Abstract
Plants have to cope with changing seasons and adverse environmental conditions. Being sessile, plants have developed elaborate mechanisms for their survival that allow them to sense and adapt to the environment and reproduce successfully. A major adaptive trait for the survival of trees of temperate and boreal forests is the induction of growth cessation in anticipation of winters. In the last few years enormous progress has been made to elucidate the molecular mechanisms underlying SDs induced growth cessation in model perennial tree hybrid aspen (Populus tremula × P. tremuloides). In this review we discuss the molecular mechanism underlying photoperiodic control of growth cessation and adaptive responses.
Keywords: adaptation, growth cessation, hybrid aspen, perennial trees, photoperiod
Introduction
In temperate regions, the advent of winter is associated with a gradual fall in temperature and day length. Most of the perennial trees anticipate the advent of winter by sensing the reduction in day length while some trees such as apple and pear utilize a reduction in temperature as a signal for the advent of winter. Yet others use a combination of both day length and temperature signal to prepare themselves for winter.1-3 Trees of temperate climates perceive SDs signal and respond by ceasing growth well in advance of winter and protect their apical meristem and leaf primordia within an apical bud.4 In the last decade several studies have been carried out to unveil the molecular mechanism of photoperiodic controlled growth cessation and adaptation using the perennial tree hybrid aspen (Populus tremula × P. tremuloides) as a model plant.5-8 In this review, we discuss photoperiodic control of growth cessation and adaptive response in such trees and describe the unexpected similarities between photoperiodic growth cessation program in trees and flowering time control in model plant Arabidopsis thaliana.
Environmental consequences on seasonal plant growth
Perennial trees with life spans of a few to several hundred years are among the longest living organisms on the earth. One of the keys to their survival is the evolution of highly complicated and sensitive mechanisms to perceive changing environment and modulate their growth accordingly. In boreal and temperate regions where temperatures drop below −30°C during winter, perennial trees stop their growth well ahead of winter to ensure their survival. During autumn, when the winter is approaching, both day length and temperature fall gradually. However, there can be considerable variations in the temperatures in a given season in each year and hence the fall in temperature is not as robust a cue for approaching winter. In contrast, the daily fall in day length prior to winter is constant year after year and is therefore a major robust cue for plants in boreal and temperate regions to understand that winter is approaching in contrast to the fall in temperature as a signal. When day length falls below a critical value, plants undergo growth cessation and stop making new leaves and form an apical bud to protect the Shoot Apical Meristem (SAM). The critical day length value that determines the timing of growth varies with geographical area and comes earlier at higher latitudes9 which face winters earlier. Since it is critical for plants to get the maximum period for their development and reproduction and also enough time for undergoing adaptations to survive the winter, the timing of growth cessation is a trait of major adaptive significance.
In addition to growth cessation, the perennating organs like SAM and leaf primordia, enclosed within the apical bud, initiate physiological processes such as acquisition of cold hardiness (cumulatively referred to as adaptive response) in order to cope with extremely low temperatures (LTs) of temperate and boreal region that are crucial for their survival.4 Without cold hardiness, plants may be subjected to serious injuries due to inter-cellular ice formation which may damage the cellular membrane during winter. LT may also inactivate enzymes thereby affecting essential physiological processes like respiration, photosynthesis etc.10-14 Indeed, failure to undergo growth cessation and acquire cold hardiness severely compromises survival as shown in hybrid aspen plants that fail to sense or respond to SDs.15 Additionally, following growth cessation, SAM and leaf primordia acquire dormancy which is also induced by SDs. Dormancy is considered to be part of the mechanism contributing to survival by preventing precocious activation of growth before spring. In contrast to growth cessation, photoperiodic control of dormancy remains poorly understood and therefore this review focuses primarily on photoperiodic control of growth cessation and its co-ordination with acquisition of cold hardiness.
