Abstract
Sterol glycosyltransferases regulate the properties of sterols by catalyzing the transfer of carbohydrate molecules to the sterol moiety for the synthesis of steryl glycosides and acyl steryl glycosides. We have analyzed the functional role of TTG15/UGT80B1 gene of Arabidopsis thaliana in freeze/thaw and heat shock stress using T-DNA insertional sgt knockout mutants. Quantitative study of spatial as well as temporal gene expression showed tissue-specific and dynamic expression patterns throughout the growth stages. Comparative responses of Col-0, TTG15/UGT80B1 knockout mutant and p35S:TTG15/UGT80B1 restored lines were analyzed under heat and freeze stress conditions. Heat tolerance was determined by survival of plants at 42°C for 3 h, MDA analysis and chlorophyll fluorescence image (CFI) analysis. Freezing tolerance was determined by survival of the plants at -1°C temperature in non-acclimatized (NA) and cold acclimatized (CA) conditions and also by CFI analysis, which revealed that, p35S:TTG15/UGT80B1 restored plants were more adapted to freeze stress than TTG15/UGT80B1 knockout mutant under CA condition. HPLC analysis of the plants showed reduced sterol glycoside in mutant seedlings as compared to other genotypes. Following CA condition, both β-sitosterol and sitosterol glycoside quantity was more in Col-0 and p35S:TTG15/UGT80B1 restored lines, whereas it was significantly less in TTG15/UGT80B1 knockout mutants. From these results, it may be concluded that due to low content of free sterols and sterol glycosides, the physiology of mutant plants was more affected during both, the chilling and heat stress.
Keywords: Arabidopsis thaliana, chlorophyll fluorescence image, freeze stress, heat stress, sterol glycosyltransferases
Abbreviations
- SGT
Sterol glycosyltransferase
- NA
Non acclimatized
- CA
Cold acclimatized
- CFI
Chlorophyll fluorescence image
Introduction
Sterol glycosyltransferases (SGTs) in plants catalyze glycosylation of phytosterols and related compounds to generate their glycol-conjugates. Higher plant cell synthesizes a complex array of mixture of sterols comprising of sitosterol, campesterol, stigmasterol.1 The derivatives of sterol known as sterol glycosides (SG) and acyl sterol glycosides (ASG) are in large quantity in plants, but their specific functions are yet to be known.2 SGs are the abundant membrane components both in prokaryotes and eukaryotes since they are important in membrane fluidity and permeability and the phospholipid dependency of UDP-Glc: sterol glucosyltransferase.3-4 In the plasma membrane, the ratio of SG and ASG is consistent with the intracellular localization of the enzymes mediating the interconversion of sterol and sterol derivatives by UDP-glucose:sterol glycosyltransferase and sterol β-glucoside hydrolase activities localized in golgi dictyosomes and plasma membrane fractions.2 SGTs play an important regulatory role in the activity of sterols in higher organisms. It is clear that the glycosylation of a significant fraction of membrane bound free sterols alters the biophysical properties of the membranes.5 However, due to such consequences changes in the functional proportions of free sterols and their respective glycosides on the biological functions of a membrane are still unclear. A difference in the proportion of glycosylated versus acylated sterols were reported in 2 different Solanaceous species under cold acclimation experiment.6 It has been assumed that SGs may have a very important role in adaptation against temperature stress. Studies on glycosyltransferases and glycosylation of low-molecular weight plant compounds have been done mainly on Arabidopsis thaliana and several other plant species. After the analysis of entire Arabidopsis genome sequence, more than 100 putative GTs were found present in this model plant, which were supposed to be involved in the modification of plant secondary metabolites.7 Because of the availability of considerable biochemical and genomic data on plant GTs, the analyses of its biological roles in plants could be possible by using the techniques of functional genomics, especially the strategies of over-expression and down-regulation of the gene. The genome of A. thaliana contains 2 sterol β-glucosyltransferase genes in the subfamily UGT80 named as UGT80B1 (At1g43620) and UGT80A2 (At3g07020), which catalyzes the glycosylation of 3β-hydroxy group of sterols to produce a 3β-D-glycoside.8 Previously, it was reported that mutation in UDP-glucose:sterol glucosyltransferase (UGT80B1) in (WS)-O ecotype in A. thaliana caused transparent testa phenotype and suberization defects in seeds.9 Recent results obtained with functional characterization of plant GTs indicated that glycosyltransferases might play an important role in plant growth, development and interaction with the environment.10 Moreover, the gene expression data from Genome Cluster Database suggested that TTG15/UGT80B1 transcripts were up-regulated by cold stress.
In the present paper, we have analyzed the freeze-thaw/ heat shock injury in leaves by chlorophyll fluorescence imaging (CFI) method and also quantified the ratio of free β-sitosterol and β-sitosterol glycoside through HPLC analysis in TTG15/ UGT80B1 Arabidopsis mutants and their restored lines after cold acclimation (CA; plants survived after cold stress) condition. Chlorophyll fluorescence imaging provides spatial resolution and can rapidly be quantified. The fluorescence images clearly showed spatial heterogeneity of freeze-thaw injury in partially damaged leaves and integration of the fluorescence signals over the whole leaf area. Our results indicated that TTG15/UGT80B1 gene was induced under cold stress and important for survival of the plants. The fluorescence images clearly showed, freeze-thaw /heat shock injury more damaged TTG15/UGT80B1 mutant plants and integration of the fluorescence signals were remarkably more affected in mutants.
