Abstract
Ranitidine (RAN) is one of the drugs associated with idiosyncratic adverse drug reactions (IADRs) in human patients. In rats, cotreatment with nontoxic doses of lipopolysaccharide (LPS) and RAN causes liver injury. This is a potential animal model for RAN-induced IADRs in humans. Previous studies showed that RAN augmented serum tumor necrosis factor (TNF)-α production and hepatic neutrophil activation after LPS treatment and that both TNF-α and neutrophils are crucial for the liver pathogenesis. We tested the hypothesis that p38 mitogen-activated protein kinase activation is necessary for TNF-α production, neutrophil activation, and subsequent liver injury. LPS/RAN cotreatment caused more p38 activation compared with LPS alone. The p38 inhibitor SB 239063 [trans-1-(4-hydroxycyclohexyl)-4-(4-fluorophenyl)-5-(2-methoxypyridimidin-4-yl) imidazole] reduced liver injury in rats cotreated with LPS/RAN. This inhibitor also reduced neutrophil activation and attenuated hemostatic system activation. SB 239063 decreased serum TNF-α concentration after LPS/RAN treatment to the same level as LPS treatment. However, the inhibitor did not reduce TNF-α mRNA in liver, suggesting a post-transcriptional mode of action. This might occur through TNF-α-converting enzyme (TACE), which cleaves pro-TNF-α into its active form. Indeed, a TACE inhibitor administered just before RAN treatment reduced serum TNF-α protein. The TACE inhibitor also reduced liver injury and serum plasminogen activator inhibitor (PAI)-1. Furthermore, a PAI-1 inhibitor reduced neutrophil activation and liver injury after LPS/RAN treatment. In summary, RAN enhanced TNF-α production after LPS treatment through augmented p38 activation, and this seems to occur through TACE. The prolonged TNF-α production enhanced PAI-1 production after RAN cotreatment, and this is important for the hepatotoxicity.
Idiosyncratic adverse drug reactions (IADRs) occur during treatment with numerous drugs, typically in a small fraction of patients. These responses are seemingly unrelated to dose, and the time of onset relative to beginning of drug therapy is often variable (Uetrecht, 2007). A widely used drug associated with rare idiosyncratic hepatotoxicity is the histamine 2 (H2)-receptor antagonist ranitidine (RAN) (Bourdet et al., 2005). RAN is available over the counter for oral administration or by prescription for parenteral administration for treatment of duodenal ulcers, gastric hypersecretory diseases, and gastroesophageal reflux disease. Idiosyncratic RAN hepatotoxicity occurs in less than 0.1% of people taking the drug (Vial et al., 1991; Fisher and Le Couteur, 2001). Most liver reactions are mild and reversible; however, extensive liver damage and death have occurred in individuals undergoing RAN therapy (Cherqui et al., 1989; Ribeiro et al., 2000). Rechallenge with RAN does not necessarily result in a reoccurrence of toxicity (Halparin, 1984; Hiesse et al., 1985).
In rats, cotreatment with nontoxic doses of lipopolysaccharide (LPS) and RAN causes liver injury. This was not the case with another histamine-2 receptor antagonist, famotidine (FAM), which is not associated with IADRs in human patients (Fisher and Le Couteur, 2001). Thus, this LPS-drug interaction model in rodents could differentiate a drug that causes IADRs from a drug that does not. Previous mechanistic studies showed that RAN augmented serum tumor necrosis factor (TNF)-α production and hepatic neutrophil activation after LPS treatment, and both TNF-α and neutrophils are crucial for the liver pathogenesis (Deng et al., 2007; Tukov et al., 2007). Moreover, TNF-α is likely to be a proximal signal in the pathogenic cascade (Tukov et al., 2007). The mechanism behind RAN augmentation of TNF-α production and neutrophil activation is unknown.
TNF-α production involves gene expression of pro-TNF-α mRNA, translation of pro-TNF-α protein, and its cleavage and release of active TNF-α. LPS-induced TNF-α transcriptional activation has been well studied (Kawai and Akira, 2007). However, TNF-α production can also be regulated at a post-transcriptional level. For example, TNF-α mRNA stabilization and translation are regulated by activation of p38 mitogen-activated protein kinase (MAPK) (Neininger et al., 2002; Hitti et al., 2006). Furthermore, TNF-α-converting enzyme (TACE) cleaves the 26-kDa membrane-bound pro-TNF-α protein to generate secreted 17-kDa mature TNF-α (Aggarwal et al., 1985; Müllberg et al., 2000). This cleavage occurs at the Ala76-Val77 bond. The release of TNF-α from cells in vitro and in vivo can be selectively blocked by hydroxamate-based metalloprotease inhibitors that inhibit TACE activity (Gearing et al., 1994; Mohler et al., 1994). These TACE inhibitors protect against endotoxin-mediated lethality, in which TNF-α plays a critical role (Mohler et al., 1994).
p38 and its downstream MAPK-activated protein kinase 2 (MK-2) have been shown to be involved in the production of several cytokines and chemokines [i.e., TNF-α, macrophage inflammatory protein (MIP)-2, and interleukin 6] (Neininger et al., 2002; Numahata et al., 2003; Hitti et al., 2006) and in neutrophil activation (Nick et al., 1997). Thus, p38 activation is a potential upstream signal that leads to production of cytokines/chemokines and subsequently to downstream cascades that contribute to LPS/RAN-induced liver injury (Luyendyk et al., 2006). Here, we tested the hypothesis that p38 is necessary for TNF-α production, neutrophil activation, and subsequent liver injury caused by LPS/RAN cotreatment.These studies elucidated signaling events that are crucial to the initiation of LPS/RAN induced-liver injury.
Materials and Methods
Materials
Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO). LPS derived from Escherichia coli serotype O55:B5 with activity of 13 × 106 EU/mg (lot no. 43K4112) was used for these studies. This activity was determined using a QCL Chromogenic Limulus amoebocyte lysate endpoint assay purchased from Lonza, Inc. (Baltimore, MD).
Animals
Male, Sprague-Dawley rats [Crl:CD (SD)IGS BR; Charles River Breeding Laboratories, Portage, MI], weighing 250 to 350 g, were fed standard chow (rodent chow/Tek 8640; Harlan Teklad, Madison, WI), and they were allowed access to water ad libitum. Rats were allowed to acclimate for 1 week in a 12-h light/dark cycle before use.
Experimental Protocol
Rats fasted for 24 h were given 2.5 × 106 EU/kg LPS or its saline vehicle (Veh) i.v. at 5 ml/kg, and food was then returned. Two hours later, 30 mg/kg RAN, 6 mg/kg FAM, or their vehicle [sterile phosphate-buffered saline (PBS)] was administered (i.v.). RAN solution was administered at 2 ml/kg at a rate of approximately 0.15 ml/min. The FAM dose was selected as a pharmacologically equi-efficacious dose based on relative potencies of RAN and FAM in antagonizing H2-receptors (Luyendyk et al., 2006). At the time of sacrifice, rats were anesthetized with sodium pentobarbital (75 mg/kg i.p.). Plasma was collected by drawing 2 ml of blood from the vena cava into a syringe containing sodium citrate (final concentration, 0.38%). Another portion of blood was collected and allowed to clot at room temperature; serum was prepared from this fraction and stored at −20°C until use. Representative slices (3–4 mm in thickness) of the ventral portion of the left lateral liver lobe were collected and fixed in 10% (v/v) neutral-buffered formalin. For immunohistochemical analysis, a portion of the left medial lobe of the liver was flash-frozen in isopentane cooled by liquid nitrogen.
Treatment with Inhibitors of p38, TACE, or Plasminogen Activator Inhibitor-1
A p38 inhibitor, SB 230963, or its vehicle (acidified PBS, pH 6.0) was administered (2.5 mg/kg i.v.) at the same time as RAN and again 2 h after RAN. Two administrations of SB 230963 were given because of its short half-life (Barone et al., 2001). A TACE inhibitor, BMS-561392 (60 mg/kg; also named DPC-333; provided by Bristol-Myers Squibb Co., Princeton, NJ) (Grootveld and McDermott, 2003; Qian et al., 2007), or its vehicle (1% Tween 80 + 0.5% methylcellulose in PBS), was administered 15 min before RAN orally. This dose was shown in a preliminary study to reduce LPS (2.5 × 106 EU/kg i.v.)-induced serum TNF-α increase in rats when given 30 min before LPS. A PAI-1 inhibitor WAY-140312 (10 mg/kg; provided by Wyeth Research, Philadelphia, PA) (Crandall et al., 2004) or its vehicle (2.0% Tween 80 + 0.5% methylcellulose in PBS) was administered orally 1 h before RAN and again 1 and 3 h after RAN.
