Abstract
Many plants show heterophylly, which is variation in leaf form within a plant owing to environmental change. The molecular mechanisms underlying heterophylly have recently been investigated in several plant species. However, little is known about how plants exhibiting heterophylly sense environmental cues. Here, we used Rorippa aquatica (Brassicaceae), which shows heterophylly, to investigate whether a single leaf can sense and transit changes in ambient temperature. The morphology of newly developed leaves after single-leaf warming treatment was significantly different from that of mock-treated control leaves, suggesting that leaves are sensing organs that mediate the responses to changes in ambient temperature in R. aquatica.
Keywords: Brassicaceae, heterophylly, leaf morphology, leaves, phenotypic plasticity, Rorippa aquatica, single-leaf warming treatment
Abbreviations
- DI
dissection index
- GA
gibberellin
- KNOX1
KNOTTED1-LIKE HOMEOBOX
- LN
leaf number
- SLWT
single-leaf warming treatment.
Heterophylly in Rorippa aquatica
Many organisms show phenotypic plasticity in response to surrounding environments; this often results in variation among individuals. Heterophylly is a type of phenotypic plasticity that results in variation in leaf form within a single plant owing to environmental variation, and many land plants including ferns show patterns of heterophylly.1,2 Some such leaf form alterations are thought to be adaptive responses to environmental changes.3 Several plants showing heterophylly have been described and the underlying mechanisms have been investigated.1,4,5 These studies have shown that various hormones, such as ethylene and abscisic acid, are involved in the alteration of leaf form.
Recently, we studied the mechanism underlying heterophylly in a semi-aquatic plant, Rorippa aquatica (Eaton) EJ Palmer & Steyermark (Brassicaceae).6,7 R. aquatica is found in bays, lakes, ponds, and streams in North America, and shows drastic heterophylly. In submerged conditions, deeply dissected leaves develop, whereas in terrestrial conditions, simple leaves with smooth margins develop. Additionally, R. aquatica is closely related to Cardamine hirsuta and Arabidopsis thaliana (hereafter, Arabidopsis),8 which are the most well-studied model plants with respect to leaf development.8,9,10 Hence, R. aquatica is an ideal model plant to determine the mechanisms of heterophylly.
Changes in ambient temperature induce heterophylly in R. aquatica.7 Under terrestrial conditions, dissected leaves develop at 20°C, whereas simpler-formed leaves with smooth margins develop at 30°C (Fig. 1A). Previously, we showed that regulation of the gibberellin (GA) level via KNOTTED1-LIKE HOMEOBOX (KNOX1) is involved in this phenomenon. Moreover, a transcriptome analysis indicated that light intensity also affects leaf form alterations. Consistent with this, we have demonstrated that light intensity induces heterophylly.7 Together, these results suggest that the KNOX-GA module, which is involved in the morphological diversification of leaf form among species, may also govern variation in leaf form within a species, and even within individuals, in response to environmental changes.7
Figure 1.
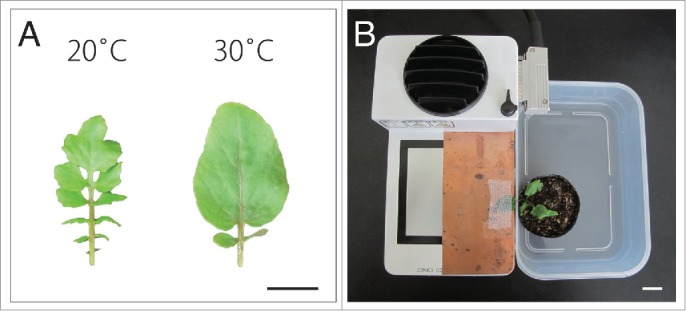
Gross morphology of Rorippa aquatica leaves. (A) A comparison of the morphology of leaf number 5 (LN5) for different temperatures. Left: 20°C; right: 30°C. (B) Experimental set up for single-leaf warming treatment using a plate-type temperature control system (CP-085; SCINICS). Bars = 2 cm.
However, the precise organs that sense environmental cues, such as ambient temperature, in R. aquatica and other plants that show heterophylly remain unclear. Thus, it is necessary to identify the organs that sense these cues to further understand the mechanisms underlying plant heterophylly.