Results
Molecular characterization of TTG15/UGT80B1 mutants and preparation of restored lines with 35S promoter
We have obtained T-DNA insertional TTG15/UGT80B1 knockout mutant lines from Arabidopsis Biological Resource Center (ABRC) to know its effect on plant development and under temperature stress conditions. The initial allele that we obtained, were designated as Salk-021175, contained T-DNA insertion at the fifth exon, downstream of the translational initiation codon (Supplementary Fig. 4A). Homozygous lines carrying T-DNA mutations were isolated by PCR analysis with kanamycin selection (Fig. 1A). RT-PCR analysis showed that absence of gene expression in homozygous lines was due to T-DNA insertion (Fig. 1B). After restoration of TTG15/UGT80B1gene in mutant we obtained 25 restored lines. Out of 25, RL21 and RL25 lines showing significant gene expression were selected and used for further functional characterization (Fig. 1C and D).
Figure 4.
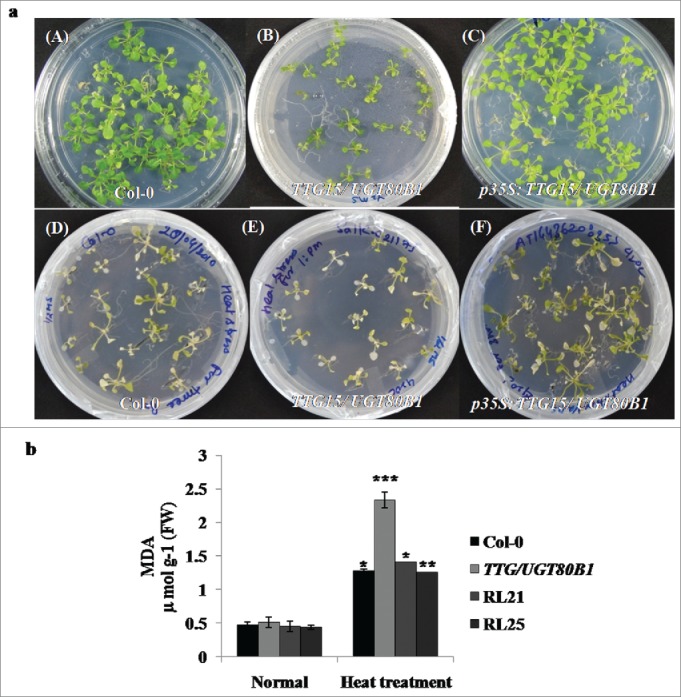
Survival analysis and lipid peroxidation assays. Survival of seedlings (A) and MDA accumulation of the Col-0, TTG15/ UGT80B1 mutant and p35S:TTG15/UGT80B1 restored lines 5 d after heat stress (B).
Figure 1.
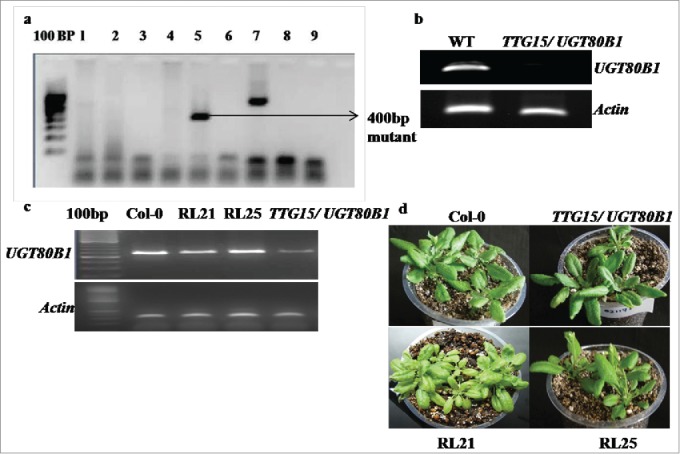
PCR analysis of homozygous lines. Lines carrying T-DNA mutations with kanamycin selection (A). RT-PCR analysis of homozygous lines of Salk-021175 mutants (B). Gene expression analysis in Col-0, TTG15/ UGT80B1 mutants and p35:TTG15/UGT80B1 restored lines (C) Restored plant RL21 and RL25 (D).
Spatial and temporal regulation of TTG15/UGT80B1 genes
Spatial and temporal gene expression patterns of TTG15/UGT80B1 were systematically examined to obtain the functional role of proteins encoded by UGT80B1. Interestingly, the TTG15/UGT80B1 gene exhibited distinct tissue-specific expression patterns through GENEVESTIGATOR software analysis (Supplementary Fig. 1A). The qRT-PCR analysis of TTG15/UGT80B1 was significantly higher in the siliques, flowers and shoots as compared to roots (Fig. 2A). TTG15/UGT80B1 gene also exhibited dynamic expression patterns throughout the growth stages. Transcript levels of TTG15/UGT80B1 gene remained relatively low in young seedlings up to 7 d after germination (DAG, Fig. 2B), beyond which the transcript levels was significantly elevated up to 14 DAG and then reaching a plateau (Fig. 2B). Together, these observations suggest that the spatial and temporal expression patterns of TTG15/UGT80B1 gene may be intimately linked to its functional role in plant development.
Figure 2.