Molecular mechanism underlying SDs induced growth cessation and adaptation
Plants modulate their growth in response to changes in day length by receiving light signals through photoreceptors like phytochromes that detect red (R)/far-red (FR) light and cryptochromes that detect blue light signal. Molmann et al.16 investigated the impact of light qualities on growth cessation and bud set of Norway spruce (Picea abies). They have shown that blue light treatment delays growth cessation and bud set but cannot prevent it completely. Phytochromes have been shown to mediate short day induced growth cessation and bud set in Populus.17 PHYOTOCHROME A (PHY A) seems to be important in the perception of short days and the subsequent response. Transgenic poplar (hybrid aspen) ectopically expressing oat PHY A had a perturbed SDs response and thus failed to cease growth and bud set.15,18 On the other hand, down regulation of endogenous PHY A in hybrid aspen led to an early response to SDs and an earlier bud set than control plants.18
However, the major breakthrough in how photoperiod controls seasonal growth in trees came through analysis of tree orthologs of Arabidopsis flowering time regulator FT. The expression of FT is modulated by photoperiod with long days inducing the transition from vegetative phase to reproductive phase through expression of FT in Arabidopsis and other plants.19-21 FT is regulated by CONSTANS (CO) protein in a photoperiod dependent manner. CO expression displays a diurnal expression pattern with a peak toward the end of the day. In SDs (long nights) CO expression peaks at night. Since CO protein is highly unstable in dark, CO can only induce FT expression, and thereby flowering, when days are long, the CO protein is stable and accumulates at levels high enough to activate FT.22
The CO/FT module also plays a central role regulating SDs mediated growth cessation in perennial trees.5 After SDs signal perception in trees, FT2 down-regulation is required to induce growth cessation.23 Transgenic poplar over-expressing either FT1 or FT2 show a perturbed SD response that abolishes growth cessation and bud set. In contrast, downregulation of FT2 results in an earlier SD response and plants show early growth cessation and bud set in comparison to control plants.7,24 In addition to down-regulation of FT, additional components also contribute to regulation of the timing of growth cessation since hybrid poplar clones that display similar downregulation of FT expression nevertheless differ in their timing of growth cessation in response to SDs.25
Until recently, the downstream targets of CO/FT module in SD mediated growth cessation were not known. Moreover, since FT by itself lacks DNA binding ability, it was not clear how FT would control the downstream targets. A large scale screen designed to identify new factors involved in photoperiodic growth cessation identified the tree ortholog of the MADS-box protein floral meristem identity gene APETALA1 (AP1), noted as LAP1 (Like-AP1), as the main downstream target of CO/FT module.7 Like FT1 and FT2 over-expressors, LAP1 over-expressors demonstrated a perturbed SDs response and failed to cease growth and bud set, whereas RNAi-mediated down-regulation of LAP1 led to an earlier response to SDs and earlier growth arrest and bud set than control plants.7 Moreover, LAP1 was shown to be regulated by FT and its downregulation could suppress the phenotype of FT over-expressors clearly indicating LAP1 as a key regulator in SDs mediated growth cessation in hybrid aspen.7 Furthermore, LAP1 was found to control the expression of a key cell cycle regulator AINTEGUMENTA-LIKE1 (AIL1), thereby explaining how FT down-regulation by SDs could induce growth cessation. These observations lead us to suggest that day length mediates 2 different developmental pathways namely flowering in annuals such as Arabidopsis and growth cessation in trees. These two pathways are conserved till AP1 and diverge downstream of AP1/LAP1 rather than FT.
Another recent finding is that the flowering time regulator FD, a partner of FT, is also involved in photoperiodic control of seasonal growth. Since FT lacks DNA binding ability it was an open question as to how FT could control LAP1 expression. Although FT lacks DNA binding capacity, it makes a complex with the bZIP transcription factor FLOWERING LOCUS D (FD) and the protein 14-3-3 to make a Florigen Activating Complex (FAC) that binds the regulatory region of the downstream component to induce transition to flowering in the SAM.26 Recently, 2 closely related FD homologs, FDL1 (FD-LIKE1) and FDL2 (FD-LIKE2) with distinct roles have been identified in hybrid aspen.8 FDL1 makes a complex with FT2 and mediates photoperiodic control of growth through control of LAP1 which is the downstream target of this complex.
