Abstract
Sugars, the end products of photosynthesis, not only fuel growth and development of plants as carbon and energy sources, but also function as signaling molecules to modulate a range of important processes during plant growth and development. We recently found that sugar can promote hypocotyl elongation in Arabidopsis in darkness and this is largely dependent on brassinosteroids (BRs), a group of essential phytohormones involved in mediation of plant cell elongation. Sugars not only positively regulate the transcription of BZR1, the gene encoding the BR-activated transcription factor BRASSINAZOLE RESISTANT1 (BRZ1), but also stabilize the BZR1 protein. Based on these results, we proposed that BZR1 may act as a converging node for crosstalk between BR and sugar signaling in regulating plant growth in darkness. In this short communication, we present some new data showing that HEXOKINASE1 (HXK1), the first identified glucose (Glc) sensor in plants, was positively involved in Glc promotion of hypocotyl elongation in Arabidopsis in the dark. It appears that the function of HXK1 is dependent on the presence of BR, suggesting that BR may act downstream of HXK1 to positively regulate Glc-induced hypocotyl elongation in Arabidopsis in darkness.
Keywords: BR, darkness, glucose HXK1, hypocotyl elongation, sugar
Abbreviations
- BL
brassinolide
- BR
brassinosteroid
- BRZ
brassinazole
- BZR1
brassinazole resistant1
- Glc
glucose
- HXK1
hexokinase1
- Mtl
mannitol
- Suc
sucrose.
Sugars not only fuel growth and development as carbon and energy sources, but in addition have acquired important regulatory roles as signaling molecules.1,2 Glucose (Glc) and sucrose (Suc), 2 kinds of metabolic sugars that are widely present in plants, have been recognized as pivotal integrating regulatory molecules that control gene expression related to plant metabolism, stress responses, and other growth and development related processes including seed germination, floral transition, fruit ripening, embryogenesis, and senescence.2-4 While little is known to the signaling mechanisms of Suc, part of the signal transduction pathways of Glc in plants has been established, which include 3 distinct pathways: HEXOKINASE1 (HXK1)-dependent pathway, HXK1-independent pathway, and glycolysis-dependent pathway that utilizes the SUCROSE NONFERMENTING RELATED KINASE1 (SnRK1)/TARGET OF RAPAMYCIN (TOR) pathway.5 Discovery of unique and global repression of photosynthetic genes by Glc in Arabidopsis led to identification of HXK1 as the first plant Glc sensor, which mediates Glc repression or promotion of gene transcription and plant growth.6,7 Later on, diverse isoforms of HXK and HXK-like (HXL) genes were found in the genomes of many plants, including rice, maize, tomato, tobacco and Arabidopsis, which suggests that plants have evolved a complex mode of Glc signaling to support various growth strategies and architectures dictated by sugar availability.4,7 Suc is the major transport sugar in higher plants and could also initiate signaling pathways leading to changes of gene expression and physiological adaptation, but a Suc sensor has not be identified.8
Brassinosteroids (BRs) are a class of polyhydroxylated sterol derivatives that regulate diverse developmental and physiological processes, including seed germination, seedling photomorphogenesis, stomata differentiation, organ boundary formation, flowering, male fertility, and even responses to biotic and abiotic stresses.9,10 In particular, BRs stimulate cell elongation by increasing cell wall plasticity and affect cell expansion via regulation of microtubule dynamics.10-12 The BR receptor BRASSINOSTEROID INSENSITIVE1 (BRI1) heterodimerizes with BRI1 ASSOCIATED KINASE1 (BAK1), the BRI1 co-receptor, after binding to BR. BRI1 and BAK1 subsequently act together to inhibit the GSK3-like kinase BRASSINOSTEROID INSENSITIVE2 (BIN2), a negative regulator of BR signaling.10,13 In the absence of BR, BIN2 phosphorylates and inactivates BRASSINAZOLE RESISTANT1 (BZR1) and BRI1 EMS-SUPRESSOR1 (BES1), 2 key transcription factors mediating BR responses. In the presence of BR, BZR1 and BES1 are dephosphorylated and activated for regulation of BR-inducible gene expression, including many genes related to cell elongation, such as PACLOBUTRAZOL RESISTANCEs (PREs), XYLOGLUCAN ENDOTRANSGLYCOSYLASE/HYDROLASEs (XTHs) and EXPANSINs (EXPs).10,12,14,15