Hepatotoxicity Assessment
Hepatic parenchymal cell injury was estimated as an increase in serum alanine aminotransferase (ALT) activity. ALT activity was determined spectrophotometrically using Infinity-ALT reagent from Thermo Electron Corporation (Louisville, CO). Previous studies in this LPS/RAN model have shown that serum ALT activity reflects histopathologic evidence of hepatocellular necrosis (Luyendyk et al., 2003).
Evaluation of Hepatic Phospho-p38 and Phospho-MK-2
A liver section of 5-mm length from the right lateral lobe was homogenized in 1 ml of lysis buffer (1 mM EDTA, 0.5% Triton X-100, 5 mM NaF, 6 M urea, 10 µg/ml leupeptin, 10 µg/ml pepstatin, 100 µM phenylmethylsulfonyl fluoride, 3 µg/ml aprotinin, 2.5 mM sodium pyrophosphate, and 1 mM activated sodium orthovanadate). After sitting on ice for 15 min, the tissue homogenates were sonicated for 10 s. After centrifugation at 14,000 rpm for 10 min, the supernatants were collected. Phospho-p38 and total p38 in the tissue homogenates were evaluated using a commercial enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN), and p38 activation was expressed as a ratio of phosphorylated p38 to total p38.
For MK-2 evaluation, the protein concentration of homogenates was determined by a Bradford assay (Bio-Rad, Hercules, CA). Ten micrograms of protein was loaded and separated by SDS-polyacrylamide gel electrophoresis. Phospho-MK-2 and total-MK-2 were detected by Western analysis using rabbit polyclonal anti phospho-MK-2 (Thr222) and anti total-MK-2 antibody (Cell Signaling Technology Inc., Danvers, MA) (both at 1:1000 dilution, overnight at 4°C). MK-2 activation was expressed as the density ratio of phospho-MK-2 to total MK-2.
Evaluation of Coagulation System Activation and Plasma PAI-1
Plasma thrombin-antithrombin (TAT) concentration was used as a marker for coagulation activation. Plasma TAT concentration was determined by an ELISA using a kit from Dade Behring Inc. (Deerfield, IL). The concentration of functionally active PAI-1 in plasma was assessed using a commercially available ELISA kit purchased from Molecular Innovations (Southfield, MI).
Serum TNF-α and MIP-2 Evaluation
Serum TNF-α concentration was evaluated using a commercial ELISA kit (BD Biosciences, San Diego, CA). Serum MIP-2 concentration was also evaluated by commercial ELISA kit (BioSource International, Camarillo, CA).
Fibrin Immunohistochemistry and Quantification
Fibrin immunohistochemistry and quantification were performed as described previously (Copple et al., 2002). This protocol solubilizes all fibrinogen and fibrin except for cross-linked fibrin (Schnitt et al., 1993). Therefore, only cross-linked fibrin stains in sections of liver. The positive area fraction refers to the fraction of area with positive staining in the total microscopic field.
Evaluation of Liver Polymorphonuclear Neutrophils and Polymorphonuclear Neutrophil Activation
PMNs accumulated in liver were visualized by immunohistochemical staining and quantified as described previously (Yee et al., 2003). PMN activation was measured by staining of hypochlorous acid (HOCl)-protein adducts in liver. The monoclonal antibody (clone 2D10G9; subtype IgG2bk) is specific for HOCl-modified epitopes generated in vivo (Malle et al., 1997) and in vitro (Malle et al., 1995). Clone 2D10G9 does not cross-react with other oxidative protein modifications. Frozen liver sections were fixed in 4% (v/v) formalin for 10 min at room temperature with gentle rocking. The slides were washed three times, 5 min each, with PBS, and then they were blocked for 1 h at room temperature with 3% (v/v) goat serum (Invitrogen, Carlsbad, CA) in PBS. Antibody (diluted 1:1 in 3% goat serum) was added and incubated for 2 h at room temperature with gentle rocking. The slides were washed three times, 5 min each, with PBS. Alexa Fluor 488-labeled goat anti-mouse secondary antibody (diluted 1:500 in 3% goat serum; Invitrogen) was applied, and the slides were incubated for 3 h at room temperature. After washing three times, 5 min each with PBS, they were examined microscopically. Staining was quantified as for fibrin staining described above and presented as positive area fraction.
Evaluation of Hepatic TNF-α mRNA and TACE Activity
Total RNA was extracted from frozen liver tissues using the MELT Total Nucleic Acid isolation system (Ambion, Austin, TX) according to the manufacturer’s instructions. First-strand cDNA was synthesized from isolated RNA using oligo(dT)12-18 primer and Superscript II reverse transcriptase (Invitrogen). In the real-time PCR step, cDNA was amplified using the TaqMan universal PCR master mix and TaqMan predeveloped gene expression assay reagents for rat TNF-α (Applied Biosystems, Foster City, CA). β-Actin was measured as an endogenous control, using TaqMan endogenous control assay Rat ACTB (VIC/MGB Probe; Primer Limited, Pismo Beach, CA). Quantification was conducted on the Applied Biosystems StepOne real-time PCR system, according to the manufacturer’s protocols. A standard curve for each gene was made of 4-fold serial dilutions of total RNA from one sample. The curve was then used to calculate the relative amounts of target mRNA in the samples. The ratio between TNF-α mRNA and β-actin was used as an indicator for TNF-α mRNA abundance. The TNF-α mRNA level in each liver sample was expressed as ratio versus one Veh/Veh-treated liver for Fig. 6 and versus one LPS/Veh/Veh-treated liver for Fig. 7B.
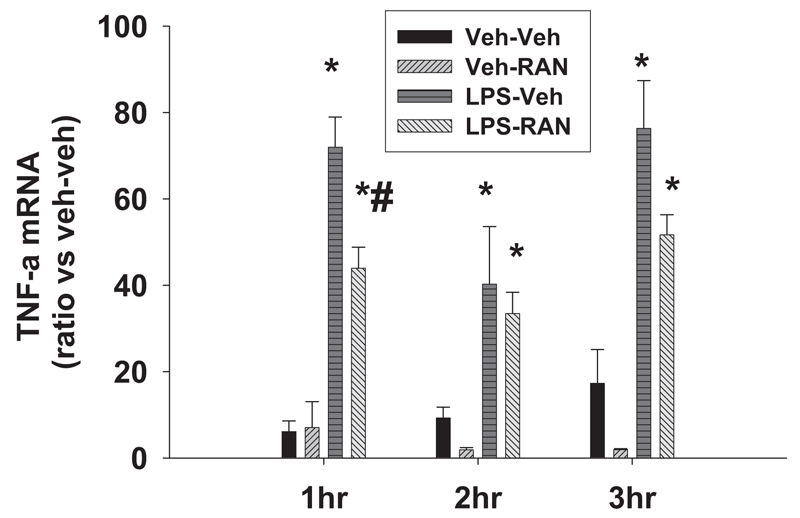
Fig. 6. Hepatic TNF-α mRNA after LPS/RAN treatment.
Rats were treated with LPS or Veh and cotreated with RAN or Veh as described in Fig. 1. Hepatic TNF-α mRNA was evaluated 1, 2, and 3 h after the drug treatment. *, significantly different from the respective treatment without LPS. #, significantly different from the respective treatment without RAN. n = 4 to 8.
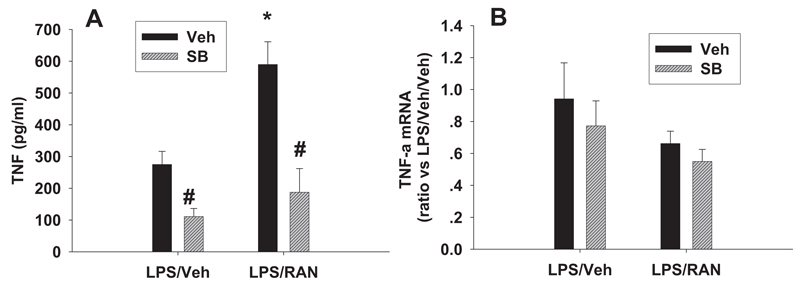
Fig. 7. Effects of a p38 inhibitor on serum TNF-α protein and hepatic TNF-α mRNA.
Rats were treated with LPS/RAN or LPS/Veh, and SB 239063 (SB) or its vehicle was also administered as described in Fig. 3. Serum TNF-α protein (A) and hepatic TNF-α mRNA (B) were evaluated at 2 h after RAN or Veh treatment. *, significantly different from the respective treatment without RAN. #, significantly different from the respective treatment without SB 239063. n = 4 to 7.