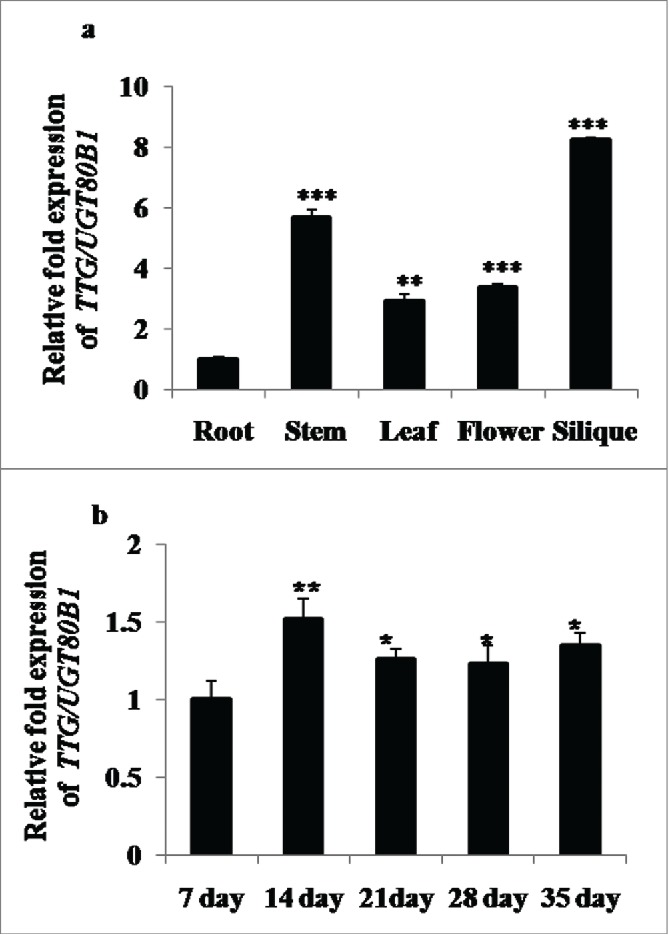
Spatial and temporal expression analyses of UGT80B1 transcripts by real time–PCR. qRT-PCR of UGT80B1 gene in root, stem, leaves, flowers and silique of the plants (A). Expression analysis of UGT80B1 gene in different time period of the developmental stages (B).
Phenotypic effect of NA and CA conditions on Col-0, mutant and restored lines
GENEVESTIGATOR software analysis showed the expression of TTG15/UGT80B1 gene was enhanced after 24 h of cold stress (Supplementary Fig. 1B). Phenotypic analysis for basal cold tolerance (NA) was performed in growing plates of 14-days-old seedlings of Col-0, mutants and p35S:TTG15/UGT80B1 restored lines of A. thaliana which were transferred directly to a freezing chamber at -1°C. During NA treatment, all seedlings of different genotypes were severely damaged. Only 25% seedlings could survive (Fig. 3A[D-F] and 3B). Following CA treatment, survival percentage of mutant seedlings were significantly reduced (48%) than both Col-0 and restored lines (Fig. 3A [G-I], 3B). For CA, the plates were first transferred to a cold room set at 4 ± 2°C, under constant light for 7 d.
Figure 3.
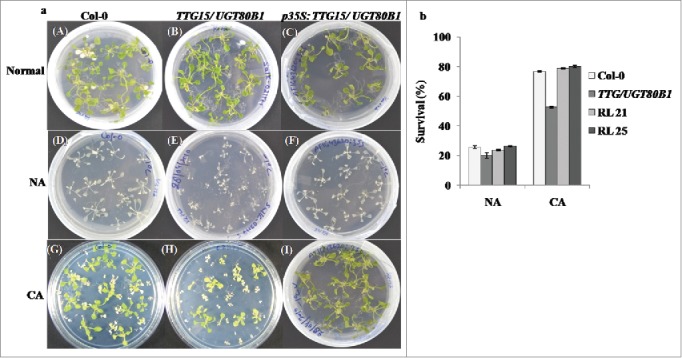
Freeze stress analysis of the plants. Stress analysis of the normal, CA and NA condition of Col-0, TTG15/UGT80B1 mutant and p35S:TTG15/UGT80B1 restored lines i.e., RL21 and RL25 (A). Survival percentage of the plants after cold stress (B) in Col-0, TTG15/ UGT80B1 mutant and RL21, RL25 restored lines.
Heat shock damage on mutant and restored lines
Phenotypic analysis of mutant and p35S:TTG15/UGT80B1 restored lines of A. thaliana were showing heat sensitivity at 14-d-old seedling stage in petriplates (Fig. 4A). We have observed 20–23% survival of mutants whereas survival percentage of p35S:TTG15/UGT80B1 restored lines was enhanced up to 32–34%, which was not significant. GENEVESTIGATOR analysis also did not show significant expression of TTG15/UGT80B1 gene during heat stress (Supplementary Fig. 1C). Leaves of mutants were more bleached as compared to restored lines after heat stress during visual analysis (Fig. 4A [D-F]).
Lipid peroxidation assay
As a test of heat sensitivity and measure of oxidative damage, heat stressed mutants, Col-0 and restored lines of A. thaliana were assayed for accumulation of MDA. MDA content was accumulated 77.96% more in mutant lines as compared to normal condition, whereas 65–67% and 63.61% was elevated in restored lines (RL25, RL21) and Col-0 plants, respectively. This result suggested that due to heat stress, maximum peroxide activity occurred in TTG15/UGT80B1 as compared to p35S:TTG15/UGT80B1 and Col-0 plants (Fig. 4B).