Sugars repress plant growth in the light, whereas they promote growth in the dark, indicating that the sugar regulation of growth is mediated through different pathways in the light and dark.4,15-19 In Arabidopsis, Phytochrome-Interacting Factors (PIFs) and the plant hormone gibberellin (GA) have been shown to play important roles in sugar-induced hypocotyl elongation under both dark and short photoperiod conditions.17-19 In a recent study, we found that sugar-induced hypocotyl elongation and the expression of genes important for cell elongation in Arabidopsis seedlings could be significantly repressed by the treatment with brassinazole (BRZ), a biosynthetic inhibitor of BRs, suggesting that BR is important for sugar regulation of plant growth in darkness.15 Furthermore, we found that exogenous sugar treatment could enhance the transcription of BZR1 and BES1, the 2 BR transcription factor genes, and that sugar treatment could maintain a higher level of BZR1 proteins in plants grown in the dark, implying that sugar promotion of plant growth in darkness is mediated by the BZR1- and BES1-dependent pathways.15
Here, we report that sugar could promote plant growth in Arabidopsis not only in the dark but also under shade conditions. The results in (Fig. 1) showed that after the 7-day-old Col-0 seedlings (grown up under a continuous light condition [100 μmol photons m−2 s−<τβρ>1</tbr>]) were treated with 90 mM Suc, 90 mM Glc, or 90 mM mannitol (Mtl) and then transferred to a shade condition (∼10 μmol photons m−2 s−<τβρ>1</tbr>) for another 7 days, the Suc- or Glc-treated seedlings grew much bigger than the Mtl-treated control seedlings. However, whether the mechanisms for sugar promotion of plant growth in shade are identical to that in darkness remain to be determined. Moore et al. (2003) reported that when the Arabidopsis gin2-1 mutant (a null mutant of HXK1) was grown under a shade condition, it showed defects in hypocotyl elongation compared to its wild-type Ler,7 indicating that HXK1 might play a role during shade promotion of plant growth.
Figure 1.

Representative images of 7-day-old Col-0 seedlings that were grown up under white light (100 μmol photons m−2 s−<τβρ>1</tbr>) and were then treated with or without 90 mM sucrose (Suc), 90 mM glucose (Glc) or 90 mM mannitol (Mtl), followed by additional growth in a shade condition (10 μmol m−2 s−<τβρ>1</tbr>) for 7 days. Bar = 0.4 cm.
To test whether HXK1 also functions during Glc promotion of plant growth in darkness, we grew both the gin2-1 and Ler plants in a constant light condition in 1/2 MS media without any sugars for 4 days, and then treated the plants with various concentrations of Glc for another 2 days in darkness, before the hypocotyl lengths of seedlings were determined. The results (Fig. 2A) showed that the Glc-induced hypocotyl elongation was obviously impaired in gin2-1 as compared with Ler. For instance, in the absence of Glc, the ratio of hypocotyl lengths of Ler and gin2-1 was only 1.06, but in the presence of 30 mM and 60 mM of Glc, the ratio was increased to 1.24 and 1.38, respectively (Fig. 2A), suggesting that HXK1 is indeed playing a positive role in Glc promotion of hypocotyl elongation in darkness. We have recently shown that sugar-induced hypocotyl elongation is greatly impaired in the BR biosynthetic mutant det2-1, indicating that BR is necessity for sugar promotion of hypocotyl elongation.15 To identify the relationship between HXK1 and BRs in this process, we tested by yeast 2-hybrid assay for possible interactions between BZR1 and 3 sugar signaling components HXK1, VHA-B1 (vacuolar H+-ATPase B1), and RPT5B (19S regulatory particle of proteasome subunit), which have been shown to form a Glc signaling complex core to modulate sugar-regulated gene transcription likely through interaction with other transcriptional factors.6 However, no interaction was observed.15 On the other hand, the hypocotyl elongation of the gin2-1 mutant was shown to be less sensitive to the treatment of BRZ, a BR biosynthetic inhibitor. For example, without BRZ treatment, the hypocotyl length ratio of the wild-type Ler seedlings to the gin2-1 seedlings treated with Glc in darkness (2d) was 1.40, whereas in the presence of BRZ, the ratio was decreased to 1.19. Moreover, when the Glc- and BRZ-treated plants were supplied with exogenous BL (brassinolide, the most active form of BR), the hypocotyl ratio increased again (Fig. 2B). These results suggest that BRs may function downstream of HXK1 in mediating Glc promotion of hypocotyl elongation. Similar to our findings, Gupta et al. (2015) recently reported that BR signaling functions downstream of Glc to control lateral root production and emergence in Arabidopsis.20 The same laboratory also reported that under etiolated conditions, Glc and BR signals may converge at auxin signaling machinery to regulate etiolated hypocotyl elongation.11 All these results suggest that the crosstalk between sugars and BRs are important for regulation of different growth and developmental processes in plants.
