Abstract
Phytohormones are key players in signaling environmental stress conditions. Hormone profiling together with proline accumulation were studied in leaves and roots of different mutant lines of Arabidopsis. Regulation of proline accumulation in this system seems complex and JA-deficient (jar1-1) and JA-insensitive (jai1) lines accumulating high levels of proline despite their very low ABA levels seems to discard an ABA-dependent response. However, the pattern of proline accumulation in jai1 seedlings parallels that of ABA. Under stress conditions, there is an opposite pattern of ABA accumulation in roots of jar1-1/coi1-16 (in which ABA only slightly increase) and jai1 (in which ABA increase is even higher than in WT plants). This also makes JA-ABA crosstalk complex and discards any lineal pathway that could explain this hormonal interaction.
Keywords: ABA, coi1-16, drought, hormone signal transduction, JA-Ile, jar1-1, proline
Proline accumulation is commonly used as a marker of plant responses to stress conditions such as drought,1 and salinity,2 and a causal relationship between proline synthesis and hyperosmotic stress tolerance has been demonstrated.3 It was postulated that proline might play a role as an organic osmotic regulator,4 nevertheless, the contribution of proline accumulation to osmotic adjustment in some Species seems to be quite small. Besides its role in osmotic regulation, proline can function as a chaperonine, protecting proteins and membranes from degradation. There are also studies linking proline accumulation to ROS scavenging activity.5
Whereas proline accumulation under stress conditions is extensively documented, the molecular basis of the signaling underlying its regulation has not been clearly established. Proline accumulation seems to be mediated by both ABA-dependent and ABA-independent pathways. The dominant role of ABA in mediating plant responses to water stress has been questioned several times, basically due to a poor correlation between ABA concentrations and physiological and growth parameters such as stomatal conductance or inhibition of shoot growth.6 This led to research focused on the ABA-independent pathways regulating water stress responses.7
In a previous publication it was shown that jasmonates and in particular Jasmonic acid Isoleucine (JA-Ile) accumulation and, hence JA-dependent signaling, was necessary for consistent ABA accumulation in roots of Arabidopsis.8 Although plants defective in JA-Ile accumulation (jar1-1) and JA-Ile dependent signaling (coi1-16) were able to accumulate ABA in roots after dehydration, accumulation was significantly lower compared to their respective WT. Interestingly, in complementary experiments using the same system, JA transiently accumulates in roots of dehydrated plants but this increase is coupled with a decrease in the hormone concentration in shoots. It is also important to note that JA-dependent build-up of ABA seems restricted to the roots, at least in short-term responses. However, according to,9 leaf ABA accumulation in Arabidopsis is also influenced by JA in long-term responses. Moreover, exogenous treatment with jasmonates increased ABA levels in different Species both under control conditions or exposed to drought.10,11 The reciprocal treatment with exogenous ABA had no significant effect on endogenous JA levels in citrus plants.11
In order to understand the effect of JA on plant responses to desiccation, proline accumulation after dehydration was analyzed in different Arabidopsis seedlings: a line defective in JA-Ile biosynthesis (jar1-1), 2 JA insensitive lines (jai1 and coi1-16), and an ABA insensitive line (abi2).
Relative water content (RWC) was similar in well-watered plants of the different genotypes. After stress imposition, this parameter decreased a 25% in average with no significant differences among genotypes with the exception of jar1-1where lower values were recorded (38% of reduction with respect control plants).
After 300 min of desiccation all lines but jar1-1 accumulated high concentrations of JA-Ile in roots (Fig. 1, bottom). In leaves, only jai1 and coi1-16 accumulated JA-Ile after dehydration (Fig. 1, top). ABA increased in roots of all lines except in jar1-1 and coi1-16; surprisingly, jai1 (defective in one of the 2 branches of JA signaling) not only accumulated ABA to the same extent that WT plants but levels were the highest among lines. It is interesting that even under well-watered conditions jai1 seedlings had a significantly higher ABA content in roots (Fig. 2, bottom). On the other hand, ABA accumulation in leaves was similar among the different lines (Fig. 2, top). It is also important to point out that abi2 mutants accumulated the same amount of JA-Ile than WT plants, indicating that ABA-dependent signaling has no influence on jasmonate biosynthesis under dehydration.
Figure 1.
