls, the glycoarray screen reached a diagnostic sensitivity of 60% and a specificity of 97%; a lower sensitivity (32%) was reported using a conventional glycolipid ELISA. To address the possible pathogenic role for anti-GM2 Abs in ACP, we identified GM2 in canine sciatic nerve by both mass spectrometry and thin layer chromatography overlay. In immunohistological studies, GM2 was localized predominantly to the abaxonal Schwann cell membrane. The presence of anti-GM2 Abs in ACP suggests that it may share a similar pathophysiology with GBS, for which it could thus be considered a naturally occurring animal model.
Keywords: antibody, autoimmune neuropathy, dog, ganglioside, Guillain-Barreé syndrome
Introduction
Historically, acute canine polyradiculoneuritis (ACP) has been likened to the human peripheral nerve disorder Guillain-Barré syndrome (GBS) (Cummings and Haas, 1967; 1972; Northington and Brown, 1982; Cuddon, 2002; Olby, 2004). Like GBS, ACP is an immune-mediated disorder affecting peripheral myelin, axons, or both (Cummings and Haas, 1967; Cummings et al., 1982; Northington and Brown, 1982; de Lahunta and Glass, 2009). Canine patients present with an acute onset of lower motor neuron paraparesis, which usually progresses rapidly to tetraparesis or tetraplegia and may be accompanied by cranial nerve (CN) involvement, evident by dysphonia and facial paresis (Cummings et al., 1982; Cuddon, 2002). There is no clear manifestation of sensory loss; hyperesthesia, however, may be seen (Cuddon, 2002; Olby, 2004). Historically, this disorder was first described in dogs used to hunt raccoons, hence the original nomenclature of ‘‘coonhound paralysis’’ (Cummings and Haas, 1967). Following the observation of ACP in dogs not exposed to raccoons (Vandevelde et al., 1981; Northington and Brown, 1982), the disease was preliminarily sub-classified into coonhound paralysis, idiopathic polyradiculoneuritis, and post-vaccination polyradiculoneuritis (Olby, 2004).
Anti-ganglioside antibodies (Abs) are observed in GBS patients (Yuki and Hartung, 2012) and are considered as important mediators of the disorder (Willison and Yuki, 2002; Willison, 2005). Gangliosides are glycosphingolipids found on plasma membranes throughout the body, but in a higher concentration in neural tissues (Hamberger and Svennerholm, 1971). Once anti-ganglioside Abs have bound their target, one mechanism by which they induce injury is through the activation of the complement cascade, which culminates in the formation of a membrane attack complex on the structures bound (Halstead et al., 2005). The membrane attack complex pore allows an uncontrolled flux of ions and water along the diffusion gradient, resulting in pathological changes and dysfunction of the structures targeted (O’Hanlon et al., 2001; Halstead et al., 2004; McGonigal et al., 2010; Rupp et al., 2012).
Depending on the screening parameters and GBS subtypes under investigation, the prevalence of the wide spectrum of anti-glycolipid Abs found in GBS sera ranges from 38% to 100% (Chiba et al., 1992; Alaedini et al., 2002; Caudie et al., 2002; Mata et al., 2006; Yu et al., 2006; Meena et al., 2010). The Abs are predominantly of immunoglobulin (Ig) G subclass (Caudie et al., 2002; Mata et al., 2006; Kaida et al., 2007; Meena et al., 2010), with IgG1 and IgG3 dominating (Willison and Yuki, 2002).
In this study, the sera of dogs diagnosed with ACP based on history and clinical, neurological, and electrophysiological features were screened for anti-ganglioside IgG Abs using glycoarrays and conventional immunoassays. Geographically matched and unmatched neurological and non-neurological control canine patients were also investigated. Clinical data was used to search for epidemiological or clinical differences between those dogs exhibiting anti-ganglioside Abs and those without Abs.
Materials and Methods
Patients
Between October 2008 and October 2011, 28 dogs were diagnosed with ACP at the Veterinary Clinic of the University of Parma, Italy. All dogs were examined by two of the authors (E. B. and M. D.), and the diagnosis was reached by evaluation of history, clinical and neurological examinations, supplemented by electrophysiological investigations under general anaesthesia using conventional electrodiagnostic equipment (Myoquick, Micromed S.p.A., Treviso, Italy). Briefly, electromyography recordings were obtained from the appendicular, epaxial, and head muscles, whereas for motor and sensory nerve conduction studies either the tibial and/or ulnar nerve were stimulated.
Most dogs also underwent auxiliary investigations to rule out other causes of muscle weakness and ataxia. These included MRIs of the spinal cord (n = 8), thyroid profiles (n = 9), and screening for Toxoplasma gondii (n = 14), Neospora caninum (n = 13), Ehrlichia canis (n = 5), Leishmania infantum (n = 6), Rickettsia (n = 4), Borrelia burgdorferi (n = 3), and anti-acetylcholine receptor Abs (n = 5). In four dogs, muscle biopsies were examined too.
Sera of all patients were stored at −80°C immediately after collection.
Controls
The sera of 19 local age-, sex-, and breed-matched non-neurological control dogs as determined by clinical and neurological examinations were collected in May and June 2011 at the Teaching Hospital of the Veterinary Faculty, University of Parma, Italy. Sera were stored at −80°C immediately after collection.
Additionally, the sera of 15 dogs diagnosed with idiopathic epilepsy at the Small Animal Hospital of the School of Veterinary Medicine in Glasgow, Scotland were used as a control for other neurologic diseases (OND). These samples were obtained between June 2006 and May 2011 and stored at −80°C until required.
Anti-ganglioside IgG antibody glycoarrays and ELISAs
All serum samples were stored in the required aliquots for each test at −20°C and each sample was screened in triplicate by glycoarray as described previously (Rinaldi et al., 2009; Galban-Horcajo et al., 2013) (see also Appendix S1, Supporting Information). Briefly, polyvinyl difluoride membranes were spotted with glycolipids and incubated with dog serum and appropriate secondary Ab before development using a chemiluminescent reaction and autoradiography. Spot intensities were calculated using TotalLab image analysis software (Nonlinear Dynamics Ltd, Newcastle upon Tyne, UK) and expressed as arbitrary units of intensity (AIU).
Additionally, ACP and local non-neurological control sera were screened in triplicate by ELISA using the methodology of the Glasgow Diagnostic Neuroimmunology Laboratory (Willison et al., 1999). Mean optical densities (ODs) larger than 0.1 were considered positive.
Liquid chromatography tandem mass spectrometry and thin layer chromatography overlay of canine peripheral nerve extract
Folch upper phase extracts of canine peripheral nerves and nerve roots dissected from a neurologically healthy, 8-year-old male German Shepherd dog, and murine sciatic nerve were prepared by standard methods (Folch et al., 1957) (see Appendix S1). Commercial ganglioside extract (Avanti Polar Lipids, Alabaster, AL, USA) prepared at 0.5 mg/ml in methanol served as control.
Liquid chromatography tandem mass spectrometry (LCMS) was carried out in triplicate for each tissue sample on a reverse phase Acclaim C30 column (Dionex-Thermo Scientific, Sunnyvale, CA, USA) operated by an Ultimate 3000 HPLC system according to standard techniques (see Appendix S1 for details and settings). Detection was performed using a LTQ Velos Orbitrap (Thermo Scientific, Sunnyvale, CA, USA) with HESI probe and operated in negative ion mode under standard settings.
Canine sciatic nerve extract produced as described above and GM2 standard (1 mg/ml; Avanti Polar Lipids, Alabaster, AL, USA) were separated by thin layer chromatography (TLC) and then immunostained with a murine monoclonal anti-GM2 Ab (kindly provided by K. Furukawa, Nagoya University Graduate School of Medicine, Japan) and GM2-reactive ACP serum. Methods were applied as previously described (Bethke et al., 1986; Scandroglio et al., 2009) (see Appendix S1).
Immunostaining of canine peripheral nerve with anti-GM2 antibody
Teased and cryostat-sectioned canine and murine sciatic nerves were incubated with mouse monoclonal anti-GM2 Ab (100 µg/ml) or neat anti-GM2 Ab-containing ACP serum, respectively (for details see Appendix S1). Following fixation of the nerves, fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgM-Ab (Southern Biotech, Birmingham, AL, USA: 1 : 200 in PBS) or FITC-conjugated anti-dog IgG-Ab (AbD Serotec, Kidlington, UK; 1 : 200) was applied. Other teased and sectioned fixed murine and canine sciatic nerves were incubated with FluoroMyelin™ (Invitrogen, Eugene, OR, USA; 1 : 300). All nerves were imaged with a Zeiss AxioImager with optional ApoTome function (Zeiss, Goettingen, Germany).
Statistics
To compare the patient demographics, clinical, and electrophysiological features of the reactive and non-reactive patients groups, Mann-Whitney U-tests and Fisher’s exact tests (Minitab statistical software, Version 16.1, Minitab Ltd., Coventry, UK and GraphPad Prism, Version 5.03, GraphPad Prism Software Inc, San Diego, CA, USA) were applied.