Figure 2.
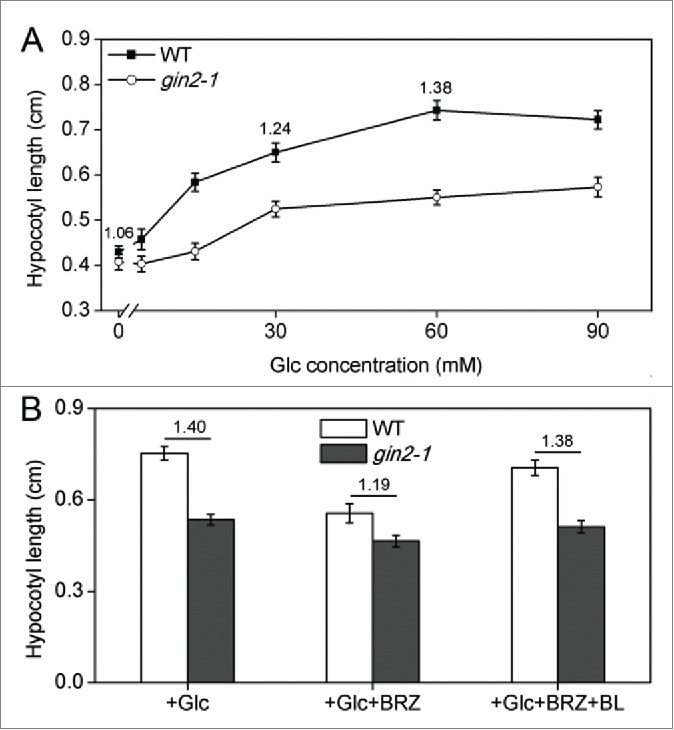
(A) Role of HXK1 in Glc-induced hypocotyl elongation in darkness. Hypocotyl lengths of 4-day-old light-grown seedlings of Ler and gin2-1 that were first treated with various concentrations of Glc (from 0 to 90 mM) and then transferred to darkness for 2 days. (B) Role of BR in HXK1-mediated Glc induction of hypocotyl elongation. Hypocotyl lengths of 4-day-old light-grown seedlings of Ler and gin2-1 that were treated with 60 mM Glc (+Glc), 60 mM Glc plus 2 ìM BRZ (+Glc+BRZ), or 60 mM Glc plus 2 ìM BRZ and 0.1 μM BL (+Glc+BRZ+BL) for 2 days. Each value is the mean of 25 seedlings ±SE. The numbers above the bars indicate the ratios for hypocotyl lengths of Ler related to gin2-1.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Arabidopsis Biological Resource Center (ABRC) for providing the gin2-1 mutant (CS6383).
Funding
This work was supported by the General Research Fund (CUHK codes 465410 and 464412) and an AoE grant (AoE/M-05/12) from the Research Grants Council (RGC) of Hong Kong, a grant from the National Natural Science Foundation of China (No. 91125027), the Shenzhen Science & Technology Research & Development Funding - Peacock Scheme, to JH, and the Fundamental Research Funds for the Central Universities (lzujbky-2015-91) to YZ.