Intriguingly, FDL1 is also involved in SDs induced transcriptional changes in adaptive responses and bud maturation of these 2 pathways.8 Functional analysis of FDL1 showed that in over-expressors, expression of the adaptive response marker genes such as OSM (OSMOTIN) and LEA (LATE EMBRYOGENESIS ABUNDANT), was much higher than in control plants after SDs treatment, whereas in FDL1-RNAi plants, the expression of these marker genes was lower than in control plants after SDs.8 SDs induced bud maturation is accompanied by accumulation of the phenylpropanoids in the bud scales that gives a dark color to the bud and functions as a good marker. The expression of phenylpropanoid marker genes such as CHS (CHALCONE SYNTHASE), C4H (CINNAMATE 4-HYDROXYLASE) was up-regulated after SDs in control plants but not as prominently in FDL1 over-expressors after SDs. The buds in FDL1 over-expressors were also greener compared to controls. FDL1-RNAi plants showed the opposite phenotype,8 suggesting the role of FDL1 in SDs mediated transcriptional control of adaptive responses and bud maturation pathways.
Conclusions
Timing of growth cessation before the advent of winter in perennial trees of temperate and boreal forests is a major adaptive trait. Photoperiodic signals are perceived in leaves and transduced via CO/FT module to control growth. Repression of FT2 expression is required to induce SDs mediated growth cessation and bud set. Expression of the MADS-box transcription factor LAP1 is positively regulated by FAC, while reduction in FT expression causes reduction in LAP1 expression which results in the downregulation of AIL1. Since core cell-cycle genes are positively regulated by AIL1, its down-regulation results in reduction in expression of core cell-cycle genes which induces growth cessation and bud set. Based on the molecular information available for photoperiodic growth control in perennial trees we propose a model which is adopted from Azeez et al.7 and modified according to Tylewicz et al.8 (Fig. 1A). According to this model, short days lead to growth cessation through suppression of FTs but SDs mediated transcriptional control of adaptive responses to cold and bud maturation pathways requires FDL1 (Fig. 1B).
Figure: 1.
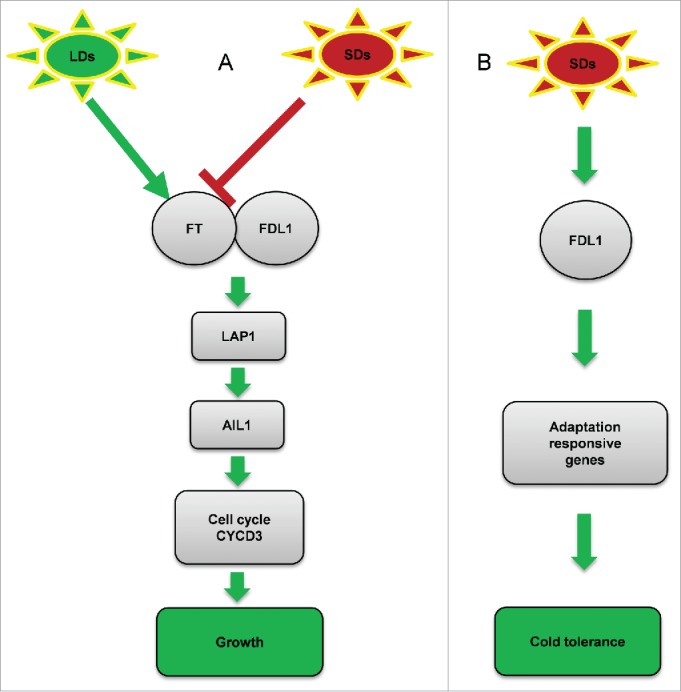
Model for photoperiodic growth control in perennial trees (adopted from Azeez et al.7 and Tylewicz et al.8)
Although the major components of SDs mediated growth control are now known, the site of signal perception is not yet understood. FT in Arabidopsis is expressed in leaves and then transported to the shoot apex where it induces flowering. But there is still no information on FT movement in trees to regulate growth. Another major lacuna is regarding hormonal control of photoperiodic growth. For example, gibberellins (GA) and auxin27 play a role in photoperiodic control of growth but the interplay between CO/FT and GA (or auxin) signaling is still not entirely clear. These and other questions not covered here such as the role of clock will hopefully be answered in the coming years.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Dr. Frantisek Baluska for kindly inviting this mini review and Prof. Rishikesh P. Bhalerao for critical reading of the review. We thank Padmashri B. H. Jain for providing necessary facilities and encouragement.