Lipid reactivities on the heat map underwent hierarchical clustering with average linkage and Pearson correlation for distance measurement (MEV software, Dana-Farber Cancer Institute, Boston, MA, USA). Additionally, receiver operating characteristic (ROC) analysis to evaluate differences in the assay performance (glycoarray vs. ELISA) was performed using the Hanley and McNeil methodology (Hanley and McNeil, 1982) and 95% confidence intervals (MedCalc software for Windows, Mariakerke, Belgium). The cut-off value for a reliable marker for disease was extrapolated from a likelihood ratio of LR +2.0 and LR −0.5, which is equivalent to an area under the curve (AUC) of 0.75. The coefficient of determination (R2) between glycoarray and ELISA was calculated using GraphPad Prism.
Results
Patient demographics
Three of the 28 ACP dogs were excluded from the reported study, one due to incomplete clinical information and two due to the chronicity of the neurological disorder (n = 2). In the latter cases, the dogs had been presented at the clinic (and serum samples collected) 194 days and 262 days after the onset of symptoms.
The age of the remaining 25 dogs ranged from 1 to 14 years (median 8.0 years). Many types of breeds including toy breeds (e.g., Chihuahua) and large breeds (e.g., German Shepherd dog) were represented, with cross-breeds slightly overrepresented (32%). There were 16 males and 9 females.
The time between onset of disease and acquisition of serum sample ranged from 2 to 61 days with a median of 10 days.
Neurological examinations and history
In most dogs (92%), impaired limb movement could be observed in all four legs. Ten dogs (40%) exhibited symmetrical weakness but were still able to walk (ambulatory paresis), whereas 13 dogs (52%) needed support to be able to walk (non-ambulatory paresis). Two dogs were completely paralysed. Decreased or absent tendon reflexes were observed in all dogs.
Many dogs (80%) also exhibited involvement of CNs; these were CNs V (4%), VII (48%), X (72%), and XII (12%). Vagal involvement manifested itself as dysphonia. In some dogs (24%) hyperesthesia was noted. Two dogs suffered from concurrent pneumonia, one of which, and another dog, also exhibited respiratory compromise and died (total n = 2; 8%).
Four dogs (16%) had experienced a gastrointestinal infection (as reported by the owner) prior to the onset of neurological symptoms. In one of the dogs, this was accompanied by a respiratory infection. None of the dogs had been vaccinated within 3 weeks before onset of the neurological deficits.
Electrophysiologic examinations
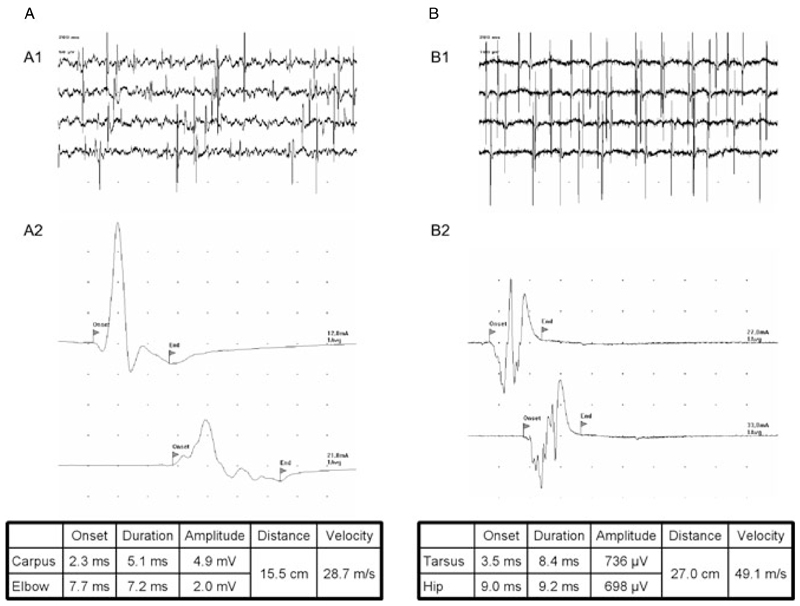
Twenty-four out of 25 dogs (96%) exhibited fibrillations and positive sharp waves, most evident in the distal appendicular muscles (Figs. 1A1 and 1B1). Tibial motor nerve conduction velocities ranged from 22.6 to 65.0 m/s (mean 50.1 m/s; median 52.2 m/s), whilst tibial sensory nerve conduction velocities ranged from 36.4 to 84.3 m/s (mean 58.7 m/s; median 55.7 m/s). The distally evoked compound muscle action potential (CMAP) amplitudes were reduced in 21 dogs (84%) and the CMAPs dispersed in 13 dogs (52%) (Figs. 1A2 and 1B2). Sensory action potentials were measured in 19 dogs, two of which (10.5%) exhibited dispersions. F-waves were normal in 2 dogs (8%), showed a prolonged latency in 20 dogs (80%), and were not recordable in 3 dogs (12%). Thus, in many dogs parameters indicative of both demyelinating and axonal neuropathy were seen.
Figure 1.
Examples for results obtained in electrophysiologic investigations of acute canine polyradiculoneuritis (ACP) dogs (both dogs exhibited anti-GM2 Abs). (A) Dog 15; (B) dog 16. A1/B1, spontaneous activity in electromyographic assessments of the tibial cranial muscle; A2/B2, motor nerve conduction studies of ulnar (A2) and sciatic/tibial (B2) nerve. Note the reduction of CMAP-amplitude and CMAP dispersion in the second trace of dog A. This dog also exhibited a reduced motor nerve conduction velocity (MNCV; 28.7 m/s). Dog B exhibited a vast reduction of its CMAP amplitude in both traces (<1 mV) with a MNCV (49.1 m/s) at the lower end of the physiological reference rage. Divisions on the abscissa are 2 ms (A2) and 5 ms (B2). Divisions on the ordinate are 1 mV (A2) and 200 µV (B2).
Imaging, infection titres, and muscle biopsies
Diagnostic imaging of the spinal cord was unremarkable in all dogs where conducted (n = 8), whilst serology for protozoan infections, conducted in a total of 15 dogs, was found negative in all but two cases (87%); one dog had a positive titre (1 : 40) for T. gondii, the other dog was positive for E. canis (1 : 64). Screening for acetylcholine receptor Abs and thyroid investigations was negative in all dogs where conducted, and in all four dogs which underwent muscle biopsies, neurogenic muscle atrophy was diagnosed.
Serology
Glycoarrays
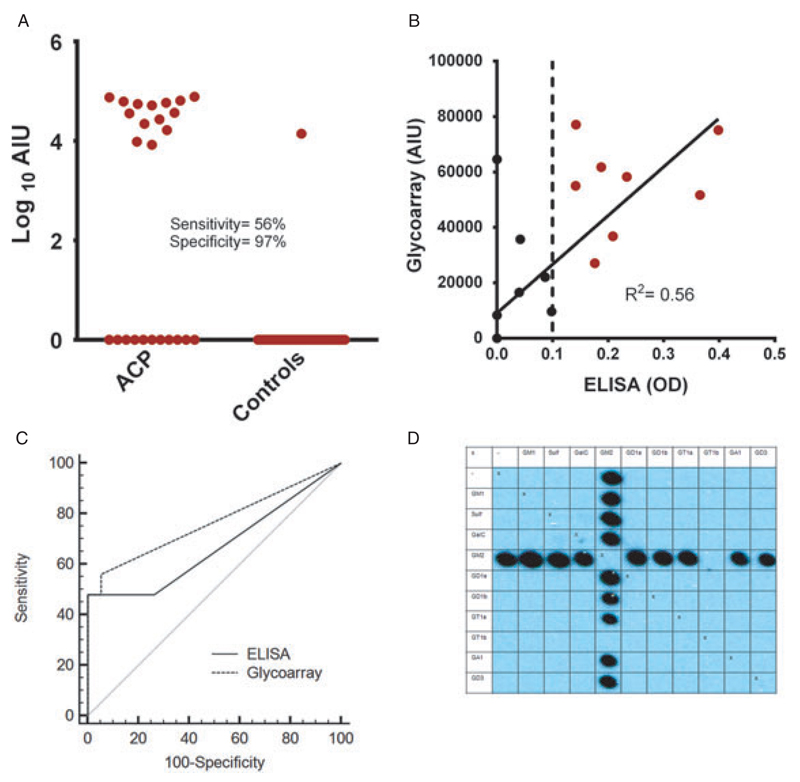
Fifteen of the 25 ACP dogs (60%) had detectable anti-GM2 ganglioside (14 dogs) or anti-GA1 (1 dog) IgG Abs, whereas all controls except for one of the local healthy controls were negative for the incidence of anti-ganglioside IgG Abs (p < 0.001). In this population, the glycoarray reached a sensitivity of 60% and a specificity of 97%.
In all but one of the positive cases (n = 15, inclusive of the one healthy control dog), the Abs were reactive for GM2 and most GM2-associated heterodimeric complexes (Fig. 3A). Whilst a reactivity with GM2 alone and an inhibition of reactivity with the GM2 : GT1b complex was observed in all dogs, about half of the GM2-positive sera reacted to GM2 regardless of which other glycolipid it was associated with, whereas the other half of sera (including the reactive control dog) favoured binding to GM2 : sulfatide and GM2 : galactocerebroside complexes (Figs. 2 and 3D).
Figure 3.