Protein extraction for TACE activity measurement was conducted as follows: Protein was extracted from liver tissue by homogenization in radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH 7.2, 150 mM NaCl, 1% deoxycholic acid, 1% Triton X-100, and 0.1% SDS) containing 10 mM 4-nitrophenyl phosphate, 20 mM β-glycerophosphate, 500 µM Pefabloc, 2 µg/ml aprotinin, 50 µM sodium orthovanadate, and 0.5 µg/ml leupeptin. Homogenization was followed by a 30-min tumbling period and a 10-min centrifugation at 14,000g (both at 4°C). Supernatant was collected, and protein concentration was determined by Bradford assay. The TACE activity was measured using a commercial TACE activity kit (Calbiochem, San Diego, CA).
Statistical Analysis
A two-way analysis of variance with Tukey’s post hoc test was used for all data analyzed, except where only two groups were present, in which case Student’s t test was applied. For all studies, results are expressed as mean ± S.E.M., and the criterion for statistical significance was p < 0.05.
Results
p38 Activation after LPS/RAN Treatment
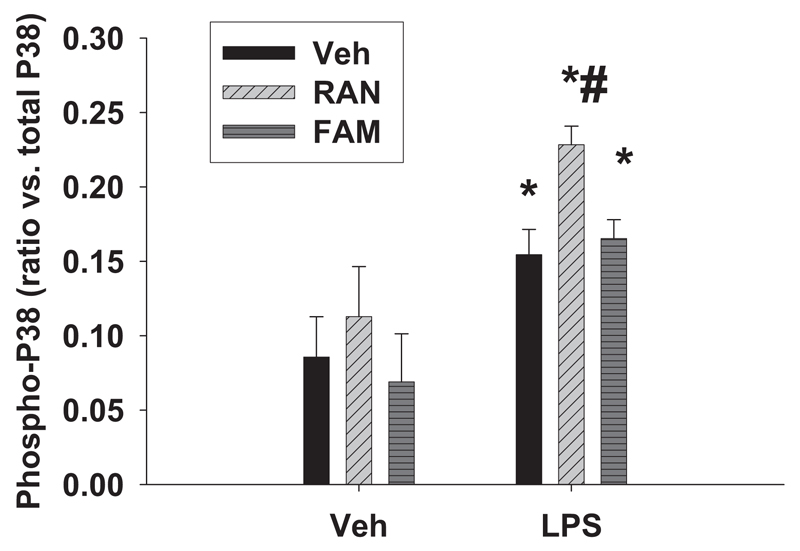
Because activation of MAPK is a potential upstream signal that leads to production of cytokines and subsequent cascades, we first tested whether activation of p38 occurred during LPS/RAN-induced liver injury. Rats were given either LPS or its vehicle and cotreated 2 h later with RAN, FAM (at a dose equiefficacious to RAN), or vehicle. Hepatic p38 activation was evaluated 1 h after drug treatment. Neither RAN nor FAM alone caused p38 activation as reflected by the ratio of phospho-p38 to total p38 (Fig. 1). LPS alone increased p38 activation. Treatment of LPS-exposed rats with RAN augmented p38 activation compared with LPS alone, whereas FAM cotreatment had no additional effect (Fig. 1).
Fig. 1. Activation of p38 MAPK after LPS/RAN treatment.
Rats were given either LPS or its vehicle and cotreated with RAN, FAM (at a dose pharmacologically equi-efficacious to RAN), or vehicle 2 h later. Hepatic p38 activation was evaluated 1 h after drug treatment by ELISA. *, significantly different from the respective treatment without LPS. #, significantly different from the respective treatment without the drug. n = 5 to 9.
Effects of p38 Inhibitor on Hepatotoxicity
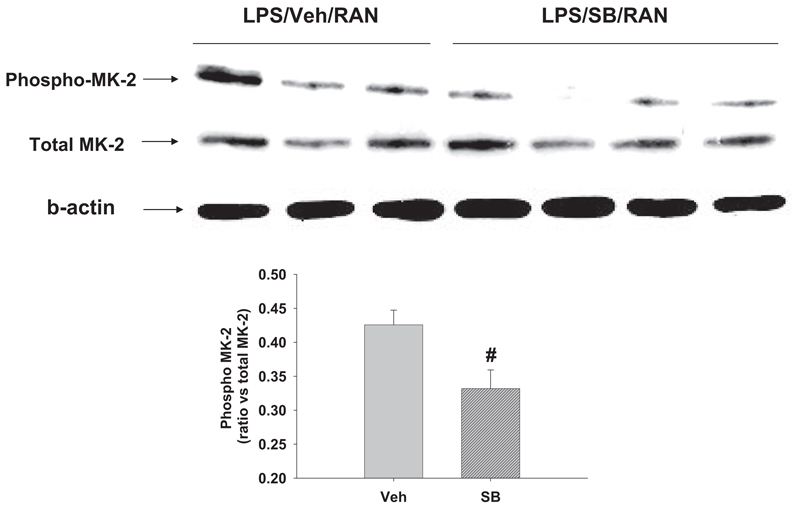
Because activation of MK-2 is a downstream consequence of p38 activation, we tested whether inhibition of p38 impaired phosphorylation of MK-2. For these studies, rats were given LPS and RAN as described above, and SB 239063, a p38 kinase inhibitor (Barone et al., 2001), was administered at the same time as RAN and again 2 h after RAN. Phosphorylation of hepatic MK-2 protein was measured 2 h after RAN treatment as an indicator of p38 activation, and serum ALT activity was evaluated at 6 h as a marker of hepatotoxicity. The p38 inhibitor reduced phospho-MK-2 protein (Fig. 2), indicating attenuation of p38 activation. The inhibitor also significantly reduced the increase in serum ALT activity (Fig. 3).
Fig. 2. Effects of a p38 inhibitor on MK-2 phosphorylation.
Rats were given LPS and RAN as described in Fig. 1. A p38 inhibitor, SB 239063 (SB), was administered at the same time as RAN and again 2 h later. Hepatic phospho-MK-2 protein and total MK-2 were measured 2 h after RAN treatment as an indicator of p38 activation. #, significantly different from the respective treatment without SB 239063. n = 3 to 4.
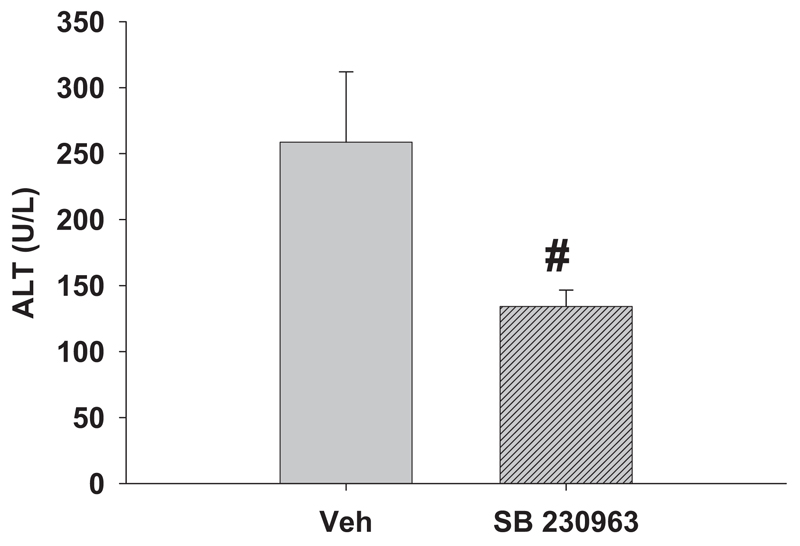
Fig. 3. Effect of a p38 inhibitor on serum ALT activity.
Rats were given LPS and RAN as in Fig. 1. SB 239063, was administered at the same time as RAN and again 2 h later. Serum ALT activity was evaluated 6 h after RAN. #, significantly different from the respective treatment without SB 239063. n = 5 to 7.