Leaves may function as temperature sensors
Previous studies have demonstrated that some developing leaf phenotypes, such as stomatal density, palisade tissue size, and leaf thickness, are independent of local light irradiance. However, in addition to a change in CO2 concentration, irradiance to mature leaves affects the phenotypes of developing leaves.11-14 Additionally, the effect from mature to developing leaves is conserved among eudicots and monocots,14 suggesting that the system is relatively common in angiosperms. As indicated previously, light intensity also affects leaf morphology in R. aquatica.7 Hence, leaves are candidate organs with respect to the ability to sense changes in the surrounding environment, resulting in heterophylly of R. aquatica.
In this study, we investigated the morphology of newly developed leaves after warming single leaves within the same R. aquatica plant. To warm an individual leaf, a plate-type temperature control system (CP-085; SCINICS) was used (Fig. 1B). This single-leaf warming treatment (SLWT) has been successfully performed using Arabidopsis leaves.15 Leaf number 3 (LN3) was warmed at 30°C by the system for 30 days in a chamber that was maintained at 20°C. On the other hand, in mock-treated control, LN3 was cooled at 20°C for 30 days in a chamber that was maintained at 20°C. All plants were cultivated at 20°C without treatment for an additional 30 days (60 days in total) to ensure full lamina expansion. To investigate the complexity in leaf morphology, the dissection index (DI), which is an index of leaf complexity, was calculated as . To measure the leaf area and perimeter, mature leaves (LN4–7) were photographed using a digital camera (PowerShot G11; Canon). Leaf areas and perimeters were calculated using ImageJ v1.48 (http://rsb.info.nih.gov/ij/).
As expected, the form of newly developed leaves (LN5 and 6) after the SLWT (30°C) was simpler than that of leaves in mock-treated control (Fig. 2). This result suggests that there is a long distance signal from older leaves to newly developed leaves in the heterophylly of R. aquatica, as has been suggested for anatomical alterations between leaves exposed to sun and shade in some plants.11,12 However, we did not observe a statistically significant decrease in DI for LN4. This may be due to the determinacy of LN4 primordia. A previous study showed that the form of leaf primordia appears to be determined between stages P4 and P5.7 When the SLWT was initiated, the form of LN4 seemed to be determined already. Hence, we might not expect to observe a significant decrease in the DI of LN4. Interestingly, although we observed a statistically significant decrease in the DI of LN5 and LN6, the DI of LN7 did not show a decrease (Fig. 2B), suggesting that not all newly developed leaves were affected by the treatment applied to LN3. These results suggest a few potential interpretations. One possibility is that the long distance signal is only generated at a certain developmental stage of leaves. After the stage elapses, the signaling mechanism becomes less effective. Another possibility is that the signal from a leaf subjected to the warming treatment becomes weak owing to signals from other, newly developed leaves that are not treated. Indeed, the SLWT (30°C) did not totally mimic leaf form of plants maintained at 30°C (Fig. 2B). The result suggests that the signal from a leaf subjected to the treatment is not enough to mimic the leaf form of plants maintained at 30°C, and that signals from multiple leaves may be needed to fully mimic. In fact, both mechanisms may affect the form of newly developed leaves. In R. aquatica, GA is involved in leaf form alterations,7 suggesting that the relationship between GA and the long distance signal should be investigated. Although a recent study showed a gradation in the concentration of GA in maize leaves,16 little is known about their translocation and effect on other leaves. Therefore, the detailed mechanisms require further investigation.
Figure 2.