Fluorescence response (Fv/Fm) in NA, CA and heat shock conditions
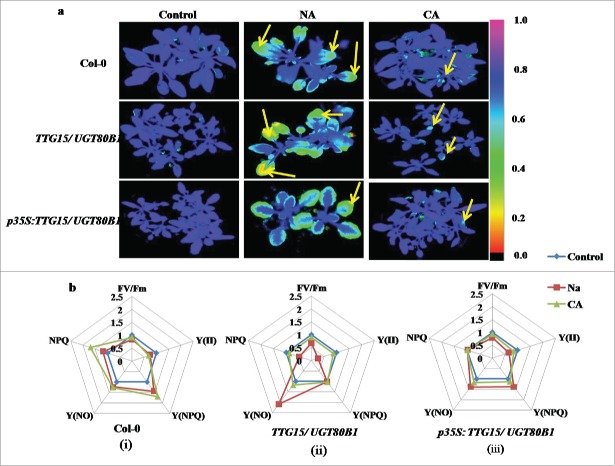
Figures 5A and 6A are showing false color images of chlorophyll fluorescence yield (FV/FM) from freeze and heat stressed Arabidopsis leaves, respectively. For freeze stress, we have compared leaves from plants grown under NA with CA conditions. The images clearly show increased inactivation of photosynthesis with decreased freezing temperature in all the cases (Fig. 5A). Leaves from both the mutants and restored lines increased freezing tolerance during CA, as clearly shown by the shift of the onset of changes in the coloration (i.e. in FV/FM) to lower temperatures in the acclimated as compared to the NA leaves (Fig. 5A, Lane 2).
Figure 5.
Differential effect of cold treatments on fluorescence response. Freezing damage in Arabidopsis leaves visualized by CFI (A). Spider plots showing relative changes of mean values of selected fluorescence parameters during cold stress in Col-0 (i), TTG15/ UGT80B1 mutant (ii) and p35S:TTG15/UGT80B1 restored lines (iii) (B).
Figure 6.
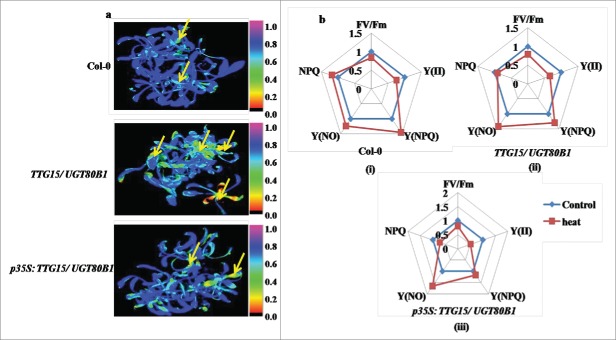
Differential effect of heat treatment on fluorescence response. Heat shock damages in Arabidopsis leaves visualized by CFI (A). Spider plots showing relative changes of mean values of selected fluorescence parameters during heat stress in Col-0 (i), TTG15/ UGT80B1 mutant (ii) and p35S:TTG15/UGT80B1 restored lines (iii) (B). NPQ is a non photochemical quenching measurement, Y (NPQ) is a measure of the fraction of photons absorbed by PSII antennae, NPQY is an indication of protective strategies at PSII, Y (NO) is the fraction of photons dissipated by dissociation of light-harvesting complex II.
The CFI has been shown under the selected fluorescence parameters, and the variability of the fluorescence response parameters has been shown through spider plots (Figure 5B and 6B). All spider plots show the dynamics of conventional fluorescence parameters during the cold and heat stress. Each spider plot consisted of different parameters, some measured with dark-adapted plants (Fv/Fm) while Kautsky induction was measured with low light intensity (80 µmol m−2 s−1). The distance from the center of the spider plot indicated the relative change of the fluorescence parameter during the treatment as compared to untreated plants. Clearly, under normal conditions all genotypes show approximately similar fluorescence response. In spider plot 5b and 6b, those representing mutant plants, the florescence dynamics of NA and heat stress was different from the control, while in CA conditions, the dynamics did not change much (Fig. 5B ii and 6B ii). In spider plot (iii), which represented restored plants, the florescence dynamics did not change much as compared to mutants in CA condition (Fig. 5B, iii). In other words, the restored plants show more adaptation to chilling stress as well as heat stress conditions. Level of significance was considered as P < 0.05.
Analysis of β-sitosterol and sitosterol glycoside in CA mutant seedlings
We have investigated the quantity of free β-sitosterol and their respective glycosides (SG) in normal, CA seedlings and their restored lines through HPLC analysis. β-sitosterol is the principal sterol moiety for the modification with glucose. There was no significant difference of free sterol molecule among all genotypes during normal growth, however, steryl glycoside quantity reduced in mutant seedlings as compared with other genotypes (Fig. 7A). Following CA condition, both β-sitosterol and sitosterol glycoside quantities were more in RL21 and RL25 restored lines and Col-0. However, the total quantity of free sterol and sterol glycoside decreased in TTG15/UGT80B1 mutant as compared to restored and Col-0 plants resulting in significant decrease in the ratio of free sterol / sterol glycoside (Fig. 7B and Fig. S2A-H).
Figure 7.