References
- 1.Samish RM. Dormancy in woody plants. Ann Rev Plant Physiol 1954; 5:183-204; http://dx.doi.org/ 10.1146/annurev.pp.05.060154.001151 [DOI] [Google Scholar]
- 2.Junttila O. Effect of photoperiod and temperature on apical growth cessation in two ecotypes of Salix and Betula. Physiologia Plantarum 1980; 48:347-52; http://dx.doi.org/ 10.1111/j.1399-3054.1980.tb03266.x [DOI] [Google Scholar]
- 3.Heide OM, Prestrud AK. Low temperature, but not photoperiod, controls growth cessation and dormancy induction and release in apple and pear. Tree Physiol 2005; 25:109-14; PMID:15519992; http://dx.doi.org/ 10.1093/treephys/25.1.109 [DOI] [PubMed] [Google Scholar]
- 4.Rohde A, Prinsen E, De Rycke R, Engler G, Van Montagu M, Boerjan W. PtABI3 impinges on the growth and differentiation of embryonic leaves during bud set in poplar. Plant Cell 2002; 14:1885-901; PMID:12172029; http://dx.doi.org/ 10.1105/tpc.003186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Böhlenius H, Huang T, Charbonnel-Campaa L, Brunner AM, Jansson S, Strauss SH, Nilsson O. CO/FT Regulatory Module Controls Timing of Flowering and Seasonal Growth Cessation in Trees. Science (New York, NY) 2006; 312:1040-3; http://dx.doi.org/ 10.1126/science.1126038 [DOI] [PubMed] [Google Scholar]
- 6.Karlberg A, Bako L, Bhalerao RP. Short day-mediated cessation of growth requires the downregulation of AINTEGUMENTALIKE1 transcription factor in hybrid aspen. PLoS Genet 2011; 7:e1002361; PMID:22072988; http://dx.doi.org/ 10.1371/journal.pgen.1002361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azeez A, Miskolczi P, Tylewicz S, Bhalerao RP. A tree ortholog of APETALA1 mediates photoperiodic control of seasonal growth. Curr Biol 2014; 24:717-24; PMID:24656832; http://dx.doi.org/ 10.1016/j.cub.2014.02.037 [DOI] [PubMed] [Google Scholar]
- 8.Tylewicz S, Tsuji H, Miskolczi P, Petterle A, Azeez A, Jonsson K, Shimamoto K, Bhalerao RP. Dual role of tree florigen activation complex component FD in photoperiodic growth control and adaptive response pathways. Proc Natl Acad Sci U S A 2015; 112:3140-5; PMID:25713384; http://dx.doi.org/ 10.1073/pnas.1423440112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howe GT, Hackett WP, Furnier GR, Klevorn RE. Photoperiodic responses of a northern and southern ecotype of black cottonwood. Physiologia Plantarum 1995; 93:695-708; http://dx.doi.org/ 10.1111/j.1399-3054.1995.tb05119.x [DOI] [Google Scholar]
- 10.Uemura M, Gilmour SJ, Thomashow MF, Steponkus PL. Effects of COR6.6 and COR15am polypeptides encoded by COR (cold-regulated) genes of Arabidopsis thaliana on the freeze-induced fusion and leakage of liposomes. Plant Physiol 1996; 111:313-27; PMID:8685271; http://dx.doi.org/ 10.1104/pp.111.1.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uemura M, Joseph RA, Steponkus PL. Cold acclimation of arabidopsis thaliana (effect on plasma membrane lipid composition and freeze-induced lesions). Plant Physiol 1995; 109:15-30; PMID:12228580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webb MS, Steponkus PL. Freeze-induced membrane ultrastructural alterations in rye (secale cereale) leaves. Plant Physiol 1993; 101:955-63; PMID:12231747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivanov AG, Sane PV, Zeinalov Y, Malmberg G, Gardestrom P, Huner NP, Oquist G. Photosynthetic electron transport adjustments in overwintering Scots pine (Pinus sylvestris L.). Planta 2001; 213:575-85; PMID:11556790; http://dx.doi.org/ 10.1007/s004250100522 [DOI] [PubMed] [Google Scholar]