Analysis of serological data. (A) Individual values of anti-GM2 IgG Ab-reactivity obtained in glycoarrays for acute canine polyradiculoneuritis (ACP)-patients and controls (local non-neurological controls and other neurologic diseased [OND] dogs have been combined). The values are presented in arbitrary units of intensity (AIU). (B) Correlation analysis of anti-GM2 Ab glycoarray and anti-GM2 Ab ELISA, which reached a coefficient of determination (R2) of 0.56 when screening sera at a dilution of 1 : 100. Patients positive for both methodologies are represented by red dots (n = 8). (C) Comparison of receiver operating characteristic (ROC) curves for anti-GM2 Ab glycoarray and anti-GM2 Ab ELISA investigations, which show a trend in favour of the glycoarray’s diagnostic performance (AUC > 0.75). (D) Glycoarray blot of an anti-GM2 IgG Ab-positive ACP-dog. The anti-GM2 Abs in this dog react with GM2 alone and all heterodimeric GM2-complexes apart from the GM2:GT1b complex.
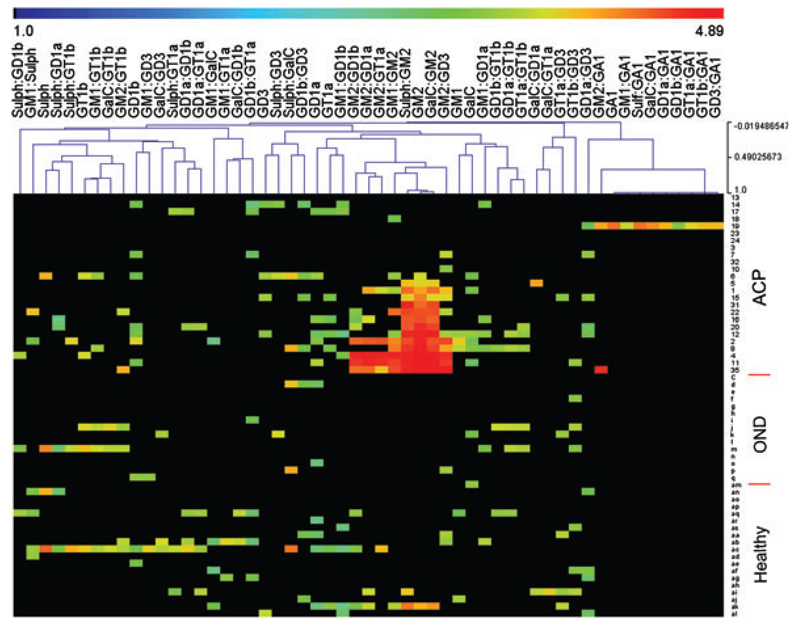
Figure 2.
Heat map showing the (log10 scaled) reactivity of IgG anti-ganglioside Abs in acute canine polyradiculoneuritis (ACP) patients (n = 25; numbers), local non-neurological controls (n = 19; double letters), and dogs suffering from idiopathic epilepsy (OND, n = 15; single letters). Black corresponds to Ab-negative signals, whereas the intensity of Ab-reactivity increases over blue to green and red, with red corresponding to the strongest signals.
The anti-GA1 Ab-positive dog (n = 1) was reactive for GA1 (single lipid) and all associated heterodimeric complexes (Fig. 2).
Enzyme-linked immunosorbent assay (ELISA)
Using a conventional anti-glycolipid Ab ELISA to assess the prevalence of anti-GM2 IgG Abs in ACP and local non-neurological control dog sera, GM2 reactivity was seen in 8/44 sera examined at a serum dilution of 1 : 100 (p = 0.007) and an additional three positive sera (total n = 11) at a dilution of 1 : 50 (p = 0.006). All positive sera, when screened at a dilution of 1 : 100 were from ACP cases, whereas one of the additional sera containing anti-GM2 Abs at a serum dilution of 1 : 50 was from a local non-neurological control dog. The ELISA, thus, had a sensitivity of 32% and a specificity of 100% when screening the samples at a dilution of 1 : 100, and a sensitivity of 40% and specificity of 95% when screening the serum samples at a dilution of 1 : 50.
Correlation of glycoarray and ELISA assays at a serum dilution of 1 : 100
Fourteen ACP serum samples (56%) were positive for anti-GM2 IgG Abs by glycoarray; eight ACP-serum samples (32%) were positive for anti-GM2 IgG Abs by ELISA. All sera exhibiting a GM2 reactivity in ELISA were also positive for anti-GM2 Abs on the glycoarray. The ELISA, therefore, confirmed the glycoarray data, whereas glycoarrays identified six additional anti-GM2 Ab-positive sera (Figs. 3A and 3B; R2 = 0.56). ROC analysis showed a slight, but not significant (p = 0.25), improvement of diagnostic performance when comparing glycoarray (AUC = 0.764) with ELISA (AUC = 0.672) (Fig. 3C). The glycoarray’s performance was positioned above the threshold of diagnostic value acceptance (AUC > 0.75).
Correlation of clinical and serological investigations
As the one dog with anti-GA1 Abs was clinically indistinguishable from the anti-GM2 Ab-positive dogs, the patient groups were combined for correlative analysis.
Comparing the demographics and history of the reactive and non-reactive patient groups, no statistical significant differences in age, sex distribution, and the incidence of preceding gastrointestinal/respiratory infections or vaccinations were observed (Table 1). Both groups included toy and large breed dogs; more cross-breed dogs were found in the non-reactive group (40%) when compared to the reactive group (26.7%; p = 0.669). The time between disease onset and acquisition of serum samples was insignificantly different (p = 0.51) when comparing the anti-ganglioside Ab-positive and Ab-negative group.
Table 1.
Patient demographics, clinical features, preceding events, and electrophysiological features in anti-GM2 antibody reactive and non-reactive dogs.
| Abs present n = 15 |
Abs absent n = 10 |
p | ||
|---|---|---|---|---|
| Patient demographics | Age (median) | 7.5 years | 9 years | 0.200* |
| Sex | 9m 6f | 7m 3f | 0.691† | |
| Sampling | Onset to serum acquisition | 10 days | 9.5 days | 0.504* |
| Clinical features | Ambulatory tetraparesis | 26.7% (n = 4) | 60.0% (n = 6) | 0.122† |
| Non-ambulatory paraparesis | 6.7% (n = 1) | 10.0% (n = 1) | 1.000† | |
| Non-ambulatory tetraparesis | 60.0% (n = 9) | 20.0% (n = 2) | 0.099† | |
| Tetraplegia | 6.7% (n = 1) | 10.0% (n = 1) | 1.000† | |
| Areflexia | 60.0% (n = 9) | 20.0% (n = 2) | 0.099† | |
| Hyperesthesia | 33.3% (n = 5) | 10.0% (n = 1) | 0.341† | |
| Involvement of CNs | 86.7% (n = 13) | 70.0% (n = 7) | 0.358† | |
| Respiratory compromise | 13.3% (n = 2) | 0.0% (n = 0) | 0.500† | |
| Pneumonia | 6.7% (n = 1) | 10.0% (n = 1) | 1.000† | |
| Death | 13.3% (n = 2) | 0.0% (n = 0) | 0.500† | |
| Preceding events | GIT infection | 13.3% (n = 2) | 20.0% (n = 2) | 1.000† |
| Respiratory infection | 6.7% (n = 1) | 0.0% (n = 0) | 1.000† | |
| Vaccination | 0.0% (n = 0) | 0.0% (n = 0) | − | |
| Electrophysiological features | Spontaneous activity in EMG | 100% (n = 15) | 90% (n = 9) | 0.400† |
| CMAP dispersion | 53.3% (n = 8) | 50.0% (n = 5) | 1.000† | |
| CMAP amplitude decreased | 93.3% (n = 14) | 70.0% (n = 7) | 0.267† | |
| F-waves normal | 0.0% (n = 0) | 20.0% (n = 2) | 0.150† | |
| F-waves prolonged latency | 80.0% (n = 12) | 80.0% (n = 8) | 1.000† | |
| F-waves not recordable | 20.0% (n = 3) | 0.0% (n = 0) | 0.250† |
Abs, antibodies; CMAP, compound muscle action potential; CNs, cranial nerves; EMG, electromyography; GIT, gastrointestinal.
Mann-Whitney U-test.
Fisherˊs exact test.
However, when examining the clinical presentation in the Ab-reactive and non-reactive patient groups a strong (yet statistically not significant) trend towards a more pronounced clinical phenotype in the group exhibiting anti-ganglioside Abs was observed when compared to the group without Abs, with a higher percentage of dogs unable to walk unaided, presented with a loss of reflexes, hyperesthesia, involvement of CNs, and respiratory compromise (leading to death).
Decreased distally evoked CMAP amplitudes and CMAP dispersions were observed in similar proportions in reactive- and non-reactive ACP patients, whereas normal F-waves (occurrence and latency) were only observed in anti-ganglioside Ab-negative dogs and absent F-waves were only observed in anti-ganglioside Abs positive dogs. Although not statistically significant, these observations were correlated with the trend towards more severe disease in the presence of anti-ganglioside Abs.