Effects of p38 Inhibitor on Biomarkers of Hemostasis and Liver PMNs
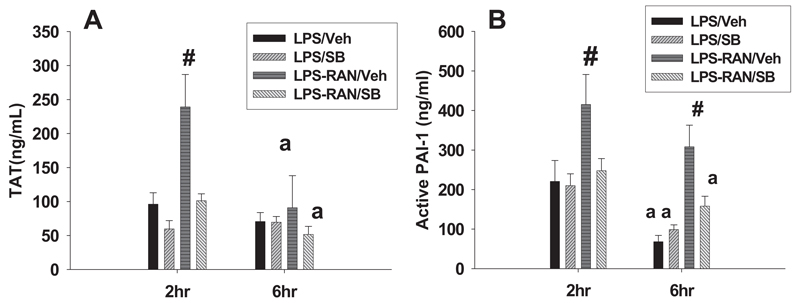
Since previous findings showed that the hemostatic system and PMN activation are crucial for liver injury caused by LPS/RAN (Luyendyk et al., 2004; Deng et al., 2007), the effect of p38 inhibition on these events was evaluated. Two hours and 6 h after RAN or its vehicle, TAT and active PAI-1 were measured as biomarkers of coagulation and fibrinolysis, respectively (Levi et al., 2003). LPS/RAN treatment caused a more pronounced increase in plasma TAT and active PAI-1 compared with LPS/Veh treatment 2 h after RAN (Fig. 4), data in line with previous results (Luyendyk et al., 2004). SB 239063 reduced both plasma TAT and active PAI-1 to the same levels as LPS/Veh treatment. At 6 h, active PAI-1 remained elevated after LPS/RAN treatment, and SB 239063 eliminated this increase. SB 239063 had no effect on plasma TAT or active PAI-1 after LPS/Veh treatment at either 2 or 6 h (Fig. 4).
Fig. 4. Effects of a p38 inhibitor on biomarkers of hemostasis.
Rats were treated with LPS, and 2 h later they were treated with RAN or its vehicle. SB 239063 or its vehicle was administered as in Fig. 3. TAT (A) and active PAI-1 (B) were measured 2 and 6 h after RAN or its vehicle. #, significantly different from all the groups at the same time. a, significantly different from the respective treatment at 2 h. n = 4 to 6.
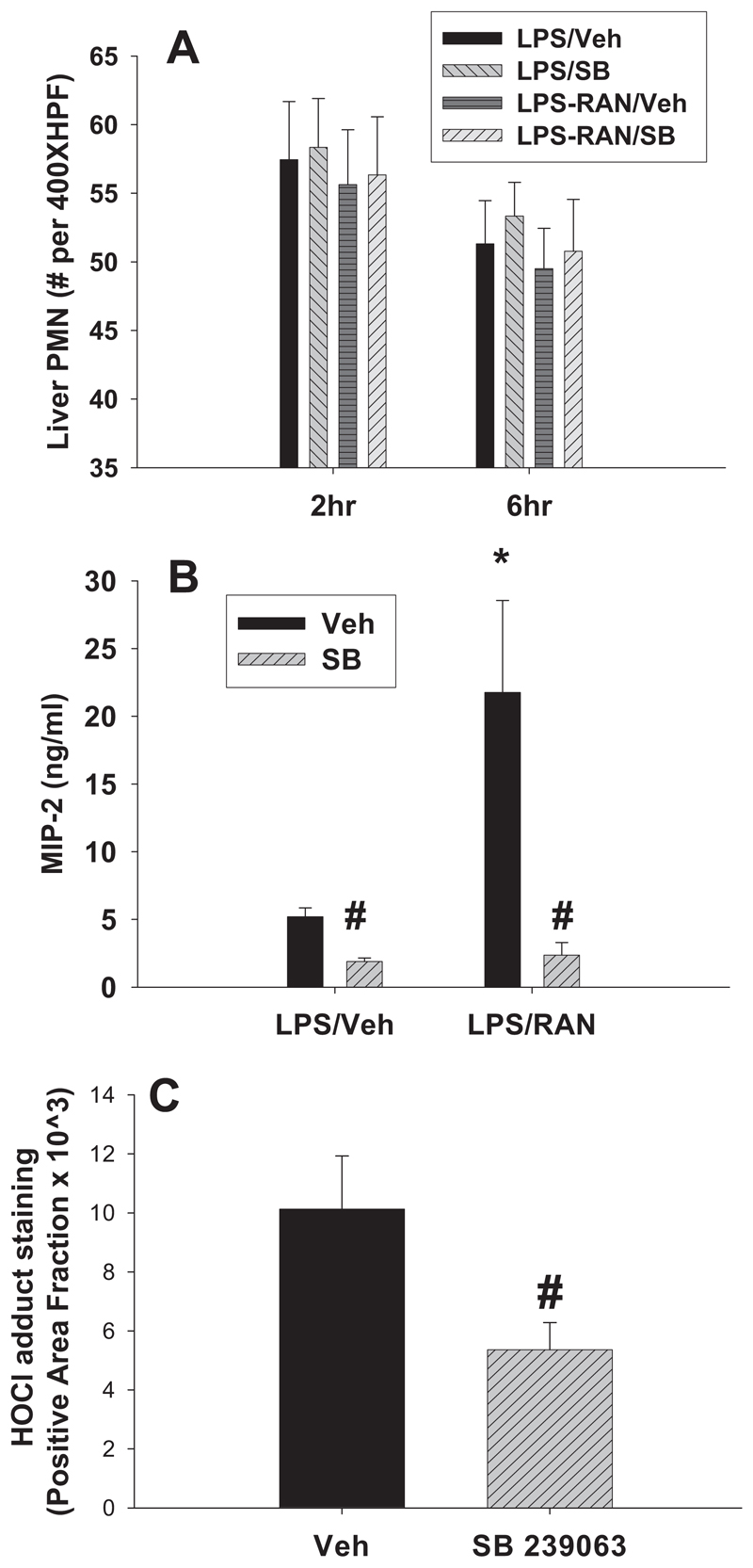
In inflammatory liver injury, PMNs accumulate in sinusoids, and then they transmigrate into parenchyma in response to stimuli and become activated to injure hepatocytes (Springer, 1994). LPS/RAN treatment did not change hepatic PMN accumulation compared with LPS/Veh treatment at either 2 or 6 h, and SB 239063 had no effect on hepatic PMN accumulation (Fig. 5A). Serum MIP-2, a PMN chemokine, was elevated at 2 h by RAN in LPS-treated rats, and this increase was prevented by SB 239063 (Fig. 5B). Upon activation of PMNs, hydrogen peroxide is converted to the potent oxidant HOCl by myoperoxidase in the presence of physiological chloride concentrations. Therefore, the occurrence of HOCl-modified epitopes/proteins can be considered a footprint for activated PMNs. HOCl adduct staining was measured 6 h after RAN (Fig. 5C), since PMNs are not activated until 3 h after RAN (Deng et al., 2007). SB 239063 significantly reduced HOCl adduct staining, suggesting attenuation of PMN activation.
Fig. 5. Effects of a p38 inhibitor on PMN biomarkers.
Rats were treated with LPS, and 2 h later they were treated with RAN or its vehicle. SB 239063 (SB) or its vehicle was administered as in Fig. 3. A, PMNs in the liver were enumerated 2 and 6 h after RAN or its vehicle. B, serum MIP-2 was measured 2 h after RAN or its vehicle. C, staining for HOCl-modified epitopes/proteins was measured 6 h after RAN. *, significantly different from the respective treatment without RAN. #, significantly different from the respective treatment without SB 239063. n = 4 to 6.
Hepatic TNF-αmRNA after LPS/RAN Treatment
For the next set of experiments, rats were treated with LPS or Veh and cotreated with RAN or Veh as in Fig. 1. Hepatic TNF-α mRNA was evaluated 1, 2, and 3 h after drug treatment. RAN alone did not affect expression of TNF-α mRNA at any of the times examined (Fig. 6). By contrast, LPS alone increased TNF-α mRNA at all the times examined. LPS/RAN treatment decreased TNF-α mRNA compared with LPS/Veh treatment at 1 h after RAN, but it had no significant effect at 2 or 3 h (Fig. 6).
Effects of p38 Inhibitor on Serum TNF-α Protein and Hepatic TNF-α mRNA after LPS/RAN Treatment
Rats were treated with LPS/RAN or LPS/Veh, and SB 239063 or its vehicle was administered as shown in Fig. 4. Serum TNF-α protein and hepatic TNF-α mRNA was evaluated at 2 h after RAN or Veh treatment. LPS/RAN treatment caused a significant increase in serum TNF-α concentration compared with LPS/Veh treatment (Fig. 7A). SB 239063 reduced serum TNF-α concentration both after LPS/RAN and after LPS/Veh treatment. In contrast, LPS/RAN treatment did not affect hepatic TNF-α mRNA compared with LPS treatment, and SB 239063 did not change TNF-α mRNA expression after either treatment (Fig. 7B).