- 14.Ivanov AG, Sane PV, Zeinalov Y, Simidjiev I, Huner NP, Oquist G. Seasonal responses of photosynthetic electron transport in Scots pine (Pinus sylvestris L.) studied by thermoluminescence. Planta 2002; 215:457-65; PMID:12111228; http://dx.doi.org/ 10.1007/s00425-002-0765-x [DOI] [PubMed] [Google Scholar]
- 15.Olsen JE, Junttila O, Nilsen J, Eriksson ME, Martinussen I, Olsson O, Sandberg G, Moritz T. Ectopic expression of oat phytochrome A in hybrid aspen changes critical daylength for growth and prevents cold acclimatization. Plant J 1997; 12:1339-50; http://dx.doi.org/ 10.1046/j.1365-313x.1997.12061339.x [DOI] [Google Scholar]
- 16.Molmann JA, Junttila O, Johnsen O, Olsen JE. Effects of red, far-red and blue light in maintaining growth in latitudinal populations of Norway spruce (Picea abies). Plant, Cell Environ 2006; 29:166-72; http://dx.doi.org/ 10.1111/j.1365-3040.2005.01408.x [DOI] [PubMed] [Google Scholar]
- 17.Howe GT, Gardner G, Hackett WP, Furnier GR. Phytochrome control of short-day-induced bud set in black cottonwood. Physiologia Plantarum 1996; 97:95-103; http://dx.doi.org/ 10.1111/j.1399-3054.1996.tb00484.x [DOI] [Google Scholar]
- 18.Kozarewa I, Ibanez C, Johansson M, Ogren E, Mozley D, Nylander E, Chono M, Moritz T, Eriksson ME. Alteration of PHYA expression change circadian rhythms and timing of bud set in Populus. Plant Mol Biol 2010; 73:143-56; PMID:20229130; http://dx.doi.org/ 10.1007/s11103-010-9619-2 [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi Y, Weigel D. Move on up, it's time for change–mobile signals controlling photoperiod-dependent flowering. Gen Dev 2007; 21:2371-84; PMID:17908925; http://dx.doi.org/ 10.1101/gad.1589007 [DOI] [PubMed] [Google Scholar]
- 20.Turck F, Fornara F, Coupland G. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Ann Rev Plant Biol 2008; 59:573-94; PMID:18444908; http://dx.doi.org/ 10.1146/annurev.arplant.59.032607.092755 [DOI] [PubMed] [Google Scholar]
- 21.Wigge PA. FT, a mobile developmental signal in plants. Curr Biol 2011; 21:R374-8; PMID:21549960; http://dx.doi.org/ 10.1016/j.cub.2011.03.038 [DOI] [PubMed] [Google Scholar]
- 22.Suarez-Lopez P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 2001; 410:1116-20; PMID:11323677; http://dx.doi.org/ 10.1038/35074138 [DOI] [PubMed] [Google Scholar]
- 23.Hsu CY, Adams JP, Kim H, No K, Ma C, Strauss SH, Drnevich J, Vandervelde L, Ellis JD, Rice BM, et al.. FLOWERING LOCUS T duplication coordinates reproductive and vegetative growth in perennial poplar. Proc Natl Acad Sci U S A 2011; 108:10756-61; PMID:21653885; http://dx.doi.org/ 10.1073/pnas.1104713108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu CY, Liu Y, Luthe DS, Yuceer C. Poplar FT2 shortens the juvenile phase and promotes seasonal flowering. Plant Cell 2006; 18:1846-61; PMID:16844908; http://dx.doi.org/ 10.1105/tpc.106.041038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Resman L, Howe G, Jonsen D, Englund M, Druart N, Schrader J, Antti H, Skinner J, Sjodin A, Chen T, et al.. Components acting downstream of short day perception regulate differential cessation of cambial activity and associated responses in early and late clones of hybrid poplar. Plant Physiol 2010; 154:1294-303; PMID:20847139; http://dx.doi.org/ 10.1104/pp.110.163907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science (New York, NY) 2005; 309:1052-6; http://dx.doi.org/ 10.1126/science.1115983 [DOI] [PubMed] [Google Scholar]
- 27.Baba K, Karlberg A, Schmidt J, Schrader J, Hvidsten TR, Bako L, Bhalerao RP. Activity-dormancy transition in the cambial meristem involves stage-specific modulation of auxin response in hybrid aspen. Proc Natl Acad Sci U S A 2011; 108:3418-23; PMID:21289280; http://dx.doi.org/ 10.1073/pnas.1011506108 [DOI] [PMC free article] [PubMed] [Google Scholar]