GM2 presence and location in the canine peripheral nerve
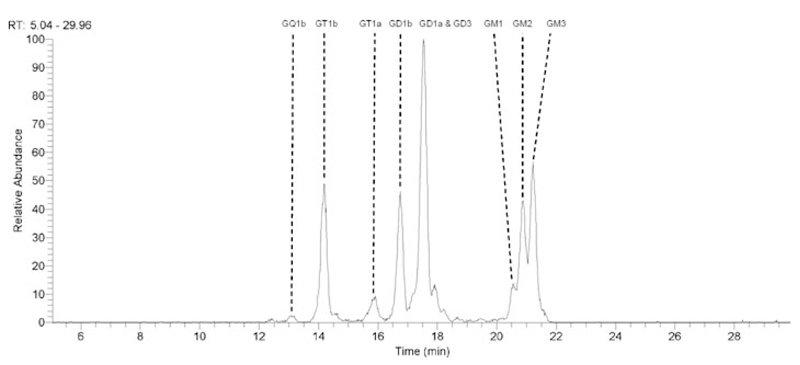
Using LCMS, all major gangliosides including GM1, GM2, GM3, GD1a, GD1b, GT1a/b, and GD3 were putatively identified in canine motor, sensory and mixed peripheral nerves, and also in both dorsal and ventral spinal roots (representative image for the sciatic nerve: Fig. 4). Whilst LCMS data is not quantitative, based on the relative signal intensities GM2 appeared to be of a similar abundance as GD1b and GT1b, lower than that of GD1a, GM3, and GD3, and greater than that of GM1, GT1a, and GQ1b. Very low levels of GalNAc-GD1a and GalNAc-GM1b could also be putatively identified (not illustrated).
Figure 4.
Extracted ion chromatogram of major gangliosides in canine sciatic nerve. All major gangliosides can be putatively detected. GalNAc-GD1a and GalNAc-GM1b were also identified (not shown).
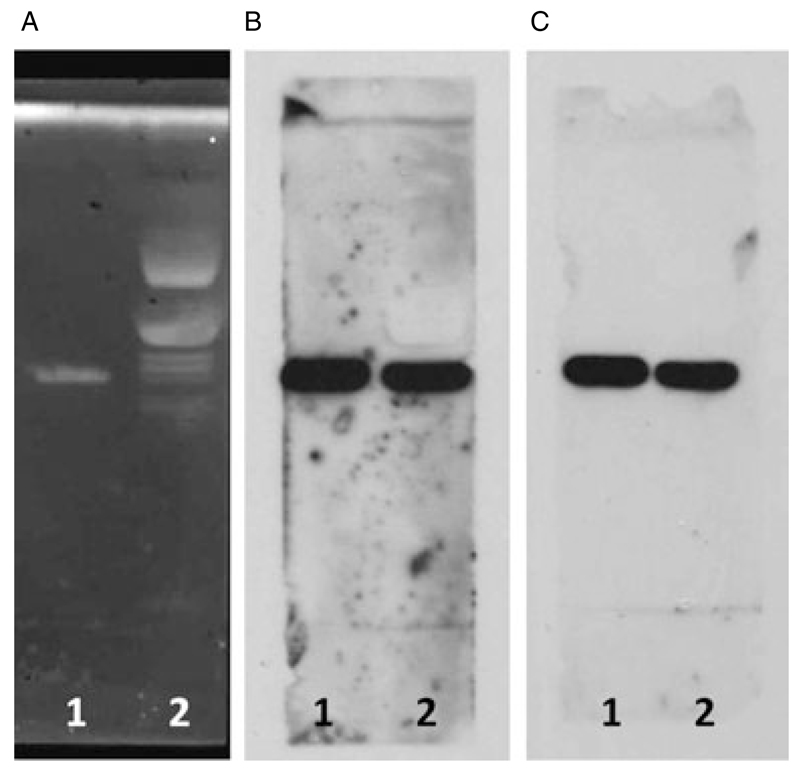
In the TLC-overlay experiments on canine sciatic nerve lipid extract, both the murine anti-GM2 Ab and canine GM2-reactive ACP serum exhibited a strong reactivity with a single glycolipid band that migrated identically with the authentic GM2 standard, confirming this band to be GM2 (Fig. 5).
Figure 5.
Thin layer chromatography (TLC) and TLC overlay of canine sciatic nerve. 1: GM2 standard; 2: canine sciatic nerve extract. (A) Ganglioside composition of canine sciatic nerve. (B) Overlay with mouse monoclonal anti-GM2 Ab. (C) Overlay with canine anti-GM2 reactive serum. A mono-specificity for GM2 both for the mouse monoclonal Ab and the canine GM2-reactive serum is observed.
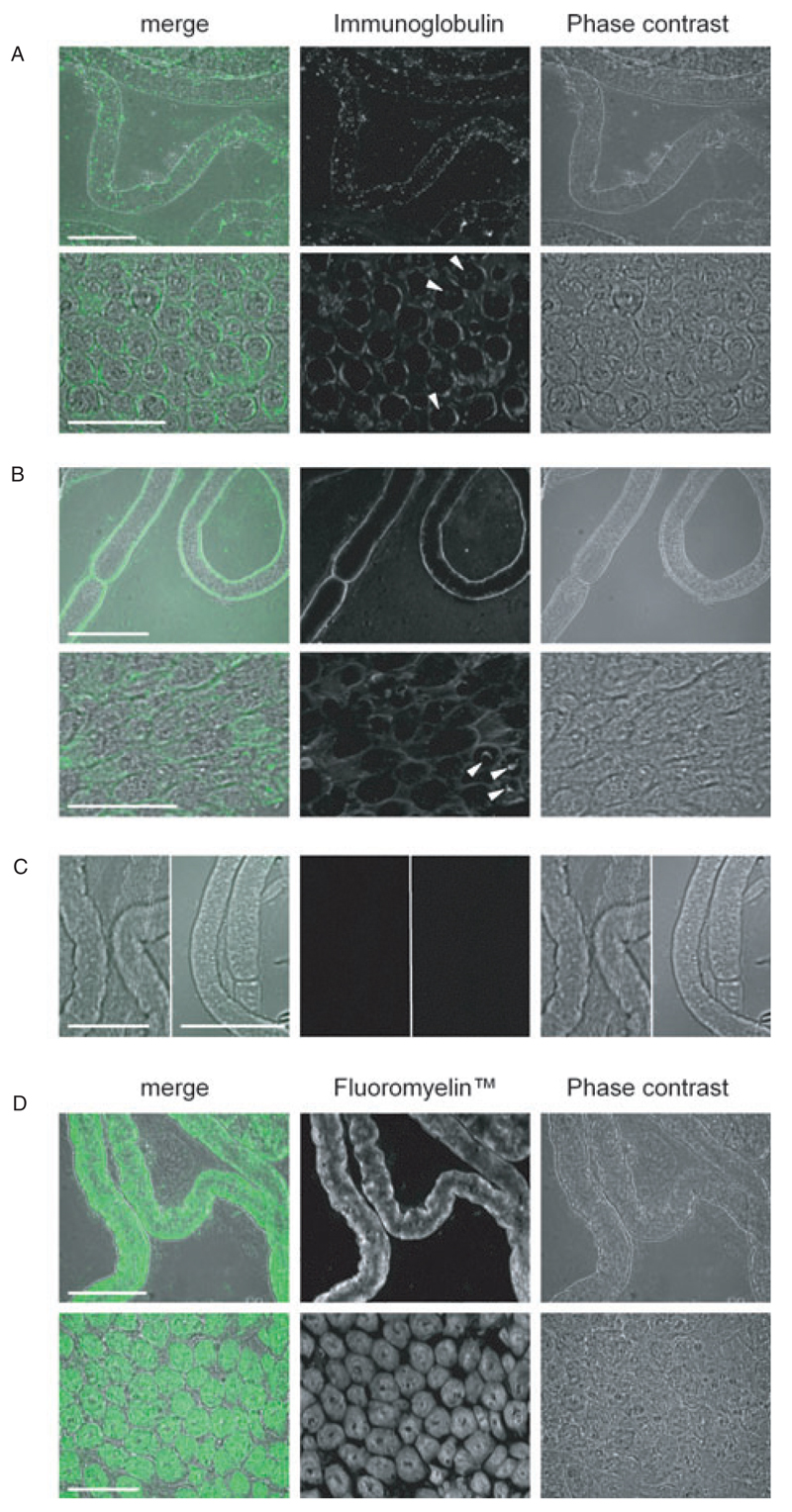
The labelling of teased and cross-sectioned canine and murine sciatic nerves with mouse monoclonal anti- GM2 Ab and canine GM2-reactive serum, respectively, revealed dense Ab-deposits on the abaxonal Schwann cell membrane (Figs. 6A and 6B). This staining pattern contrasted with that seen using the myelin marker FluoroMyelin where extensive staining of compact myelin was observed (Fig. 6D). In both canine and murine sciatic nerve cross-sections, an additional faint stain of some axonal areas was seen following the application of anti-GM2 Ab/canine GM2-reactive serum (Figs. 6A and 6B). Secondary Ab staining was negative (Fig. 6C).
Figure 6.
Staining profiles of anti-GM2 Abs. (A) Murine anti-GM2 Ab on canine sciatic nerve. (B) Canine anti-GM2 reactive acute canine polyradiculoneuritis (ACP)-serum on murine sciatic nerve. (C) Sole application of secondary Abs. (D) Fluoromyelin on canine sciatic nerve. Note the similarity in the staining profiles between (A) and (B) with the dense Ab-deposits observed on the abaxonal Schwann cell membrane; arrowheads indicate stained axonal profiles. In contrast to this, Fluoromyelin stains the material within the Schwann cells. No staining of tissue is observed following the sole application of secondary Abs. Top panels in (A), (B), and (D) are teased nerves, whereas the bottom panels are cross-sections; in (C) the left half of each panel is fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgM-Ab applied to teased canine nerve, whereas the right half of each panel is FITC-conjugated anti-dog IgG-Ab applied to teased murine nerve. Immunoglobulin/Fluoromyelin is green in merged images. Scale bars: 50 µm.