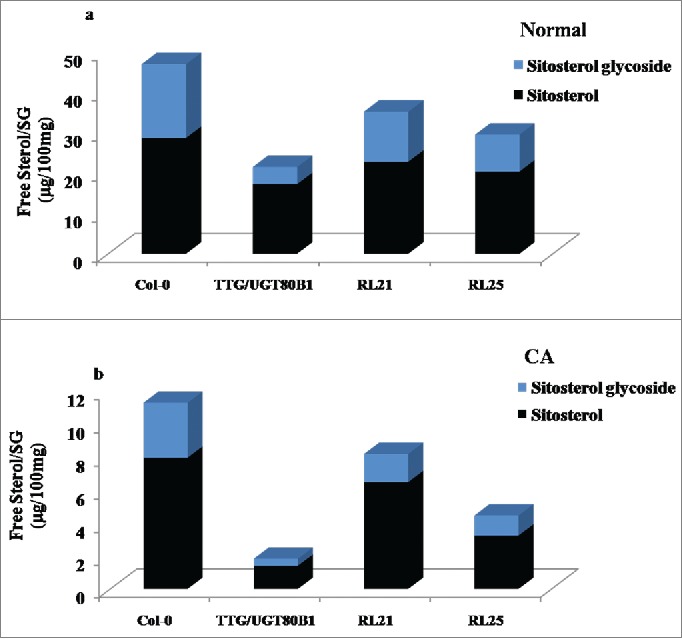
HPLC analysis of normal and CA plants. Ratio of free sterol and steryl glucoside in control condition (A) Ratio of free sterol and steryl glucoside in CA condition.
Discussion
Sterols in higher plants occur in minimum of 3 different forms: as free sterols, steryl esters, and steryl glycosides. Biosynthesis of steryl glycoside was done by UDP-sterol glycosyltransferase enzyme. It may or may not be acylated.11 Most of these studies have shown a correlation between alterations in physiological or morphological parameters and changes in the amount/proportions of SGs in the respective organism. But no causal connection could be drawn between SGs and any biological phenomenon on a molecular level. In the present work, TTG15/UGT80B1 gene was physiologically and biochemically characterized under cold and heat stress conditions, using T-DNA knockout mutants and their restored lines p35S:TTG15/UGT80B1. During spatial and temporal regulation, TTG15/UGT80B1 gene exhibited both dynamic and static expression patterns in different plant tissues of all the developmental stages. TTG15/UGT80B1 gene was expressed with high transcript levels in the siliques and flowers, while moderately in roots and leaves. These observations further support that the TTG15/UGT80B1 proteins may play certain roles in plant development. It is currently unclear whether these distinct patterns of spatial expression of TTG15/UGT80B1 genes are related to modulation of steryl glycosides under different abiotic stress conditions. The physiological importance of sterols/sterol glycosides in higher plants is due to their involvement in structural components of membranes and controlling the permeability of membranes. Due to T-DNA insertion, mutants have significantly reduced SG and ASG.9 As evident we have shown the quantity of sitosterol glycoside significantly reduced in mutant through HPLC analysis during normal growth (Fig. 7A). In particular, glycosylation was catalyzed by a super-family of GTs which play significant roles in modulating the solubility, stability, bioavailability and bioactivity of various small molecules.12 This plasticity depends on the integration of growth, development, and metabolism, and the evolution of diverse mechanisms to regulate cellular homeostasis. These modifications do help in adaption of the plants to fit better in the environment.13 In this study, we have shown the heat and chilling sensitivity of mutant and their restored lines by phenotypic observations and fluorescence response.
During cold stress, the survival percentage of restored lines and Col-0 was more than mutants under CA condition. However, plant survival of all the genotypes was almost similar under NA condition. Under cold stress, the membrane sterols play very important roles. It was observed that in response to environment condition, the sterol content of plant membranes has been changed which suggested that alterations in the sterol composition of plasma membrane may play a role in the CA process. Thus, a key function of CA is to stabilize those membranes via multiple mechanisms. For example, alterations in membrane lipid composition were correlated with membrane cryostability.14 As evident we have shown during CA, mutant lines have less quantity of membrane bound sterol and sterol glycoside during HPLC analysis, consequently in these lines, photosynthesis system was comparatively more damaged than Col-0 and restored lines (Fig. 7B). It has been reported that the concentration of membrane sterols increased with respect to decreasing sterol glycoside and acyl sterol glycoside during CA in winter rye seedlings.15 In wheat, ESTs corresponding to several enzymes involved in sterol metabolism were overexpressed suggesting major lipid modifications in membranes during CA.16
In the context of photosynthesis, chlorophyll is an essential factor and a driving force for plant growth and biomass production. Stability of chlorophyll during stress allows cells to maintain a functional chloroplast so that photosynthesis can be revived after the plant’s recovery from stress.17 Hence, estimation of total chlorophyll content can reflect the tolerance of plants to several abiotic stresses (Fig. S3). Chlorophyll biosynthesis was affected by high temperature stress, in both mutant and restored lines. In the presence of cold stress, during NA condition, chlorophyll loss was maximum in all the genotypes, whereas, significantly less in CA condition. Due to this reason, reaction centers were damaged and fluorescence response (Fv/Fm) also decreased. CFI offers the additional advantage of providing spatial information about freezing damage.18 During cold stress, the Fv/Fm ratio, as well as Y (II) was reduced indicating a structural and functional disorder of the photosynthetic apparatus and damage to the PSІІ. In our study, mutant showed lowering of Fv/Fm as compared to other genotypes. Reductions in the Fv/Fm ratio and Y (II) under cold stress suggested that an important portion of the PSII reaction center was damaged in the mutants. These damages were associated with structural modifications in PSII.19 Mutants were severely damaged during heat stress due to maximum accumulation of ROS, however, MDA level was also enhanced. Whereas, in restored lines MDA level was comparatively less accumulated. Earlier reports from Myxoamoeba and human fibroblastoma cell lines showed the activation of sterol glycosyltransferase and the production SG following heat stress. The SG has been reported to induce the signal transduction pathway, leading to the synthesis of heat shock proteins during heat stress.20
Conclusion
The present work has provided information enhancing the knowledge about the involvement of TTG15/UGT80B1 gene of A. thaliana in response to environmental stresses. CFI analysis has also been validated in this study. β-sitosterol and sitosterol glycoside quantity was analyzed higher in Col-0 and p35S:TTG15/UGT80B1 restored lines, whereas it was significantly less in TTG15/UGT80B1 knockout mutants. This change might be considered for altered physiology of mutant plants during chilling and heat stress. However, the precise mechanism for the involvement of free sterol and SG in regulating membrane permeability, characteristics and membrane function are yet to be understood. More in-depth studies are needed for complete understanding of structural modifications under different abiotic stress conditions which needs further research.