Hepatic TACE Activity after LPS/RAN Treatment
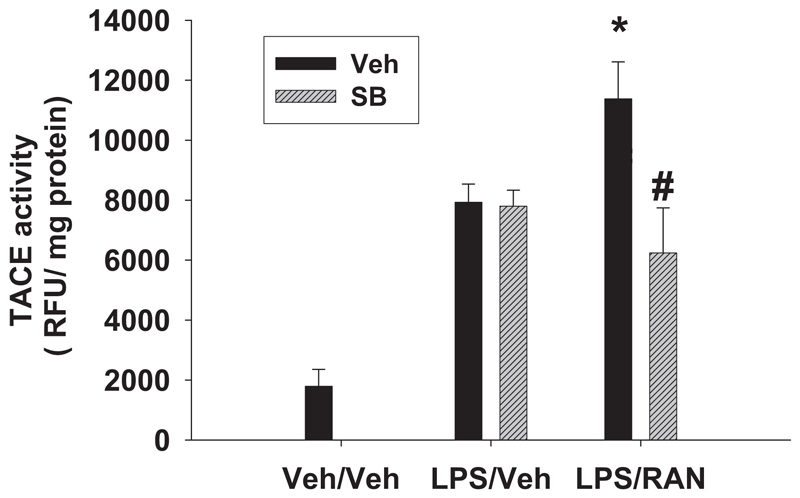
Given that SB 239063 did not reduce TNF-α mRNA in liver, a post-transcriptional mode of action could be involved, perhaps through TACE. Therefore, hepatic TACE activity was measured 2 h after RAN or Veh treatment. LPS/Veh treatment caused a significant increase in hepatic TACE activity compared with Veh/Veh treatment (Fig. 8). RAN treatment enhanced TACE activity in livers of LPS-treated rats. SB 239063 had no effect on hepatic TACE activity after LPS/Veh treatment, but it reduced TACE activity after LPS/RAN treatment to the same level as LPS/Veh treatment.
Fig. 8. Hepatic TACE activity after LPS/RAN treatment.
Rats were treated with LPS/RAN or LPS/Veh, and SB 239063 (SB) or its vehicle was also administered as in Fig. 4. Hepatic TACE activity was measured 2 h after RAN or Veh treatment. *, significantly different from the respective treatment without RAN. #, significantly different from the respective treatment without the SB compound. n = 4 to 7.
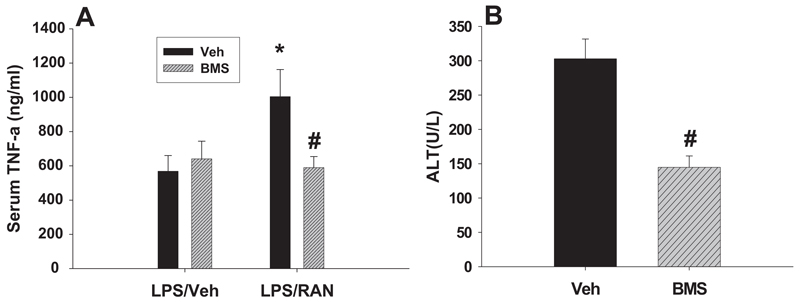
Effects of TACE Inhibitor on Serum TNF-α and Liver Injury
To confirm that TACE is involved in this process, a selective TACE inhibitor (BMS-561392; DPC-333) or its vehicle was administered 15 min before RAN or its vehicle. Serum TNF-α concentration was measured 1 h after RAN treatment, and serum ALT activity was measured at 6 h. LPS/RAN treatment caused a significant increase in serum TNF-α concentration compared with LPS/Veh treatment (Fig. 9A), confirming previous results (Tukov et al., 2007). BMS-561392 did not affect serum TNF-α concentration after LPS/Veh treatment. By contrast, it decreased serum TNF-α concentration after LPS/RAN treatment to the same level as LPS/Veh treatment at both 1 h (Fig. 9A) and 2 h (data not shown). BMS-561392 also reduced serum ALT activity after LPS/RAN treatment, indicating attenuation of liver injury (Fig. 9B).
Fig. 9. Effects of a TACE inhibitor on serum TNF-α and liver injury.
Rats were treated with LPS/RAN or LPS/Veh as described in Fig. 3. The selective TACE inhibitor BMS-561392 (BMS) or its vehicle was administered 15 min before RAN or its vehicle. Serum TNF-α was measured 1 h after RAN treatment (A), and serum ALT activity was measured at 6 h (B). *, significantly different from the respective treatment without RAN. #, significantly different from the respective treatment without BMS-561392. n = 6 to 10.
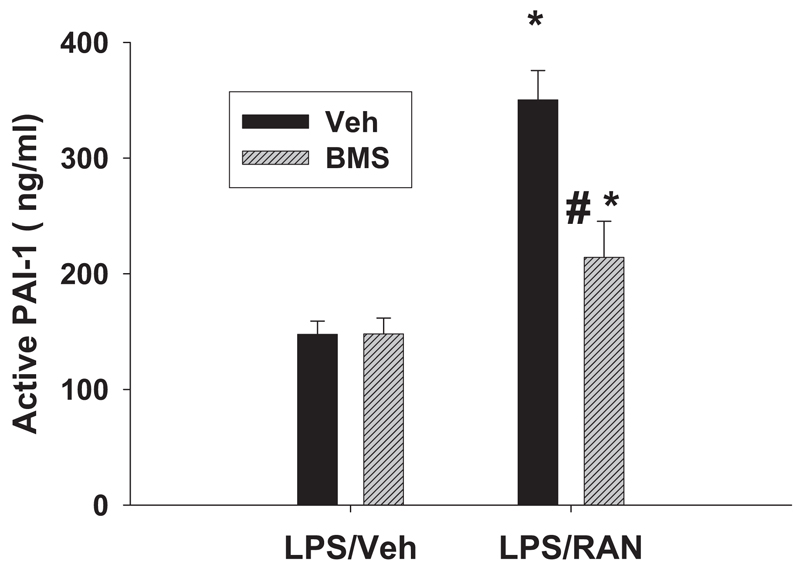
Effects of TACE Inhibitor on Plasma PAI-1
To determine whether TACE is involved in the hemostasis, rats were treated with LPS/RAN or LPS/Veh and with the TACE inhibitor as shown in Fig. 9. Since previous results showed that TNF-α was important for PAI-1 production (Tukov et al., 2007), plasma active PAI-1 concentration was measured 2 h after RAN treatment. Confirming previous results (Luyendyk et al., 2006), LPS/RAN treatment caused a significant increase in plasma active PAI-1 concentration compared with LPS/Veh treatment (Fig. 10). The TACE inhibitor decreased plasma active PAI-1 concentration after LPS/RAN treatment to almost the same level as LPS/Veh treatment.
Fig. 10. Effects of TACE inhibitor on plasma active PAI-1.
Rats were treated with LPS/RAN or LPS/Veh and with the TACE inhibitor as in Fig. 9. Plasma active PAI-1 was evaluated 2 h after RAN treatment. *, significantly different from the respective treatment without RAN. #, significantly different from the respective treatment without BMS-561392 (BMS). n = 6 to 10.
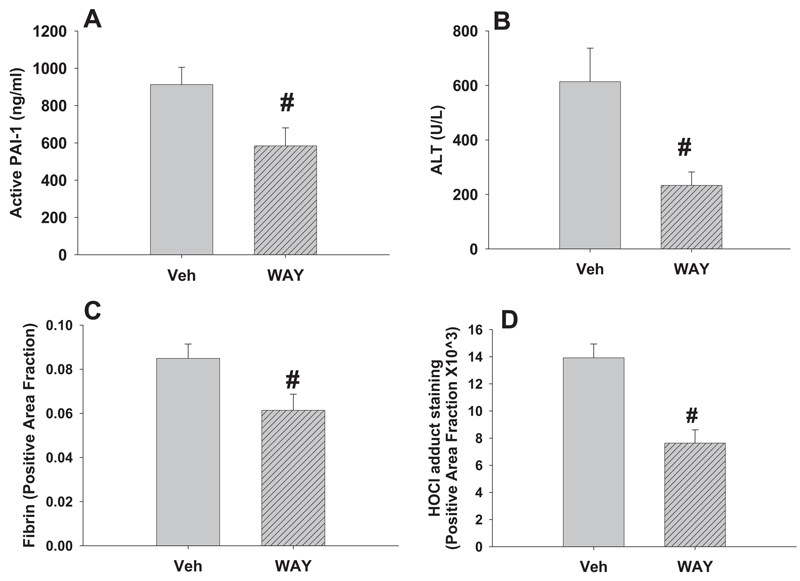
Effects of PAI-1 Inhibitor on Hepatotoxicity and Markers of Hemostasis and PMNs
To explore the involvement of PAI-1, a PAI-1 inhibitor (WAY-140312) was administered 1 h before RAN and again 1 and 3 h later. Hepatotoxicity, plasma active PAI-1 concentration, hepatic fibrin deposition, and liver HOCl adduct were measured 6 h after RAN treatment. WAY-140312 reduced plasma active PAI-1 concentration and serum ALT activity (Fig. 11, A and B). It also decreased hepatic fibrin deposition and staining for HOCl-modified epitopes/proteins (Fig. 11, C and D). The PAI-1 inhibitor did not affect hepatic PMN accumulation or serum cytokine-induced neutrophil chemoattractant-1 or MIP-2 concentrations after LPS/RAN treatment (data not shown).