Discussion
Comparison of ACP and GBS – epidemiology, clinical features, and treatment
Despite being considered the most common acute canine polyneuropathy (Cuddon, 2002; Olby, 2004) and affecting most dog breeds (Cuddon, 1998; Holt et al., 2011; Hirschvogel et al., 2012) including mongrels and cross-breeds, ACP is a rare disease (Hirschvogel et al., 2012). Similarly, GBS is considered the most common cause of acute acquired paralysis in the Western World since the eradication of viral poliomyelitis (Hahn, 1998), yet with an incidence of between one and four per 100,000 (Hughes et al., 1999; Yu et al., 2006) is also a very rare disease (Sejvar et al., 2011).
Both GBS and ACP are characterized by a bilateral symmetrical ascending weakness of the limbs which can culminate in a generalized paralysis and require the patient to be ventilated (Winer, 2001; Cuddon, 2002; Rutter et al., 2011). Additionally, an involvement of CNs VII, X, or XII might be observed (Cummings and Haas, 1967; Asbury et al., 1969; Northington and Brown, 1982; McKhann et al., 1991; van Doorn et al., 2008). In ACP, the progressive phase usually lasts 5 – 10 days (Cuddon, 2002), whereas in GBS the progressive phase may last up to 4 weeks (Winer, 2001). Both for ACP and GBS, multiple episodes are possible (Northington and Brown, 1982; McKhann et al., 1993; de Lahunta and Glass, 2009; Kuitwaard et al., 2009; Hirschvogel et al., 2012).
Routinely, canine patients receive no treatment. All efforts are put into supportive care and rehabilitation (Cuddon, 2002; Olby, 2004) and most dogs make a complete recovery within 6 months (Northington and Brown, 1982; Cuddon, 2002; de Lahunta and Glass, 2009). In GBS patients, immunotherapy is delivered in the form of plasma exchange or IVIg, alongside supportive care (van Doorn et al., 2008; Vucic et al., 2009). Despite these efforts, 4% – 15% of patients die within 1 year of disease onset (Vucic et al., 2009), of which 8% die in the acute stage (Winer, 2001). In ACP patients, death due to respiratory paralysis or concurring pneumonia occurs in 12% of dogs (Hirschvogel et al., 2012).
Differential diagnoses for ACP and GBS include central nervous system abnormalities, peripheral neuropathies, myopathies, and neuromuscular junction abnormalities (Hughes and Cornblath, 2005; van Doorn et al., 2008). In ACP, the possible peripheral aetiologies more specifically include botulism, myasthenia gravis, tick paralysis, tick-borne (E. canis, Rickettsia rickettsii, B. burgdorferi) or protozoal (T. gondii, N. caninum, L. infantum) polymyositis/polyradiculoneuritis, hypothyroidism-associated myopathy, snake envenomation, and organophosphate toxicity (Cuddon, 2002; de Lahunta and Glass, 2009).
Comparison of ACP and GBS – pathology
The pathological changes in ACP dogs closely resemble those observed in GBS patients suffering from the demyelinating forms of GBS (acute inflammatory demyelinating polyradiculoneuropathy – AIDP) with the most striking pathological changes observed in the distal ventral roots. Histologically, the myelin of large-diameter fibres appears either swollen and pale, or fragmented into globules (Cummings and Haas, 1967; Northington and Brown, 1982); where myelin is completely lost, empty Schwann cell sheaths are found. The axons might look quite normal despite a loss of myelin; alternatively, axon degeneration is observed (Cummings and Haas, 1967).
Anti-GM2 antibodies
In this study, the anti-ganglioside Abs detected in dogs suffering from ACP were predominantly of one specificity, namely GM2. In contrast to this, a wide spectrum of anti-ganglioside Abs is observed in GBS patients, including anti-GM1, anti-GM2, anti-GD1a, anti-GD1b, anti-GT1b, and anti-GQ1b Abs (Caudie et al., 2002) or Abs against ganglioside complexes (Kaida et al., 2007). Anti-GM1 and anti-GD1a Abs have been associated with motor axonal forms of GBS, whereas anti-GQ1b Abs are considered diagnostic for Miller Fisher syndrome (Chiba et al., 1992; Caudie et al., 2002; Willison and Yuki, 2002; Ariga and Yu, 2005). No specific Ab has been detected yet for AIDP.
In GBS patients, the prevalence of anti-GM2 Abs comprises <10% of all Abs detected (Caudie et al., 2002). They usually are of IgM subtype (O’Hanlon et al., 2000) and have been shown to be strongly associated with preceding Cytomegalovirus infection (Khalili-Shirazi et al., 1999). A recent study investigating childhood GBS in India identified anti- GM2 IgM Abs as the most common Ab in this cohort and revealed a roughly even distribution of this Ab both in demyelinating and axonal forms. Seven of 43 children also exhibited anti-GM2 IgG Abs (Kannan et al., 2011).
In experimental settings, surface lipopolysaccharides of different Campylobacter jejuni strains can initiate the production of anti-GM2 IgM or IgG Abs (Ritter et al., 1996; Ang et al., 2002). Also, the onset of GBS (associated with anti-GM2 IgM Abs) following a snake bite due to a cross-reactivity between snake venom proteins and GM2 has recently been described (Neil et al., 2012).
The prevalence for C. jejuni in dogs is 1.2% in the UK and 40% in The Netherlands (Koene et al., 2004; Parsons et al., 2010), whereas the prevalence for Campylobacter upsaliensis, which has been linked to motor axonal forms of GBS in two case reports (Ho et al., 1997; Prasad et al., 2001), is higher (UK: 37.7%, Parsons et al., 2010, The Netherlands: 53%, Koene et al., 2004). For both C. jejuni and C. upsaliensis, no significant association between diarrhoea and the detection of the microorganisms in canine faecal samples or rectal swabs has been shown (Wieland et al., 2005; Parsons et al., 2010), which indicates that in our ACP dogs, the production of anti-GM2 Abs could have been associated with a preceding Campylobacter infection, despite only 2/14 anti-GM2 Ab-positive ACP dogs having experienced a preceding gastrointestinal infection.
Canine polyradiculoneuritis has also been described to be associated with protozoal infections (Cummings et al., 1988) such as Toxoplasma (T.)-infections (McGlennon et al., 1990). Recent investigations have discussed T. gondii as a possible aetiological agent for Ab-production in ACP (Holt et al., 2011), whilst a T. gondii infection associated with GBS has also been reported (Bossi et al., 1998). In this study, only 1/14 ACP dogs assessed for a T. gondii infection was positive for T. gondii Abs and at the same time exhibited anti-GM2 Abs. Considering a further nine ACP dogs with anti-GM2 Abs were found sero-negative for a T. gondii infection, it might be surmised that in this one dog the T. gondii infection was incidental and that it seems unlikely that a T. gondii infection induces the production of anti-GM2 Abs.
Cytomegaloviruses have a relatively restricted host range (Staczek, 1990; Schleiss, 2006) with no or only very little cross-reactivity of Abs produced against the different strains (Staczek, 1990). Thus, the trigger for the production of anti-GM2 Abs in this study remains unknown.
GM2 in the canine peripheral nerve and presumptive pathophysiological mechanism
In humans, GM2 can be found in all CNs and both dorsal and ventral roots of the spinal nerves, albeit at a much lower concentration when compared to other major gangliosides such as GD1a, GD1b, LM1, and GT1b (Chiba et al., 1997). In the CNS, GM2 is localized within the myelin (Ilyas et al., 1988), whereas the staining of human peripheral nerves with anti-GM2 Ab-containing sera revealed Ab-deposits at the abaxonal region of the myelin sheath/Schwann cell of large fibres, plus a weaker labelling of the inner aspect of the sheath, the adaxonal membranes, and the axonal area (O’Hanlon et al., 2000). Considering human, canine, and murine neurons have been shown to accumulate GM2 under pathological conditions (Walkley et al., 1995; Huang et al., 1997; Tamura et al. 2010), they probably also contain GM2 under physiological conditions.
In this study, the application of a mouse monoclonal anti-GM2 Ab and GM2-reactive ACP serum to canine and murine sciatic nerves led to a very faint staining of the axonal cross-sectional areas and very dense Ab-deposits on the abaxonal Schwann cell surface. Combining this observation with the complement-dependent cyototoxic effect of anti-GM2 Abs on cells expressing membrane-contained GM2 (O’Hanlon et al., 2000) and the hypothesis that Schwann-cell injury drives segmental demyelination (McDonald, 1963; Weller, 1965), it could be surmised that in some ACP patients segmental demyelination is driven by anti-GM2 Abs and complement. This hypothesis also correlates well with the complement products observed only early in the disease-process on the abaxonal Schwann cell surface in AIDP patients (Hafer-Macko et al., 1996; Putzu et al., 2000). Whilst it is not clear how complement deposition can lead to demyelination without damaging the Schwann cell, it is thought that complement can stimulate Schwann cells to proliferate (Benn et al., 2001), a feature habitually observed in ACP dogs and AIDP patients (Cummings and Haas, 1967; Asbury et al., 1969).