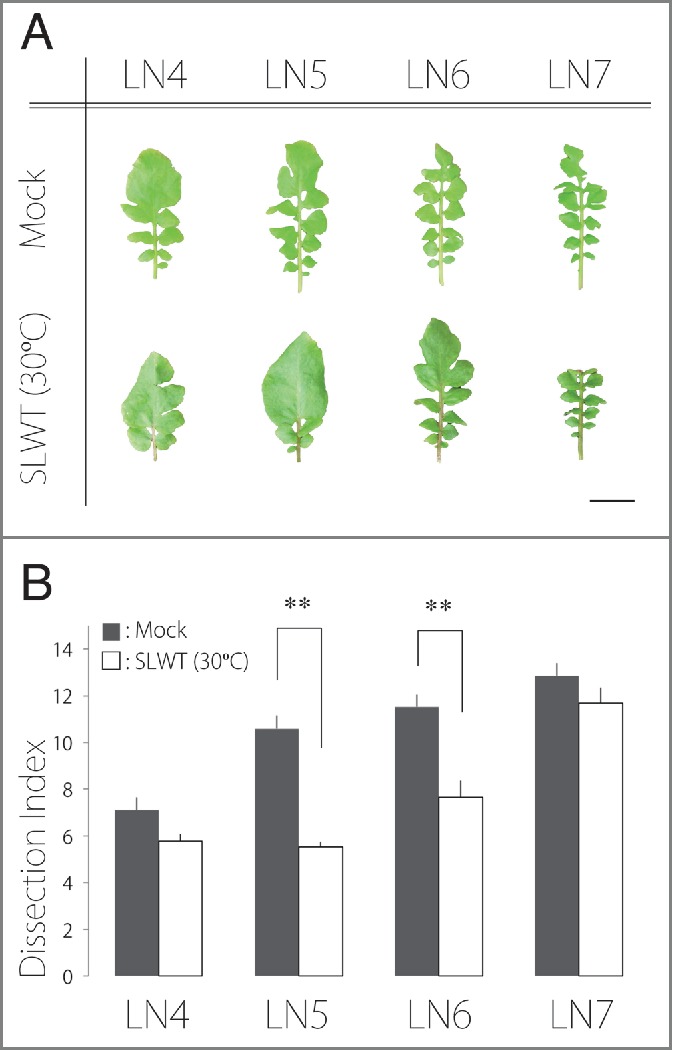
Leaf morphology and dissection index after single-leaf warming treatment. (A) Effects of single-leaf warming treatment (SLWT). LN3 was warmed at 30°C or cooled at 20°C using a plate-type temperature control system for 30 days in chamber maintained at 20°C. Upper: leaves from plants grown at 20°C with SLWT (20°C; mock-treated control); Lower: leaves from plants grown at 20°C with SLWT (30°C). The oldest leaf is shown on the left and the youngest on the right. Bar = 2 cm. (B) Dissection index (DI) of leaves. Error bars represent the standard error (SE); ** = p < 0.01 based on Welch's t-tests (n = 4; 2 plants were treated per single experiment.).
In this study, we demonstrated that the morphology of newly developed leaves is regulated by signals from older leaves in the heterophylly of R. aquatica. Thus, an understanding of heterophylly will provide new insights into the relationship between developed and developing leaves with respect to the formation of the appropriate final leaf morphology at the individual plant level.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Mitsutomo Abe for helpful suggestions regarding the single-leaf treatment using a plate-type temperature control system.
Funding
This work was partially supported by Grants-in-Aid from the Japan Society for the Promotion of Science (JSPS) (KAKENHI Grant Numbers 22870031 and 24770047), the Science Research Promotion Fund from the Promotion and Mutual Aid Corporation for Private Schools of Japan to S.K., MEXT-Supported Program for the Strategic Research Foundation at Private Universities and by a Research Fellowship from JSPS to H.N.