Methods
Plant materials and growth conditions
Columbia background (Col-0) of A. thaliana plants were used in the present study. Plants were grown in a controlled culture room set at 22°C under long day (LD) conditions (16 h light and 8 h dark) with white light illumination (120 μmol m−2s−1). For phenotypic analysis and growth assays, seedlings were exposed to light for 1 h and grown in either continuous light or complete darkness at 22°C on plates containing 0.5X Murashige and Skoog’s21 mineral salts (Sigma) and 0.8% agar. The sterol glycosyltransferase T-DNA insertional knockout mutant, TTG15/UGT80B1 of A .thaliana was obtained from a pool available from the Arabidopsis Biological Resource Center (ABRC, Ohio State University).
Identification of T-DNA insertions in TTG15/UGT80B1 knockout mutant
Genomic DNA was isolated from 4-weeks-old Arabidopsis leaves using CTAB method. The Salk homozygous T-DNA insertion mutation in TTG15/UGT80B1 was identified.22 Screening primers for TTG15/UGT80B1 resulted in 400 and 1000 bp mutant and Col-0 products using T-DNA primers LBa1. Primers used for PCR and qRT-PCR analysis in the present study have been mentioned in Supplementary Table 1.
Preparation of restored lines
Preparation of restored lines (gene complementation) was done by restriction digestion/ligation method in pIG121 vector (clonetech). We have cloned the coding region (1.84 kb) of TTG15/UGT80B1 gene into pTZ57R/T cloning vector (Thermo) (Fig. S4B). Positive clone sequence was isolated through digestion, subsequently ligated in pIG121 vector containing 35S promoter (Fig. 5A-C). Positive lines were selected through hygromycin and confirmed through sequencing (Fig. 5D). TTG15/UGT80B1 gene expression was analyzed in restored lines by RT-PCR. T3 generation was used for further study. For each experiment, 3 biological and 2 technical replicates were used.
Transcript level analysis
The modulation of UGT80B1 transcript levels were measured by real time qRT-PCR. For transcript analysis, Col-0 plants were grown for 14 d under 16 h light/8 h dark. Heat treatment was given at 42°C for 3 h and cold treatment was given at 4°C for 24 h. Total RNA samples were extracted using the RNeasy Plant Total RNA Isolation Kit (Qiagen, USA) and treated with RNase-free DNase I (Ambion). The first-strand cDNA was synthesized using Superscript II reverse transcriptase (Invitrogen). The UGT80B1 gene expression was normalized to Actin (At3g18780) as an internal (housekeeping gene) control along with RT and non-template controls. All the qRT-PCR runs were repeated at least 3 times and a representative result was displayed for individual assays.
Freeze thaw treatment and determination of freezing tolerance
Monitoring of plant survival by whole plant freezing test was performed separately with the seedlings grown on petriplates.23 Assessment of injury (and the freezing tolerance) was done by measuring electrolyte leakage from freeze-thaw injured tissues24 in the NA (just prior to administering cold acclimation) and CA plants.
Heat shock treatment and lipid peroxidation assay
Heat shock analysis was done by procedure reported by Larkindale et al.25 All the heat treatments were performed in the dark. Analysis of lipid peroxidation assay by malondialdehyde (MDA) content which is a measure of heat stress was performed by the previous modified protocol.13 The absorbance was measured at 532 and 600 nm. MDA concentration was calculated using the Lambert-Beer law with an extinction coefficient €M = 155 mM−1 cm−1.
Chlorophyll estimation of all phenotypes
Estimation of chlorophyll in all normal grown plants and treated plants was done by Arnon protocol.26 In the present study, 80% acetone was used for determination of chlorophyll and absorption was recorded at 645 and 663Å.