Lesion localization and correlation with clinical findings
In ACP patients, the majority of pathological changes are described in the nerve roots, and all anti-GM2 Ab-positive dogs exhibited nerve root involvement as demonstrated by electrophysiological examinations. Concurrently, however, similar to literature reports, all our anti-GM2 Ab-positive dogs also exhibited spontaneous activity in electromyographic investigations, and the muscle biopsies of our and other ACP dogs showed neurogenic muscle atrophy (Cummings and Haas, 1967; Hirschvogel et al., 2012), both of which are indicative of peripheral axonal involvement.
In experimental segmental demyelination, a pure loss of myelin without involvement of any other structures occurs only very rarely. In many cases, axonal degeneration is also observed (McDonald, 1963; Morgan-Hughes, 1968; Weller and Nester, 1972; Said et al., 1981), also seen in necropsies of ACP and AIDP patients (Cummings and Haas, 1967; Asbury et al., 1969). The relative paucity of pathological changes in peripheral nerve biopsies of ACP dogs (Northington and Brown, 1982; Hirschvogel et al., 2012) and the much stronger involvement of ventral roots when compared to peripheral nerves (Cummings and Haas, 1967), however, indicate that other mechanisms inducing electrophysiological changes suggestive of axonal involvement and muscle denervation need to be considered.
In mice, GM2 is found on the perisynaptic Schwann cells and the motor nerve terminals (Santafe et al., 2005; 2008). Application of anti-GM2 Abs leads to a complement-independent partial block of evoked neurotransmission (Ortiz et al., 2001; Santafe et al., 2008) with a reduction in CMAP amplitudes and no latency change, indicative of a motor axonal or neuromuscular disorder (Santafe et al., 2005) and surmised to be due to the functional perturbation of gangliosides following the binding of anti-ganglioside Abs (Santafe et al., 2008). Concomitant application of anti-GM2 Ab and complement results in a spontaneous release of acetylcholine indicating disruption of the axonal membrane and nerve terminal injury (Santafe et al., 2008).
Alternatively, other Abs or disease mechanisms should be considered because anti-GM2/anti-ganglioside Abs were not observed in all our ACP patients despite dogs with and without Abs sampled at comparable time points and presented with very similar clinical complaints.
Serological and technical considerations
Anti-GM2 IgG Abs found in a neurologically healthy control dog might correspond to the finding that neurologically healthy humans occasionally also exhibit low anti-GM2 Ab titres (Khalili-Shirazi et al., 1999). Alternatively, considering the dog in question is a pound dog and a complete history for this dog is not available, the anti-ganglioside Ab titre could indicate that this dog had previously suffered from ACP. In human GBS patients, a marked decline of anti-ganglioside Ab titres usually is observed within 4 months of disease. Occasional low titres, however, may persist longer (Odaka et al., 2003).
Corresponding well with the results obtained in GBS patients (van Koningsveld et al., 2002), an overall trend towards a stronger clinical phenotype was noted in anti-ganglioside Ab-positive dogs when compared to Ab-negative dogs.
Additionally, our investigations exhibit a higher sensitivity for anti-ganglioside Abs when investigated with glycoarrays and compared to conventional ELISA investigations; this correlates with a previous comparison of these two techniques (Galban-Horcajo et al., 2013). The glycoarray’s performance in the detection of anti-GM2 Abs indicates that it can serve as a potential diagnostic tool in confirming the diagnosis of ACP.
Conclusion
The incidence of serum anti-ganglioside Abs in ACP dogs very strongly suggests that this canine peripheral nerve disorder (or at least some forms of it) indeed is the canine equivalent to GBS. In their epidemiology and clinical symptoms, ACP and GBS are very similar to one another. The pathological changes described in ACP dogs closely resemble those of GBS patients suffering from the demyelinating form.
The predominant Ab target to be found in our ACP cohort was GM2, whose occurrence in canine peripheral and spinal nerves was confirmed by LCMS and TLC overlay investigations; in canine sciatic nerve, GM2 seems to be localized to the abaxonal Schwann cell area. Further investigations to examine the pathogenic capacity and mechanisms of anti-GM2 IgG Abs in dogs are warranted to determine whether anti-GM2 Ab-mediated ACP might be considered a naturally occurring model for anti-ganglioside Ab-mediated demyelination.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Materials and methods.
Acknowledgements
Professor K. Furukawa (Nagoya University Graduate School of Medicine, Japan) generously supplied the monoclonal murine anti-GM2 antibody. A. R. is funded by a Baxter/PNS fellowship award. F. G.-H. is funded by the Lord Kelvin Adam Smith postgraduate scholarship scheme. J. C. is funded by the BBSRC doctoral training account. H. W. is supported by a programme grant from the Wellcome Trust. Parts of this study were presented at the PNS/Inflammatory Neuropathy Consortium Meeting 2012 in Rotterdam and the 25th Annual Symposium of the European Society and College of Veterinary Neurology in Ghent.
References
- Alaedini A, Briani C, Wirguin I, Siciliano G, D’Avino C, Latov N. Detection of anti-ganglioside antibodies in Guillain-Barre syndrome and its variants by the agglutination assay. J Neurol Sci. 2002;196:41–44. doi: 10.1016/s0022-510x(02)00022-9. [DOI] [PubMed] [Google Scholar]
- Ang CW, Noordzij PG, de Klerk MA, Endtz HP, van Doorn PA, Laman JD. Ganglioside mimicry of Campylobacter jejuni lipopolysaccharides determines antiganglioside specificity in rabbits. Infect Immun. 2002;70:5081–5085. doi: 10.1128/IAI.70.9.5081-5085.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariga T, Yu RK. Antiglycolipid antibodies in Guillain-Barre syndrome and related diseases: review of clinical features and antibody specificities. J Neurosci Res. 2005;80:1–17. doi: 10.1002/jnr.20395. [DOI] [PubMed] [Google Scholar]
- Asbury AK, Arnason BG, Adams RD. The inflammatory lesion in idiopathic polyneuritis. Its role in pathogenesis. Medicine. 1969;48:173–215. doi: 10.1097/00005792-196905000-00001. [DOI] [PubMed] [Google Scholar]
- Benn T, Halfpenny C, Scolding N. Glial cells as targets for cytotoxic immune mediators. Glia. 2001;36:200–211. doi: 10.1002/glia.1109. [DOI] [PubMed] [Google Scholar]
- Bethke U, Muthing J, Schauder B, Conradt P, Muhlradt PF. An improved semi-quantitative enzyme immunostaining procedure for glycosphingolipid antigens on high performance thin layer chromatograms. J Immunol Methods. 1986;89:111–116. doi: 10.1016/0022-1759(86)90038-4. [DOI] [PubMed] [Google Scholar]
- Bossi P, Caumes E, Paris L, Darde ML, Bricaire F. Toxoplasma gondii-associated Guillain-Barre syndrome in an immunocompetent patient. J Clin Microbiol. 1998;36:3724–3725. doi: 10.1128/jcm.36.12.3724-3725.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudie C, Vial C, Bancel J, Petiot P, Antoine JC, Gonnaud PM. Antiganglioside autoantibody profiles in Guillain-Barre syndrome. Ann Biol Clin. 2002;60:589–597. [PubMed] [Google Scholar]
- Chiba A, Kusunoki S, Shimizu T, Kanazawa I. Serum IgG antibody to ganglioside GQ1b is a possible marker of Miller Fisher syndrome. Ann Neurol. 1992;31:677–679. doi: 10.1002/ana.410310619. [DOI] [PubMed] [Google Scholar]
- Chiba A, Kusunoki S, Obata H, Machinami R, Kanazawa I. Ganglioside composition of the human cranial nerves, with special reference to pathophysiology of Miller Fisher syndrome. Brain Res. 1997;745:32–36. doi: 10.1016/s0006-8993(96)01123-7. [DOI] [PubMed] [Google Scholar]
- Cuddon PA. Electrophysiologic assessment of acute polyradiculoneuropathy in dogs: comparison with Guillain-Barre syndrome in people. J Vet Intern Med. 1998;12:294–303. doi: 10.1111/j.1939-1676.1998.tb02125.x. [DOI] [PubMed] [Google Scholar]
- Cuddon PA. Acquired canine peripheral neuropathies. Vet Clin North Am Small Anim Pract. 2002;32:207–249. doi: 10.1016/s0195-5616(03)00086-x. [DOI] [PubMed] [Google Scholar]