References
- 1.Wanke D. The ABA-mediated switch between submersed and emersed life-styles in aquatic macrophytes. J Plant Res 2011; 124:467-75; PMID:21674229; http://dx.doi.org/ 10.1007/s10265-011-0434-x [DOI] [PubMed] [Google Scholar]
- 2.Zotz G, Wilhelm K, Becker A. Heteroblasty—A Review. Botanical Rev 2011; 77:109-51; http://dx.doi.org/ 10.1007/s12229-010-9062-8 [DOI] [Google Scholar]
- 3.Cook SA, Johnson MP. Adaptation to heterogenous environments I. Variation in heterophylly in Ranunculus flammula L. Evolution 1968; 22:496-516; http://dx.doi.org/ 10.2307/2406876 [DOI] [PubMed] [Google Scholar]
- 4.Kuwabara A, Ikegami K, Koshiba T, Nagata T. Effects of ethylene and abscisic acid upon heterophylly in Ludwigia arcuata (Onagraceae). Planta 2003; 217:880-7; PMID:12844266; http://dx.doi.org/ 10.1007/s00425-003-1062-z [DOI] [PubMed] [Google Scholar]
- 5.Iida S, Miyagi A, Aoki S, Ito M, Kadono Y, Kosuge K. Molecular adaptation of rbcL in the heterophyllous aquatic plant Potamogeton. PLoS One 2009; 4:e4633; PMID:19247501; http://dx.doi.org/ 10.1371/journal.pone.0004633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamasu A, Nakayama H, Nakayama N, Suematsu NJ, Kimura S. A developmental model for branching morphogenesis of lake cress compound leaf. PLoS One 2014; 9:e111615; PMID:25375102; http://dx.doi.org/ 10.1371/journal.pone.0111615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakayama H, Nakayama N, Seiki S, Kojima M, Sakakibara H, Sinha N, Kimura S. Regulation of the KNOX-GA gene module induces heterophyllic alteration in North American lake cress. Plant Cell 2014; 26:4733-48; PMID:25516600; http://dx.doi.org/ 10.1105/tpc.114.130229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koenig D, Weigel D. Beyond the thale: comparative genomics and genetics of Arabidopsis relatives. Nat Rev Genet 2015; 16:285-98; PMID:25854181; http://dx.doi.org/ 10.1038/nrg3883 [DOI] [PubMed] [Google Scholar]
- 9.Hepworth J, Lenhard M. Regulation of plant lateral-organ growth by modulating cell number and size. Curr Opin Plant Biol 2014; 17:36-42; PMID:24507492; http://dx.doi.org/ 10.1016/j.pbi.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 10.Vlad D, Kierzkowski D, Rast MI, Vuolo F, Dello Ioio R, Galinha C, Gan X, Hajheidari M, Hay A, Smith RS, et al.. Leaf shape evolution through duplication, regulatory diversification, and loss of a homeobox gene. Science 2014; 343:780-3; PMID:24531971; http://dx.doi.org/ 10.1126/science.1248384 [DOI] [PubMed] [Google Scholar]
- 11.Woodward F. Stomatal numbers are sensitive to increases in CO2 from pre-industrial levels. Nature 1987; 327:617-8; http://dx.doi.org/ 10.1038/327617a0 [DOI] [Google Scholar]
- 12.Yano ST, I. Separate localization of light signal perception for sun or shade type chloroplast and palisade tissue differentiation in Chenopodium album. Plant Cell Physiol 2001; 42:1303-10; PMID:11773522; http://dx.doi.org/ 10.1093/pcp/pce183 [DOI] [PubMed] [Google Scholar]
- 13.Miyazawa S, Livingston NJ, Turpin DH. Stomatal development in new leaves is related to the stomatal conductance of mature leaves in poplar (Populus trichocarpaxP. deltoides). J Exp Bot 2006; 57:373-80; PMID:16172139; http://dx.doi.org/ 10.1093/jxb/eri278 [DOI] [PubMed] [Google Scholar]
- 14.Jiang CD, Wang X, Gao HY, Shi L, Chow WS. Systemic regulation of leaf anatomical structure, photosynthetic performance, and high-light tolerance in sorghum. Plant Physiol 2011; 155:1416-24; PMID:21245193; http://dx.doi.org/ 10.1104/pp.111.172213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Notaguchi M, Abe M, Kimura T, Daimon Y, Kobayashi T, Yamaguchi A, Tomita Y, Dohi K, Mori M, Araki T. Long-distance, graft-transmissible action of Arabidopsis FLOWERING LOCUS T protein to promote flowering. Plant Cell Physiol 2008; 49:1645-58; PMID:18849573; http://dx.doi.org/ 10.1093/pcp/pcn154 [DOI] [PubMed] [Google Scholar]
- 16.Nelissen H, Rymen B, Jikumaru Y, Demuynck K, Van Lijsebettens M, Kamiya Y, Inzé D, Beemster GT. A local maximum in gibberellin levels regulates maize leaf growth by spatial control of cell division. Curr Biol 2012; 22:1183-7; PMID:22683264; http://dx.doi.org/ 10.1016/j.cub.2012.04.065 [DOI] [PubMed] [Google Scholar]