Fig. 11. Effects of a PAI-1 inhibitor on hepatotoxicity and markers of hemostasis and PMN activation.
Rats were treated with LPS and cotreated with RAN 2 h later. A PAI-1 inhibitor, WAY-140312 (WAY), was administered 1 h before RAN and again 1 and 3 h later. Hepatotoxicity, plasma active PAI-1, hepatic fibrin deposition, and hepatic staining for HOCl-modified proteins were measured 6 h after RAN treatment. #, significantly different from the respective treatment without WAY-140312. n = 6 to 9.
Discussion
At the dose used in the present study, LPS rapidly induced serum TNF-α production but failed to cause liver injury (Tukov et al., 2007). The serum TNF-α concentration peaked at approximately 1.5 h and rapidly decreased after that, returning almost to normal by 8 h. This indicates that an increase in TNF-α of this magnitude and duration is not sufficient to cause hepatocellular damage. RAN-cotreated rats had a longer lasting serum TNF-α increase compared with rats given LPS alone (Tukov et al., 2007). Pretreatment with agents that reduced serum TNF-α (i.e., pentoxifylline oretanercept) protected rats from LPS/RAN-induced liver injury, suggesting the necessity of TNF-α in producing the hepatocellular injury. However, because these agents were given before the LPS challenge, those results could not differentiate whether the LPS-induced TNF-α elevation or the RAN-induced prolongation of the TNF-α response to LPS was crucial for the pathogenesis. In addition, the mechanism of RAN-induced prolongation of TNF-α production after LPS exposure remained unknown.
Activation of the MAPK p38 and its downstream kinase MK-2 promote biosynthesis of several cytokines and chemokines, including TNF-α (Neininger et al., 2002; Numahata et al., 2003; Hitti et al., 2006). It is interesting that the p38-dependent cytokine/chemokines, such as TNF-α, MIP-2, and interleukin-6, are selectively up-regulated in LPS/RAN-treated rats compared with rats treated with LPS/FAM or only with LPS (Luyendyk et al., 2006; Tukov et al., 2007). Furthermore, TNF-α has been shown to be critical for activation of the coagulation system, PMN chemokine production, and subsequent hepatotoxicity after LPS/RAN treatment (Tukov et al., 2007). All of the above-mentioned results suggested the possibility that p38 MAPK activation might be an upstream signal leading to the pathogenic cascade in LPS/RAN hepatotoxicity. Indeed, RAN, but not FAM, selectively augmented p38 activation early after LPS treatment. A p38 inhibitor given at the same time as the drug reduced the hepatotoxicity. This suggests that activation of p38 after RAN cotreatment in LPS-treated rats is critical for the liver injury.
How RAN enhances the p38/MK-2 pathway is currently unclear. A previous study (Luyendyk et al., 2006) revealed that MK-2 mRNA was selectively and markedly increased by RAN in LPS-cotreated rats at a time before the onset of liver injury. Accordingly, enhanced transcription might contribute to the effects that are mediated through p38/MK-2 signaling. However, post-translational events, such as enhanced p38 phosphorylation and kinase activity, seem also to be important (Figs. 1 and 2).
The p38 inhibitor SB 239063 also reduced activation of the hemostatic system and PMN activation. The effect on the hemostatic system was reflected in the reduction of plasma TAT and PAI-1 concentration, whereas the effect on PMN activation was marked by the reduction in serum MIP-2 concentration and hepatic staining of HOCl-modified epitopes/proteins. Similar effects were observed after treatment with agents that reduced serum TNF-α, such as pentoxifylline or etanercept (Tukov et al., 2007). These results are consistent with the possibility that p38 activation was responsible for the prolonged TNF-α production caused by RAN cotreatment and that TNF-α precipitated the downstream effects on the hemostatic system and PMNs.
After 1 to 3 h, RAN did not enhance the hepatic TNF-α mRNA level after LPS treatment, but TNF protein released into plasma increased during this time (Tukov et al., 2007). This suggests that a post-transcriptional event led to the enhancement of TNF release by RAN. This event could be increased translation of pro-TNF mRNA, enhanced cleavage of pro-TNF and consequent release of TNF-α, decreased elimination of TNF-α, or a combination. Moreover, the reduction in serum TNF-α protein concentration after inhibition of p38 MAPK was not accompanied by diminished hepatic TNF-α mRNA, suggesting that p38 regulated TNF-α production after RAN treatment in a post-transcriptional manner. p38 and its downstream activation of MK-2 have been shown to regulate TNF-α production in macrophages mostly by increasing mRNA translation (Neininger et al., 2002; Hitti et al., 2006). This was mediated by the AU-rich 3̓-untranslated region of TNF-α mRNA. This result is consistent with our finding that a p38 inhibitor reduced TNF-α production in a post-transcriptional manner.
As mentioned in the introduction, an increase in TNF-α protein can arise from the cleavage of pro-TNF-α by TACE (Aggarwal et al., 1985; Müllberg et al., 2000). It is unlikely that the increase in TACE activity occurred because of an increase in TACE mRNA, since a previous study (Luyendyk et al., 2006) found no enhancement of TACE mRNA by RAN in LPS-treated rats. A more likely possibility is that p38 activates TACE, leading to increased TNF-α release into the circulation from Kupffer cells or other nonparenchymal cells in liver. Indeed, p38 is essential for ectodomain shedding of TNF-α in Chinese hamster ovary cells (Fan and Derynck, 1999). In the present study, LPS/RAN treatment increased hepatic TACE activity compared with LPS/Veh treatment (Fig. 8). The p38 inhibitor reduced hepatic TACE activity after LPS/RAN treatment to the same level as LPS/Veh treatment. Moreover, a TACE inhibitor similarly reduced serum TNF-α concentration and liver injury. Together, these results suggest that RAN prolonged TNF-α production after LPS treatment mainly through augmented p38-dependent TACE activity. However, we cannot rule out a contribution from p38-mediated enhancement of TNF-α translation.
As mentioned above, previous results could not differentiate whether the LPS-induced TNF elevation or the RAN-induced prolongation of the TNF-α response to LPS was crucial for the pathogenesis (Tukov et al., 2007). In the present study, the TACE inhibitor was given immediately before RAN treatment so that the inhibitor did not affect serum TNF-α level until after RAN was administered. This treatment regimen was effective in reducing hepatocellular injury, suggesting that the RAN-induced prolongation of the TNF-α response was critical for LPS/RAN-induced hepatotoxicity. In contrast to RAN, FAM did not prolong the TNF-α response or cause liver injury after LPS treatment. Thus, the increase in LPS-stimulated TNF-α production could distinguish a drug that causes human IADRs from one that does not.
In human case reports of RAN-induced idiosyncratic hepatotoxicity, no direct evidence for enhanced serum TNF-α or other cytokines has been reported. However, most clinical samples are taken after hepatotoxicity develops, when the peak of TNF-α has probably passed. Furthermore, it is interesting that in 24 of 34 human cases of RAN idiosyncratic hepatotoxicity, prodromal signs consistent with endotoxemia (i.e., increased LPS concentration in blood) or inflammation (e.g., diarrhea, fever, nausea/vomiting, and/or abdominal pain) were present (Luyendyk et al., 2003). These clinical signs are consistent with increased production of TNF-α and other inflammatory cytokines.