- Cummings JF, Haas DC. Coonhound paralysis. An acute idiopathic polyradiculoneuritis in dogs resembling the Landry-Guillain-Barre syndrome. J Neurol Sci. 1967;4:51–81. doi: 10.1016/0022-510x(67)90058-5. [DOI] [PubMed] [Google Scholar]
- Cummings JF, Haas DC. Animal model for human disease: idiopathic polyneuritis, Guillain-Barre syndrome. Animal model: coonhound paralysis, idiopathic polyradiculoneuritis of coonhounds. Am J Pathol. 1972;66:189–192. [PMC free article] [PubMed] [Google Scholar]
- Cummings JF, de Lahunta A, Holmes DF, Schultz RD. Coonhound paralysis. Further clinical studies and electron microscopic observations. Acta Neuropathol. 1982;56:167–178. doi: 10.1007/BF00690632. [DOI] [PubMed] [Google Scholar]
- Cummings JF, de Lahunta A, Suter MM, Jacobson RH. Canine protozoan polyradiculoneuritis. Acta Neuropathol. 1988;76:46–54. doi: 10.1007/BF00687679. [DOI] [PubMed] [Google Scholar]
- de Lahunta A, Glass E. Veterinary Neuroanatomy and Clinical Neurology. Saunders, Elsevier; St. Louis: 2009. pp. 90–97. [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Galban-Horcajo F, Fitzpatrick AM, Hutton AJ, Dunn SM, Kalna G, Brennan KM, Rinaldi S, Yu RK, Goodyear CS, Willison HJ. Antibodies to heteromeric glycolipid complexed in multifocal motor neuropathy. Eur J Neurol. 2013;20:62–70. doi: 10.1111/j.1468-1331.2012.03767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafer-Macko CE, Sheikh KA, Li CY, Ho TW, Cornblath DR, McKhann GM, Asbury AK, Griffin JW. Immune attack on the Schwann cell surface in acute inflammatory demyelinating polyneuropathy. Ann Neurol. 1996;39:625–635. doi: 10.1002/ana.410390512. [DOI] [PubMed] [Google Scholar]
- Hahn AF. Guillain-Barre syndrome. Lancet. 1998;352:635–641. doi: 10.1016/S0140-6736(97)12308-X. [DOI] [PubMed] [Google Scholar]
- Halstead SK, O’Hanlon GM, Humphreys PD, Morrison DB, Morgan BP, Todd AJ, Plomp JJ, Willison HJ. Anti-disialoside antibodies kill perisynaptic Schwann cells and damage motor nerve terminals via membrane attack complex in a murine model of neuropathy. Brain. 2004;127:2109–2123. doi: 10.1093/brain/awh231. [DOI] [PubMed] [Google Scholar]
- Halstead SK, Humphreys PD, Goodfellow JA, Wagner ER, Smith RA, Willison HJ. Complement inhibition abrogates nerve terminal injury in Miller Fisher syndrome. Ann Neurol. 2005;58:203–210. doi: 10.1002/ana.20546. [DOI] [PubMed] [Google Scholar]
- Hamberger A, Svennerholm L. Composition of gangliosides and phospholipids of neuronal and glial cell enriched fractions. J Neurochem. 1971;18:1821–1829. doi: 10.1111/j.1471-4159.1971.tb09587.x. [DOI] [PubMed] [Google Scholar]
- Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- Hirschvogel K, Jurina K, Steinberg T, Matiasek L, Matiasek K, Beltran E, Fischer A. Clinical course of actue canine polyradiculoneuritis following treatment with human IV immunoglobulin. J Am Anim Hosp Assoc. 2012;48:1–11. doi: 10.5326/JAAHA-MS-5651. [DOI] [PubMed] [Google Scholar]
- Ho TW, Hsieh ST, Nachamkin I, Willison HJ, Sheikh K, Kiehlbauch J, Flanigan K, McArthur JC, Cornblath DR, McKhann GM, Griffin JW. Motor nerve terminal degeneration provides a potential mechanism for rapid recovery in acute motor axonal neuropathy after Campylobacter infection. Neurology. 1997;48:717–724. doi: 10.1212/wnl.48.3.717. [DOI] [PubMed] [Google Scholar]
- Holt N, Murray M, Cuddon PA, Lappin MR. Sero-prevalence of various infectious agents in dogs with suspected acute canine polyradiculoneuritis. J Vet Intern Med. 2011;25:261–266. doi: 10.1111/j.1939-1676.2011.0692.x. [DOI] [PubMed] [Google Scholar]
- Huang JQ, Trasler JM, Igdoura S, Michaud J, Hanal N, Gravel RA. Apoptotic cell death in mouse models of GM2 gangliosidosis and observations on human Tay-Sachs and Sandhoff diseases. Hum Mol Genet. 1997;6:1879–1885. doi: 10.1093/hmg/6.11.1879. [DOI] [PubMed] [Google Scholar]
- Hughes RA, Hadden RD, Gregson NA, Smith KJ. Pathogenesis of Guillain-Barre syndrome. J Neuroimmunol. 1999;100:74–97. doi: 10.1016/s0165-5728(99)00195-2. [DOI] [PubMed] [Google Scholar]
- Hughes RA, Cornblath DR. Guillain-Barre syndrome. Lancet. 2005;366:1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- Ilyas AA, Li SC, Chou DK, Li YT, Jungalwala FB, Dalakas MC, Quarles RH. Gangliosides GM2, IV4GalNAcGM1b, and IV4GalNAcGC1a as antigens for monoclonal immunoglobulin M in neuropathy associated with gammopathy. J Biol Chem. 1988;263:4369–4373. [PubMed] [Google Scholar]
- Kaida K, Morita D, Kanzaki M, Kamakura K, Motoyoshi K, Hirakawa M, Kusunoki S. Anti-ganglioside complex antibodies associated with severe disability in GBS. J Neuroimmunol. 2007;182:212–218. doi: 10.1016/j.jneuroim.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Kannan MA, Ch RK, Jabeen SA, Mridula KR, Rao P, Borgohain R. Clinical, electrophysiological subtypes and antiganglioside antibodies in childhood Guillain-Barre syndrome. Neurol India. 2011;59:727–732. doi: 10.4103/0028-3886.86549. [DOI] [PubMed] [Google Scholar]
- Khalili-Shirazi A, Gregson N, Gray I, Rees J, Winer J, Hughes R. Antiganglioside antibodies in Guillain-Barre syndrome after a recent cytomegalovirus infection. J Neurol Neurosurg Psychiatry. 1999;66:376–379. doi: 10.1136/jnnp.66.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koene MG, Houwers DJ, Dijkstra JR, Duim B, Wagenaar JA. Simultaneous presence of multiple Campylobacter species in dogs. J Clin Microbiol. 2004;42:819–821. doi: 10.1128/JCM.42.2.819-821.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuitwaard K, van Koningsveld R, Ruts L, Jacobs BC, van Doorn PA. Recurrent Guillain-Barre syndrome. J Neurol Neurosurg Psychiatry. 2009;80:56–59. doi: 10.1136/jnnp.2008.156463. [DOI] [PubMed] [Google Scholar]
- Mata S, Galli E, Amantini A, Pinto F, Sorbi S, Lolli F. Anti-ganglioside antibodies and elevated CSF IgG levels in Guillain-Barre syndrome. Eur J Neurol. 2006;13:153–160. doi: 10.1111/j.1468-1331.2006.01161.x. [DOI] [PubMed] [Google Scholar]
- McDonald WI. The effects of experimental demyelination on conduction in peripheral nerve: a histological and electrophysiological study I. Clinical and histological observations. Brain. 1963;86:481–500. doi: 10.1093/brain/86.3.481. [DOI] [PubMed] [Google Scholar]
- McGlennon NJ, Jefferies AR, Casas C. Polyradiculoneuritis and polymyositis due to a toxoplasma-like protozoan: diagnosis and treatment. J Small Anim Pract. 1990;31:102–104. [Google Scholar]
- McGonigal R, Rowan EG, Greenshields KN, Halstead SK, Humphreys PD, Rother RP, Furukawa K, Willison HJ. Anti-GD1a antibodies activate complement and calpain to injure distal motor nodes of Ranvier in mice. Brain. 2010;133:1944–1960. doi: 10.1093/brain/awq119. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Cornblath DR, Ho T, Li CY, Bai AY, Wu HS, Yei QF, Zhang WC, Zhaori Z, Jiang Z. Clinical and electrophysiological aspects of acute paralytic disease of children and young adults in northern China. Lancet. 1991;338:593–597. doi: 10.1016/0140-6736(91)90606-p. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Cornblath DR, Griffin JW, Ho TW, Li CY, Jiang Z, Wu HS, Zhaori G, Liu Y, Jou LP. Acute motor axonal neuropathy: a frequent cause of acute flaccid paralysis in China. Ann Neurol. 1993;33:333–342. doi: 10.1002/ana.410330402. [DOI] [PubMed] [Google Scholar]
- Meena AK, Archana AD, Reddy GC, Ramakrishna D, Rao P. Anti-ganglioside antibodies in subtypes of Guillain-Barre syndrome in an Indian population. J Med Sci. 2010;10:138–142. [Google Scholar]
- Morgan-Hughes JA. Experimental diphtheritic neuropathy. A pathological and electrophysiological study. J Neurol Sci. 1968;7:157–175. doi: 10.1016/0022-510x(68)90011-7. [DOI] [PubMed] [Google Scholar]
- Neil J, Choumet V, Le CA, d’Alayer J, Demeret S, Musset L. Guillain-Barre syndrome: first description of a snake envenomation aetiology. J Neuroimmunol. 2012;242:72–77. doi: 10.1016/j.jneuroim.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Northington JW, Brown MJ. Acute canine idiopathic polyneuropathy. A Guillain-Barre-like syndrome in dogs. J Neurol Sci. 1982;56:259–273. doi: 10.1016/0022-510x(82)90147-2. [DOI] [PubMed] [Google Scholar]