Measurements of chlorophyll fluorescence image
An Imaging-PAM, M-Series Chlorophyll Fluorometer (Walz, Effeltrich, Germany), was used to study the chlorophyll fluorescence parameters and the calculations were done according previous method.25 The maximum photochemical efficiency of photosystem II (PSII), (Fv/Fm: where Fm is maximum fluorescence of the dark-adapted leaf under a light saturating flash and Fv is maximum variable fluorescence, Fm – F0 was measured on leaves after 20 min of dark adaptation. The effective quantum yield of PSII (Y) was calculated as (Fm-Fs)/Fm.13
HPLC analysis of free sterols and steryl glycosides
Sterol and steryl glycoside were analyzed quantitatively and qualitatively from cold treated leaves extract.28 Plant material was extracted in ethyl acetate by keeping overnight at room temperature with brief agitation. Homogenous extracts obtained were filtered through 0.45 μM filters (Millipore, USA). Filtered samples were evaporated (ethyl acetate) to dryness and resuspended (1 mg/ml) in 100% methanol. Differences in quantity of free sterols and steryl glycosides before and after cold acclimatization were calculated in Col-0, mutant and their restored lines. Qualitative and quantitative analysis of sterols was performed by HPLC-PDA with a Shimadzu (Japan) LC-10 system comprising an LC-10AT dual pump system, SPD-M20A PDA detector and rheodyne injection valve furnished with a 20 µl sample loop. Compounds were separated on a Merck Purospher star→ RP-C18 column (250 × 4.6 mm, 5 μm pore size) protected by guard column containing the same packing29 with flow rate of 2 ml/min and injecting 20 μl of extract.13 Sterols were eluted by isocratic solution of acetonitrile and water (95:5 v/v). Quantification of sterols were performed at 202 nm at 34ºC, 60 min retention time with 2 ml/min flow rate delivery of mobile phase by injecting 20 μl of extract. Sterols were quantified by calculating the area of individual peak compared with the peak obtained from standards obtained from M/S Sigma- Aldrich.
Statistics analysis
All statistics analysis was done by Student t-test. Values are means ± SD (n = 3) (*) for P ≤ 0 .05, (**) for P ≤ 0.01, (***) for P ≤ 0.001 or 0.005, significantly different from the control (t-test).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are thankful to the Director, CSIR-NBRI for the facilities provided.
Supplemental Material
Supplemental data for this article can be accessed on the publisher’s website.
Funding
The authors also thank the Department of Biotechnology (Project No. GAP 231225), Govt. of India, for financial support.
References
- 1.Stucky DF, Arpin JC, Schrick K. Functional diversification of two UGT80 enzymes required for steryl glucoside synthesis in Arabidopsis. J Exp Bot 2015; 66:189-201; PMID:25316063; http://dx.doi.org/ 10.1093/jxb/eru410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grille S, Zaslawski A, Thiele S, Plat J, Warnecke D. The functions of steryl glycosides come to those who wait: Recent advances in plants, fungi, bacteria and animals. Prog Lipid Res 2010; 49:262-88; PMID:20138912; http://dx.doi.org/ 10.1016/j.plipres.2010.02.001 [DOI] [PubMed] [Google Scholar]
- 3.Ury A, Benveniste P, Bouvier-Nave P. Phospholipid-dependence of plant UDP-glucose sterol beta-d-glucosyl transferase : IV. Reconstitution into small unilamellar vesicles. Plant Physiol 1989; 91:567-73; PMID:16667070; http://dx.doi.org/ 10.1104/pp.91.2.567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouvier-Navé PU P, Rimmele D, Benveniste P. Phospholipid-dependence of plant UDP-glucose-sterol-β-D-glucosyltransferase. I. Detergent-mediated delipidation by selective solubilization. Plant Science Letters 1984; 36:19-27; http://dx.doi.org/ 10.1016/0304-4211(84)90271-2 [DOI] [Google Scholar]
- 5.Thevissen K, Ferket KK, Francois IE, Cammue BP. Interactions of antifungal plant defensins with fungal membrane components. Peptides 2003; 24:1705-12; PMID:15019201; http://dx.doi.org/ 10.1016/j.peptides.2003.09.014 [DOI] [PubMed] [Google Scholar]
- 6.Palta JP, Whitaker BD, Weiss LS. Plasma membrane lipids associated with genetic variability in freezing tolerance and cold acclimation of solanum species. Plant Physiol 1993; 103:793-803; PMID:12231980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Baldauf S, Lim EK, Bowles DJ. Phylogenetic analysis of the UDP-glycosyltransferase multigene family of Arabidopsis thaliana. J Biol Chem 2001; 276:4338-43; PMID:11042215; http://dx.doi.org/ 10.1074/jbc.M007447200 [DOI] [PubMed] [Google Scholar]
- 8.Warnecke DC, Baltrusch M, Buck F, Wolter FP, Heinz E. UDP-glucose:sterol glucosyltransferase: cloning and functional expression in Escherichia coli. Plant Mol Biol 1997; 35:597-603; PMID:9349281; http://dx.doi.org/ 10.1023/A:1005806119807 [DOI] [PubMed] [Google Scholar]
- 9.DeBolt S, Scheible WR, Schrick K, Auer M, Beisson F, Bischoff V, Bouvier-Navé P, Carroll A, Hematy K, Li Y, et al. . Mutations in UDP-Glucose:sterol glucosyltransferase in Arabidopsis cause transparent testa phenotype and suberization defect in seeds. Plant Physiol 2009; 151:78-87; PMID:19641030; http://dx.doi.org/ 10.1104/pp.109.140582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaturvedi P, Mishra M, Akhtar N, Gupta P, Mishra P, Tuli R. Sterol glycosyltransferases-identification of members of gene family and their role in stress in Withania somnifera. Mol Biol Rep 2012; 39:9755-64; PMID:22744427; http://dx.doi.org/ 10.1007/s11033-012-1841-3 [DOI] [PubMed] [Google Scholar]