The TACE inhibitor also reduced plasma active PAI-1 concentration after LPS/RAN treatment to almost the same level as after LPS/Veh treatment, suggesting enhanced PAI-1 production as a possible downstream effect of RAN-augmented TNF-α production. Since PAI-1 is an important negative regulator of the fibrinolytic system (Levi et al., 2003), this is consistent with the previous finding that fibrin deposition resulting from the perturbed hemostatic system is crucial for the liver injury caused by LPS/RAN (Luyendyk et al., 2004). Indeed, in the present study a PAI-1 inhibitor reduced the hepatocellular injury caused by LPS/RAN cotreatment. The inhibitor also reduced hepatic fibrin deposition and PMN activation. Fibrin deposition can lead to hypoxia, which occurs early in this model (Luyendyk et al., 2005; Deng et al., 2007). Hypoxia could potentiate the killing of hepatocytes by proteases (e.g., elastase) released from PMNs after their activation (Luyendyk et al., 2005). Indeed, proteases released from activated PMNs have been shown to be important in the pathogenesis (Deng et al., 2007).
Although the PAI-1 inhibitor decreased PMN activation, it did not affect hepatic PMN accumulation or serum PMN chemokine concentration, suggesting a direct effect of PAI-1 on PMN activation. A recent study showed that PAI-1 directly potentiates LPS-induced PMN activation through a Jun-NH2-terminal kinase-dependent pathway (Kwak et al., 2006). In the LPS/RAN model, LPS causes PMN accumulation in the liver, and RAN somehow activates the hepatic PMNs (Deng et al., 2007). RAN itself does not directly enhance PMN activation. In fact, it has been shown to reduce PMN activation in vitro (Okajima et al., 2000, 2002). Together, these results suggest that RAN induces activation of PMNs accumulated in the liver after LPS exposure indirectly by augmenting PAI-1 production. Polymorphisms in PAI-1 in the human population have been identified (Lane and Grant, 2000), and thus PAI-1 could represent a potential interaction between genetic and environmental factors (i.e., inflammatory stress) in RAN-induced IADRs. For example, patients with a more active PAI-1-producing allele might be more susceptible to RAN-induced IADRs caused by endotoxin exposure or some other inflammatory stress.
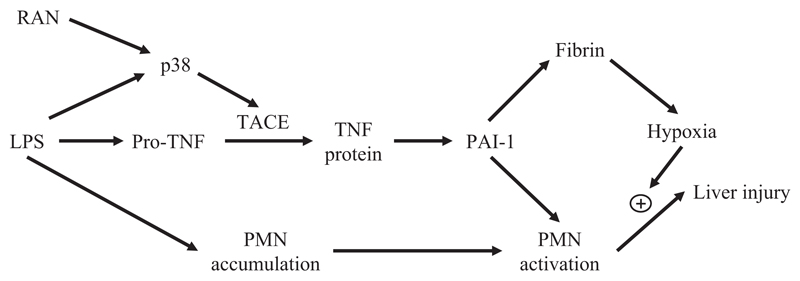
As summarized in Fig. 12, RAN augmented TNF-α production after LPS treatment in a post-transcriptional manner by enhancing p38 activation. The increase in TNF-α protein occurs through the p38-dependent activation of TACE. The prolongation of LPS-induced TNF-α production by RAN seems to be crucial for the liver injury caused by LPS/RAN cotreatment. Also involved in the injury is PAI-1, which enhances hepatic fibrin deposition and activates the PMNs accumulated in liver caused by LPS exposure. The hypoxia resulting from hepatic fibrin deposition could act synergistically with toxic proteases released from activated PMNs to kill hepatocytes.
Fig. 12. Diagram of pathogenic mechanism contributing to hepatocellular injury in the LPS/RAN model.
RAN augments TNF-α production after LPS treatment in a post-transcriptional manner by enhancing p38 activation. The increase in TNF-α protein seems to occur through the p38-dependent activation of TACE. The prolongation of LPS-induced TNF-α production by RAN causes more PAI-1 production, which enhances hepatic fibrin deposition and activates the PMNs accumulated in liver in response to LPS exposure. The hypoxia resulting from hepatic fibrin deposition could act synergistically with toxic proteases released from activated PMNs to kill hepatocytes.
ABBREVIATIONS
- IADR
idiosyncratic adverse drug reaction
- H2
histamine 2
- RAN
ranitidine
- LPS
lipopolysaccharide
- FAM
famotidine
- TNF
tumor necrosis factor
- MAPK
mitogen-activated protein kinase
- TACE
tumor necrosis factor-α-converting enzyme
- MK-2
mitogen-activated protein kinase-activated protein kinase 2
- MIP-2
macrophage inflammatory protein-2
- EU
endotoxin units
- Veh
vehicle
- PBS
phosphate-buffered saline
- PAI
plasminogen activator inhibitor
- ALT
alanine aminotransferase
- ELISA
enzyme-linked immunosorbent assay
- TAT
thrombin-antithrombin
- PMN
polymorphonuclear neutrophil
- PCR
polymerase chain reaction
- HOCl
hypochlorous acid
- SB 239063
trans-1-(4-hydroxy-cyclohexyl)-4-(4-fluorophenyl)-5-(2-methoxypyridimidin-4-yl) imidazole, C20H21N4O2F
- BMS-561392
(2R)-2-(((3R)-3-amino-3{4-[2-methyl-4-quinolinyl)methoxy]phenyl}-2-oxopyrrolidinyl)-N-hydroxy-4-methylpentanamide
- WAY-140312
[5-bromo-6-(1H-tetrazol-5-ylmethoxy)-naphthalen-2-yl]-(2-butyl-benzofuran-3-yl)-methanone
Footnotes
This work was supported by National Institutes of Health Grant DK061315 and the Austrian Science Fund FWF P19074-B05.
References
- Aggarwal BB, Kohr WJ, Hass PE, Moffat B, Spencer SA, Henzel WJ, Bringman TS, Nedwin GE, Goeddel DV, Harkins RN. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985;260:2345–2354. [PubMed] [Google Scholar]
- Barone FC, Irving EA, Ray AM, Lee JC, Kassis S, Kumar S, Badger AM, White RF, McVey MJ, Legos JJ, et al. SB 239063, a second-generation p38 mitogen-activated protein kinase inhibitor, reduces brain injury and neurological deficits in cerebral focal ischemia. J Pharmacol Exp Ther. 2001;296:312–321. [PubMed] [Google Scholar]
- Bourdet DL, Pritchard JB, Thakker DR. Differential substrate and inhibitory activities of ranitidine and famotidine toward human organic cation transporter 1 (hOCT1; SLC22A1), hOCT2 (SLC22A2), and hOCT3 (SLC22A3) J Pharmacol Exp Ther. 2005;315:1288–1297. doi: 10.1124/jpet.105.091223. [DOI] [PubMed] [Google Scholar]
- Cherqui B, Desaint B, Legendre C, Levy VG. Fatal hepatitis in a female patient treated with ranitidine. Gastroenterol Clin Biol. 1989;13:952–953. [PubMed] [Google Scholar]
- Copple BL, Banes A, Ganey PE, Roth RA. Endothelial cell injury and fibrin deposition in rat liver after monocrotaline exposure. Toxicol Sci. 2002;65:309–318. doi: 10.1093/toxsci/65.2.309. [DOI] [PubMed] [Google Scholar]
- Crandall DL, Elokdah H, Di L, Hennan JK, Gorlatova NV, Lawrence DA. Characterization and comparative evaluation of a structurally unique PAI-1 inhibitor exhibiting oral in-vivo efficacy. J Thromb Haemost. 2004;2:1422–1428. doi: 10.1111/j.1538-7836.2004.00829.x. [DOI] [PubMed] [Google Scholar]
- Deng X, Luyendyk JP, Zou W, Lu J, Malle E, Ganey PE, Roth RA. Neutrophil interaction with the hemostatic system contributes to liver injury in rats cotreated with lipopolysaccharide and ranitidine. J Pharmacol Exp Ther. 2007;322:852–861. doi: 10.1124/jpet.107.122069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Derynck R. Ectodomain shedding of TGF-alpha and other transmembrane proteins is induced by receptor tyrosine kinase activation and MAP kinase signaling cascades. EMBO J. 1999;18:6962–6972. doi: 10.1093/emboj/18.24.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AA, Le Couteur DG. Nephrotoxicity and hepatotoxicity of histamine H2 receptor antagonists. Drug Saf. 2001;24:39–57. doi: 10.2165/00002018-200124010-00004. [DOI] [PubMed] [Google Scholar]
- Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994;370:555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- Grootveld M, McDermott MF. BMS-561392. Bristol-Myers Squibb. Curr Opin Investig Drugs. 2003;4:598–602. [PubMed] [Google Scholar]
- Halparin LS. Adverse effects of ranitidine therapy. Can Med Assoc J. 1984;130:668–672. [PMC free article] [PubMed] [Google Scholar]
- Hiesse C, Cantarovich M, Santelli C, Francais P, Charpentier B, Fries D, Buffet C. Ranitidine hepatotoxicity in renal transplant patient. Lancet. 1985;1:1280. doi: 10.1016/s0140-6736(85)92354-2. [DOI] [PubMed] [Google Scholar]
- Hitti E, Iakovleva T, Brook M, Deppenmeier S, Gruber AD, Radzioch D, Clark AR, Blackshear PJ, Kotlyarov A, Gaestel M. Mitogen-activated protein kinase-activated protein kinase 2 regulates tumor necrosis factor mRNA stability and translation mainly by altering tristetraprolin expression, stability, and binding to adenine/uridine-rich element. Mol Cell Biol. 2006;26:2399–2407. doi: 10.1128/MCB.26.6.2399-2407.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Kwak SH, Wang XQ, He Q, Fang WF, Mitra S, Bdeir K, Ploplis VA, Xu Z, Idell S, Cines D, et al. Plasminogen activator inhibitor-1 potentiates LPS-induced neutrophil activation through a JNK-mediated pathway. Thromb Haemost. 2006;95:829–835. [PubMed] [Google Scholar]
- Lane DA, Grant PJ. Role of hemostatic gene polymorphisms in venous and arterial thrombotic disease. Blood. 2000;95:1517–1532. [PubMed] [Google Scholar]
- Levi M, Keller TT, van GE, ten CH. Infection and inflammation and the coagulation system. Cardiovasc Res. 2003;60:26–39. doi: 10.1016/s0008-6363(02)00857-x. [DOI] [PubMed] [Google Scholar]
- Luyendyk JP, Maddox JF, Green CD, Ganey PE, Roth RA. Role of hepatic fibrin in idiosyncrasy-like liver injury from lipopolysaccharide-ranitidine coexposure in rats. Hepatology. 2004;40:1342–1351. doi: 10.1002/hep.20492. [DOI] [PubMed] [Google Scholar]
- Luyendyk JP, Lehman-McKeeman LD, Nelson DM, Bhaskaran VM, Reilly TP, Car BD, Cantor GH, Maddox JF, Ganey PE, Roth RA. Unique gene expression and hepatocellular injury in the lipopolysaccharide-ranitidine drug idiosyncrasy rat model: comparison with famotidine. Toxicol Sci. 2006;90:569–585. doi: 10.1093/toxsci/kfj103. [DOI] [PubMed] [Google Scholar]
- Luyendyk JP, Maddox JF, Cosma GN, Ganey PE, Cockerell GL, Roth RA. Ranitidine treatment during a modest inflammatory response precipitates idiosyncrasy-like liver injury in rats. J Pharmacol Exp Ther. 2003;307:9–16. doi: 10.1124/jpet.103.054288. [DOI] [PubMed] [Google Scholar]
- Luyendyk JP, Shaw PJ, Green CD, Maddox JF, Ganey PE, Roth RA. Coagulation-mediated hypoxia and neutrophil-dependent hepatic injury in rats given lipopolysaccharide and ranitidine. J Pharmacol Exp Ther. 2005;314:1023–1031. doi: 10.1124/jpet.105.087981. [DOI] [PubMed] [Google Scholar]
- Malle E, Woenckhaus C, Waeg G, Esterbauer H, Grone EF, Grone HJ. Immunological evidence for hypochlorite-modified proteins in human kidney. Am J Pathol. 1997;150:603–615. [PMC free article] [PubMed] [Google Scholar]
- Malle E, Hazell L, Stocker R, Sattler W, Esterbauer H, Waeg G. Immunologic detection and measurement of hypochlorite-modified LDL with specific monoclonal antibodies. Arterioscler Thromb Vasc Biol. 1995;15:982–989. doi: 10.1161/01.atv.15.7.982. [DOI] [PubMed] [Google Scholar]
- Mohler KM, Sleath PR, Fitzner JN, Cerretti DP, Alderson M, Kerwar SS, Torrance DS, Otten-Evans C, Greenstreet T, Weerawarna K. Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature. 1994;370:218–220. doi: 10.1038/370218a0. [DOI] [PubMed] [Google Scholar]
- Müllberg J, Althoff K, Jostock T, Rose-John S. The importance of shedding of membrane proteins for cytokine biology. Eur Cytokine Netw. 2000;11:27–38. [PubMed] [Google Scholar]
- Neininger A, Kontoyiannis D, Kotlyarov A, Winzen R, Eckert R, Volk HD, Holtmann H, Kollias G, Gaestel M. MK2 targets AU-rich elements and regulates biosynthesis of tumor necrosis factor and interleukin-6 independently at different post-transcriptional levels. J Biol Chem. 2002;277:3065–3068. doi: 10.1074/jbc.C100685200. [DOI] [PubMed] [Google Scholar]
- Nick JA, Avdi NJ, Young SK, Knall C, Gerwins P, Johnson GL, Worthen GS. Common and distinct intracellular signaling pathways in human neutrophils utilized by platelet activating factor and FMLP. J Clin Invest. 1997;99:975–986. doi: 10.1172/JCI119263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numahata K, Komagata T, Hirasawa N, Someya K, Xiao YQ, Ohuchi K. Analysis of the mechanism regulating the stability of rat macrophage inflammatory protein-2 mRNA in RBL-2H3 cells. J Cell Biochem. 2003;90:976–986. doi: 10.1002/jcb.10710. [DOI] [PubMed] [Google Scholar]
- Okajima K, Harada N, Uchiba M. Ranitidine reduces ischemia/reperfusion-induced liver injury in rats by inhibiting neutrophil activation. J Pharmacol Exp Ther. 2002;301:1157–1165. doi: 10.1124/jpet.301.3.1157. [DOI] [PubMed] [Google Scholar]
- Okajima K, Murakami K, Liu W, Uchiba M. Inhibition of neutrophil activation by ranitidine contributes to prevent stress-induced gastric mucosal injury in rats. Crit Care Med. 2000;28:2858–2865. doi: 10.1097/00003246-200008000-00029. [DOI] [PubMed] [Google Scholar]
- Qian M, Bai SA, Brogdon B, Wu JT, Liu RQ, Covington MB, Vaddi K, Newton RC, Fossler MJ, Garner CE, et al. Pharmacokinetics and pharmacodynamics of DPC 333 ((2R)-2-((3R)-3-amino-3{4-[2-methyl-4-quinolinyl) methoxy] phenyl}-2-oxopyrrolidinyl)-N-hydroxy-4-methylpentanamide)), a potent and selective inhibitor of tumor necrosis factor α-converting enzyme in rodents, dogs, chimpanzees, and humans. Drug Metab Dispos. 2007;35:1916–1925. doi: 10.1124/dmd.107.015933. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Lucas M, Baptista A, Victorino RM. Fatal hepatitis associated with ranitidine. Am J Gastroenterol. 2000;95:559–560. doi: 10.1111/j.1572-0241.2000.t01-1-01808.x. [DOI] [PubMed] [Google Scholar]
- Schnitt SJ, Stillman IE, Owings DV, Kishimoto C, Dvorak HF, Abelmann WH. Myocardial fibrin deposition in experimental viral myocarditis that progresses to dilated cardiomyopathy. Circ Res. 1993;72:914–920. doi: 10.1161/01.res.72.4.914. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Tukov FF, Luyendyk JP, Ganey PE, Roth RA. The role of tumor necrosis factor alpha in lipopolysaccharide/ranitidine-induced inflammatory liver injury. Toxicol Sci. 2007;100:267–280. doi: 10.1093/toxsci/kfm209. [DOI] [PubMed] [Google Scholar]
- Uetrecht J. Idiosyncratic drug reactions: current understanding. Annu Rev Pharmacol Toxicol. 2007;47:513–539. doi: 10.1146/annurev.pharmtox.47.120505.105150. [DOI] [PubMed] [Google Scholar]
- Vial T, Goubier C, Bergeret A, Cabrera F, Evreux JC, Descotes J. Side effects of ranitidine. Drug Saf. 1991;6:94–117. doi: 10.2165/00002018-199106020-00002. [DOI] [PubMed] [Google Scholar]
- Yee SB, Hanumegowda UM, Hotchkiss JA, Ganey PE, Roth RA. Role of neutrophils in the synergistic liver injury from monocrotaline and bacterial lipopolysaccharide exposure. Toxicol Sci. 2003;72:43–56. doi: 10.1093/toxsci/kfg019. [DOI] [PubMed] [Google Scholar]