- Odaka M, Koga M, Yuki N, Susuki K, Hirata K. Longitudinal changes of anti-ganglioside antibodies before and after Guillain-Barre syndrome onset subsequent to Campylobacter jejuni enteritis . J Neurol Sci. 2003;210:99–103. doi: 10.1016/s0022-510x(03)00029-7. [DOI] [PubMed] [Google Scholar]
- O’Hanlon GM, Veitch J, Gallardo E, Illa I, Chancellor AM, Willison HJ. Peripheral neuropathy associated with anti-GM2 ganglioside antibodies: clinical and immunopathological studies. Autoimmunity. 2000;32:133–144. doi: 10.3109/08916930008994083. [DOI] [PubMed] [Google Scholar]
- O’Hanlon GM, Plomp JJ, Chakrabarti M, Morrison I, Wagner ER, Goodyear CS, Yin X, Trapp BD, Conner J, Molenaar PC, Stewart S, et al. Anti-GQ1b ganglioside antibodies mediate complement-dependent destruction of the motor nerve terminal. Brain. 2001;124:893–906. doi: 10.1093/brain/124.5.893. [DOI] [PubMed] [Google Scholar]
- Olby NJ. Tetraparesis. In: Platt SR, Olby NJ, editors. BSAVA Manual of Canine and Feline Neurology. British Small Animal Veterinary Association; Gloucester: 2004. pp. 214–236. [Google Scholar]
- Ortiz N, Rosa R, Gallardo E, Illa I, Tomas J, Aubry J, Sabater M, Santafe M. IgM monoclonal antibody against terminal moiety of GM2, GalNAc-GD1a and GalNAc-GM1b from a pure motor chronic demyelinating polyneuropathy patient: effects on neurotransmitter release. J Neuroimmunol. 2001;119:114–123. doi: 10.1016/s0165-5728(01)00373-3. [DOI] [PubMed] [Google Scholar]
- Parsons BN, Porter CJ, Ryvar R, Stavisky J, Williams NJ, Pinchbeck GL, Birtles RJ, Christley RM, German AJ, Radford AD, Hart CA, et al. Prevalence of Campylobacter spp. in a cross-sectional study of dogs attending veterinary practices in the UK and risk indicators associated with shedding. Vet J. 2010;184:66–70. doi: 10.1016/j.tvjl.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Prasad KN, Pradhan S, Nag VL. Guillain-Barre syndrome and Campylobacter infection. Southeast Asian J Trop Med Public Health. 2001;32:527–530. [PubMed] [Google Scholar]
- Putzu GA, Figarella-Branger D, Bouvier-Labit C, Liprandi A, Bianco N, Pellissier JF. Immunohistochemical localization of cytokines, C5b-9 and ICAM-1 in peripheral nerve of Guillain-Barre syndrome. J Neurol Sci. 2000;174:16–21. doi: 10.1016/s0022-510x(99)00328-7. [DOI] [PubMed] [Google Scholar]
- Rinaldi S, Brennan KM, Goodyear CS, O’Leary C, Schiavo G, Crocker PR, Willison HJ. Analysis of lectin binding to glycolipid complexes using combinatorial glycoarrays. Glycobiology. 2009;19:789–796. doi: 10.1093/glycob/cwp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter G, Fortunato SR, Cohen L, Noguchi Y, Bernard EM, Stockert E, Old LJ. Induction of antibodies reactive with GM2 ganglioside after immunization with lipopolysaccharides from Camplyobacter jejuni . Int J Cancer. 1996;66:184–190. doi: 10.1002/(SICI)1097-0215(19960410)66:2<184::AID-IJC8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Rupp A, Morrison I, Barrie JA, Halstead SK, Townson KH, Greenshields KN, Willison HJ. Motor nerve terminal destruction and regeneration following anti-ganglioside antibody and complement-mediated injury: an in and ex vivo imaging study in the mouse. Exp Neurol. 2012;233:836–848. doi: 10.1016/j.expneurol.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Rutter CR, Rozanski EA, Sharp CR, Powell LL, Kent M. Outcome and medical management in dogs with lower motor neuron disease undergoing mechanical ventilation: 14 cases (2003–2009) J Vet Emerg Crit Care. 2011;21:531–541. doi: 10.1111/j.1476-4431.2011.00669.x. [DOI] [PubMed] [Google Scholar]
- Said G, Saida K, Saida T, Asbury AK. Axonal lesions in acute experimental demyelination: a sequential teased nerve fiber study. Neurology. 1981;31:413–421. doi: 10.1212/wnl.31.4.413. [DOI] [PubMed] [Google Scholar]
- Santafe MM, Sabate MM, Garcia N, Ortiz N, Lanuza MA, Tomas J. Changes in the neuromuscular synapse induced by an antibody against gangliosides. Ann Neurol. 2005;57:396–407. doi: 10.1002/ana.20403. [DOI] [PubMed] [Google Scholar]
- Santafe MM, Sabate MM, Garcia N, Ortiz N, Lanuza MA, Tomas J. Anti-GM2 gangliosides IgM paraprotein induces neuromuscular block without neuromuscular damage. J Neuroimmunol. 2008;204:20–28. doi: 10.1016/j.jneuroim.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Scandroglio F, Loberto N, Valsecchi M, Chigorno V, Prinetti A, Sonnino S. Thin layer chromatography of gangliosides. Glycoconj J. 2009;26:961–973. doi: 10.1007/s10719-008-9145-5. [DOI] [PubMed] [Google Scholar]
- Schleiss MR. Nonprimate models of congenital cytomegalovirus (CMV) infection: gaining insight into pathogenesis and prevention of disease in newborns. ILAR J. 2006;47:65–72. doi: 10.1093/ilar.47.1.65. [DOI] [PubMed] [Google Scholar]
- Sejvar S, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain-Barre syndrome: a systematic review and meta-analysis. Neuroepidemiology. 2011;36:123–133. doi: 10.1159/000324710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staczek J. Animal cytomegaloviruses. Microbiol Rev. 1990;54:247–265. doi: 10.1128/mr.54.3.247-265.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura S, Tamura Y, Uchida K, Nibe K, Nakaichi M, Hossain MA, Chang HS, Rahman MM, Yabuki A, Yamato O. GM2 gangliosidosis variant 0 (Sandhoff-like disease) in a family of toy poodles. J Vet Intern Med. 2010;24:1013–1019. doi: 10.1111/j.1939-1676.2010.0564.x. [DOI] [PubMed] [Google Scholar]
- Vandevelde M, Oettli P, Rohr M. Polyradikuloneuritis beim Hund. Klinische, histologische und ultrastrukturelle Beobachtungen. Schweiz Arch Tierheilk. 1981;123:201–217. [PubMed] [Google Scholar]
- van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barre syndrome. Lancet Neurol. 2008;7:939–950. doi: 10.1016/S1474-4422(08)70215-1. [DOI] [PubMed] [Google Scholar]
- van Koningsveld R, Schmitz PI, Ang CW, Groen J, Osterhaus AD, Van der Meche FG, van Doorn PA. Infections and course of disease in mild forms of Guillain-Barre syndrome. Neurology. 2002;58:610–614. doi: 10.1212/wnl.58.4.610. [DOI] [PubMed] [Google Scholar]
- Vucic S, Kiernan MC, Cornblath DR. Guillain-Barre syndrome: an update. J Clin Neurosci. 2009;16:733–741. doi: 10.1016/j.jocn.2008.08.033. [DOI] [PubMed] [Google Scholar]
- Walkley SU, Siegel DA, Dobrenis K. GM2 ganglioside and pyramidal neuron dendritogenesis. Neurochem Res. 1995;20:1287–1299. doi: 10.1007/BF00992503. [DOI] [PubMed] [Google Scholar]
- Weller RO. Diphtheritic neuropathy in the chicken: an electron-microscope study. J Pathol Bacteriol. 1965;89:591–598. doi: 10.1002/path.1700890218. [DOI] [PubMed] [Google Scholar]
- Weller RO, Nester B. Early changes at the node of Ranvier in segmental demyelination. Histochemical and electron microscopic observations. Brain. 1972;95:665–674. doi: 10.1093/brain/95.4.665. [DOI] [PubMed] [Google Scholar]
- Wieland B, Regula G, Danuser J, Wittwer M, Burnens AP, Staerk KDC. Campylobacter spp. in dogs and cats in Switzerland: risk factor analysis and molecular characterization with AFLP. J Vet Med B. 2005;52:183–189. doi: 10.1111/j.1439-0450.2005.00843.x. [DOI] [PubMed] [Google Scholar]
- Willison HJ. The immunobiology of Guillain-Barre syndromes. J Peripher Nerv Syst. 2005;10:94–112. doi: 10.1111/j.1085-9489.2005.0010202.x. [DOI] [PubMed] [Google Scholar]
- Willison HJ, Yuki N. Peripheral neuropathies and anti-glycolipid antibodies. Brain. 2002;125:2591–2625. doi: 10.1093/brain/awf272. [DOI] [PubMed] [Google Scholar]
- Willison HJ, Veitch J, Swan AV, Baumann N, Comi G, Gregson NA, Illa I, Zielasek J, Hughes RA. Inter-laboratory validation of an ELISA for the determination of serum anti-ganglioside antibodies. Eur J Neurol. 1999;6:71–77. doi: 10.1046/j.1468-1331.1999.610071.x. [DOI] [PubMed] [Google Scholar]
- Winer JB. Guillain Barre syndrome. Mol Pathol. 2001;54:381–385. [PMC free article] [PubMed] [Google Scholar]
- Yu RK, Usuki S, Ariga T. Ganglioside molecular mimicry and its pathological roles in Guillain-Barré syndrome and related diseases. Infect Immun. 2006;74:6517–6527. doi: 10.1128/IAI.00967-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki N, Hartung HP. Guillain-Barré syndrome. N Engl J Med. 2012;366:2294–2304. doi: 10.1056/NEJMra1114525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and methods.