References
- 1.Lastdrager J, Hanson J, Smeekens S. Sugar signals and the control of plant growth and development. J Exp Bot 2014; 65:799-807; PMID:24453229; http://dx.doi.org/ 10.1093/jxb/ert474 [DOI] [PubMed] [Google Scholar]
- 2.Eveland AL, Jackson DP. Sugars, signalling, and plant development. J Exp Bot 2012; 63:3367-77; PMID:22140246; http://dx.doi.org/ 10.1093/jxb/err379 [DOI] [PubMed] [Google Scholar]
- 3.Tognetti JA, Pontis HG, Martinez-Noel GM. Sucrose signaling in plants: A world yet to be explored. Plant Signal Behav 2013; 8:e23316; PMID:23333971; http://dx.doi.org/ 10.4161/psb.23316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheen J. Master regulators in plant glucose signaling networks. J Plant Biol 2014; 57:67-79; PMID:25530701; http://dx.doi.org/; http://dx.doi.org/ 10.1007/s12374-014-0902-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu Y, Lim S, Urano D, Tunc-Ozdemir M, Phan NG, Elston TC, Jones AM. Reciprocal encoding of signal intensity and duration in a glucose-sensing circuit. Cell 2014; 156:1084-95; PMID:24581502; http://dx.doi.org/ 10.1016/j.cell.2014.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho YH, Yoo SD, Sheen J. Regulatory functions of nuclear hexokinase1 complex in glucose signaling. Cell 2006; 127:579-89; PMID:17081979; http://dx.doi.org/ 10.1016/j.cell.2006.09.028 [DOI] [PubMed] [Google Scholar]
- 7.Moore B, Zhou L, Rolland F, Hall Q, Cheng W, Liu Y, Hwang I, Jones T, Sheen J. Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 2003; 300:332-6; PMID:12690200; http://dx.doi.org/ 10.1126/science.1080585 [DOI] [PubMed] [Google Scholar]
- 8.Wind J, Smeekens S, Hanson J. Sucrose: metabolite and signaling molecule. Phytochemistry 2010; 71:1610-4; PMID:20696445; http://dx.doi.org/ 10.1016/j.phytochem.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 9.Wang W, Bai M, Wang Z. The brassinosteroid signaling network-a paradigm of signal integration. Curr Opin Plant Biol 2014; 21:147-53; PMID:25139830; http://dx.doi.org/ 10.1016/j.pbi.2014.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Bai M, Oh E, Zhu J. Brassinosteroid signaling network and regulation of photomorphogenesis. Annu Rev Genet 2012; 46:699-722; http://dx.doi.org/ 10.1146/annurev-genet-102209-163450 [DOI] [PubMed] [Google Scholar]
- 11.Singh M, Laxmi A. The interaction between glucose and brassinosteroid signal transduction pathway in Arabidopsis thaliana. Plant Physiol 2015; 168(3):1091-1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh E, Zhu J, Wang Z. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat Cell Biol 2012; 14:802-9; PMID:22820378; http://dx.doi.org/ 10.1038/ncb2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu J, Sae-Seaw J, Wang Z. Brassinosteroid signalling. Development 2013; 140:1615-20; PMID:23533170; http://dx.doi.org/; http://dx.doi.org/ 10.1242/dev.060590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, Wang C, Jiang L, Li S, Sun S, He J. An interaction between BZR1 and DELLAs mediates direct signaling crosstalk between brassinosteroids and gibberellins in Arabidopsis. Sci Signal 2012; 5:ra72; PMID:23033541 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Liu Z, Wang J, Chen Y, Bi Y, He J. Brassinosteroid is required for sugar promotion of hypocotyl elongation in Arabidopsis in darkness. Planta 2015; 881-893 [DOI] [PubMed] [Google Scholar]
- 16.Rolland F, Baena-Gonzalez E, Sheen J. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 2006; 57:675-709; PMID:16669778; http://dx.doi.org/ 10.1146/annurev.arplant.57.032905.105441 [DOI] [PubMed] [Google Scholar]
- 17.Stewart JL, Maloof JN, Nemhauser JL. PIF genes mediate the effect of sucrose on seedling growth dynamics. PLoS One 2011; 6:e19894; PMID:21625438; http://dx.doi.org/ 10.1371/journal.pone.0019894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z, Zhang Y, Liu R, Hao H, Wang Z, Bi Y. Phytochrome interacting factors (PIFs) are essential regulators for sucrose-induced hypocotyl elongation in Arabidopsis. J Plant Physiol 2011; 168:1771-9; PMID:21684034; http://dx.doi.org/ 10.1016/j.jplph.2011.04.009 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Liu Z, Wang L, Zheng S, Xie J, Bi Y. Sucrose-induced hypocotyl elongation of Arabidopsis seedlings in darkness depends on the presence of gibberellins. J Plant Physiol 2010; 167:1130-6; PMID:20430474; http://dx.doi.org/ 10.1016/j.jplph.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 20.Gupta A, Singh M, Laxmi A. Interaction between glucose and brassinosteroid during the regulation of Lateral root development in Arabidopsis. Plant Physiol 2015; 168:307-20; PMID:25810094; http://dx.doi.org/ 10.1104/pp.114.256313 [DOI] [PMC free article] [PubMed] [Google Scholar]