- 11.Grunwald C. Effects of free sterols, steryl ester, and steryl glycoside on membrane permeability. Plant Physiol 1971; 48:653-5; PMID:16657855; http://dx.doi.org/ 10.1104/pp.48.5.653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowles D, Lim EK, Poppenberger B, Vaistij FE. Glycosyltransferases of lipophilic small molecules. Annu Rev Plant Biol 2006; 57:567-97; PMID:16669774; http://dx.doi.org/ 10.1146/annurev.arplant.57.032905.105429 [DOI] [PubMed] [Google Scholar]
- 13.Mishra MK, Chaturvedi P, Singh R, Singh G, Sharma LK, Pandey V, Kumari N, Misra P. Overexpression of WsSGTL1 gene of Withania somnifera enhances salt tolerance, heat tolerance and cold acclimation ability in transgenic Arabidopsis plants. PLoS One 2013; 8:e63064; PMID:23646175; http://dx.doi.org/ 10.1371/journal.pone.0063064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Ji-Yeon, Joo Hyejoon, Choy Yoon-Hi, Ha-Lee Young-Mie, Dong-Hee L. Using specialized cDNA microarrays to analyze Arabidopsis gene expression under cold stress J Plant Bio 2010; 53:240-50. [Google Scholar]
- 15.PL Steponkus, M Uemura, RA Balsamo, T Arvinte, DV Lynch. Transformation of the cryobehavior of rye protoplasts by modification of the plasma membrane lipid composition. Proc Natl Acad Sci U S A 1988; 85:9026-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houde M, Belcaid M, Ouellet F, Danyluk J, Monroy AF, Dryanova A, Gulick P, Bergeron A, Laroche A, Links MG, et al. . Wheat EST resources for functional genomics of abiotic stress. BMC Genomics 2006; 7:149; PMID:16772040; http://dx.doi.org/ 10.1186/1471-2164-7-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker NR, Rosenqvist E. Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J Exp Bot 2004; 55:1607-21; PMID:15258166; http://dx.doi.org/ 10.1093/jxb/erh196 [DOI] [PubMed] [Google Scholar]
- 18.Ehlert B, Hincha DK. Chlorophyll fluorescence imaging accurately quantifies freezing damage and cold acclimation responses in Arabidopsis leaves. Plant Methods 2008; 4:12; PMID:18505561; http://dx.doi.org/ 10.1186/1746-4811-4-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aro Eva-Mari, Hundal Torill, Carlberg Inger, Bertil A. In vitro studies on light-induced inhibition of Photosystem II and D1-protein degradation at low temperatures. Biochimica et Biophysica Acta Bioenergetics 1990; 1019:269-75; http://dx.doi.org/ 10.1016/0005-2728(90)90204-H [DOI] [Google Scholar]
- 20.Kunimoto S, Murofushi W, Kai H, Ishida Y, Uchiyama A, Kobayashi T, Kobayashi S, Murofushi H, Murakami-Murofushi K. Steryl glucoside is a lipid mediator in stress-responsive signal transduction. Cell Struct Funct 2002; 27:157-62; PMID:12207046; http://dx.doi.org/ 10.1247/csf.27.157 [DOI] [PubMed] [Google Scholar]
- 21.Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plantarum 1962; 15:473-97; http://dx.doi.org/ 10.1111/j.1399-3054.1962.tb08052.x [DOI] [Google Scholar]
- 22.Guo L, Zhou J, Elling AA, Charron JB, Deng XW. Histone modifications and expression of light-regulated genes in Arabidopsis are cooperatively influenced by changing light conditions. Plant Physiol 2008; 147:2070-83; PMID:18550682; http://dx.doi.org/ 10.1104/pp.108.122929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xin Z, Browse J. Eskimo1 mutants of Arabidopsis are constitutively freezing-tolerant. Proc Natl Acad Sci U S A 1998; 95:7799-804; PMID:9636231; http://dx.doi.org/ 10.1073/pnas.95.13.7799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu JK. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J 2006; 45:523-39; PMID:16441347; http://dx.doi.org/ 10.1111/j.1365-313X.2005.02593.x [DOI] [PubMed] [Google Scholar]
- 25.Larkindale J, Hall JD, Knight MR, Vierling E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol 2005; 138:882–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnon DI. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol 1949; 24:1-15; PMID:16654194; http://dx.doi.org/ 10.1104/pp.24.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maxwell K, Johnson GN. Chlorophyll fluorescence–a practical guide. J Exp Bot 2000; 51:659-68; PMID:10938857; http://dx.doi.org/ 10.1093/jexbot/51.345.659 [DOI] [PubMed] [Google Scholar]
- 28.Pandey V, Niranjan A, Atri N, Chandrashekhar K, Mishra MK, Trivedi PK, Misra P. WsSGTL1 gene from Withania somnifera, modulates glycosylation profile, antioxidant system and confers biotic and salt stress tolerance in transgenic tobacco. Planta 2014; 239:1217-31; PMID:24610300; http://dx.doi.org/ 10.1007/s00425-014-2046-x [DOI] [PubMed] [Google Scholar]
- 29.Misra P, Pandey A, Tiwari M, Chandrashekar K, Sidhu OP, Asif MH, Chakrabarty D, Singh PK, Trivedi PK, Nath P, et al. . Modulation of transcriptome and metabolome of tobacco by Arabidopsis transcription factor, AtMYB12, leads to insect resistance. Plant Physiol 2010; 152:2258-68; PMID:20190095; http://dx.doi.org/ 10.1104/pp.109.150979 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.