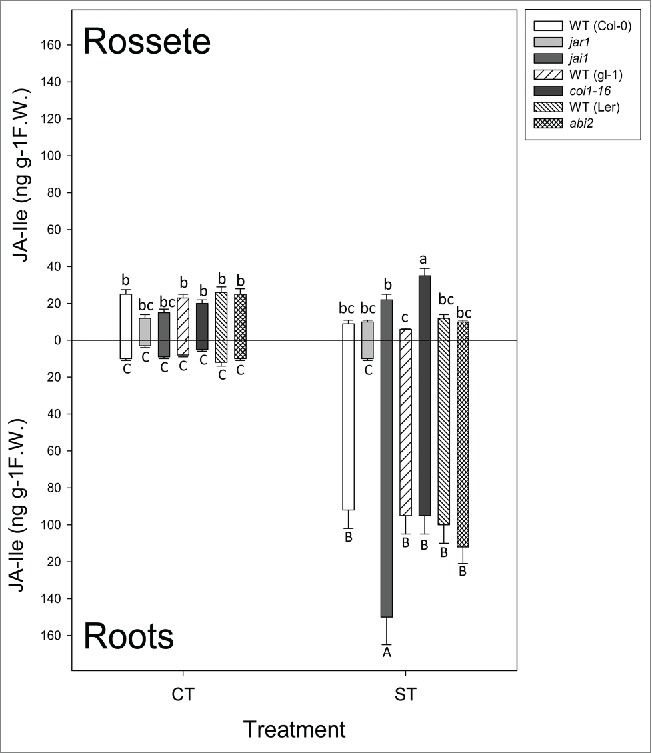
Jasmonoyl Isoleucine (JA-Ile) in leaves (top) and roots (bottom) of Arabidopsis under control conditions (CT), and after 300 min of desiccation (ST). Lines under treatment: Columbia-0 (Col-0), WT background for jar1-1 and jai1, glabra (gl-1), WT background for coi1-16, Lansberg Erecta (Ler), WT background for abi2. Data are mean values ± standard deviation of 3 independent determinations. Letters denote statistical significance after Fisher´s LSD (p ≤ 0.05) between lines under the same conditions.
Figure 2.
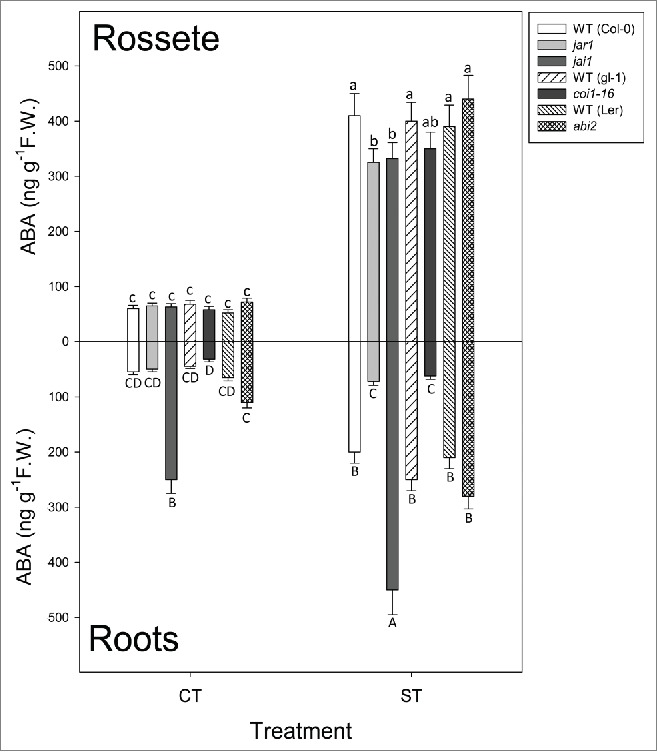
Abscisic acid (ABA) in leaves (top) and roots (bottom) under control conditions (CT), and after 300 min of desiccation (ST). Lines under treatment: Columbia-0 (Col-0), WT background for jar1-1 and jai1, glabra (gl-1), WT background for coi1-16, Lansberg Erecta (Ler), WT background for abi2. Data are mean values ± standard deviation of 3 independent determinations. Letters denote statistical significance after Fisher´s LSD (p ≤ 0.05) between lines under the same conditions.
Proline accumulation was also different among the studied genotypes after dehydration; only the JA-deficient/insensitive lines had a significant proline accumulation in roots with respect to well-watered plants, and among these lines jai1 seedlings accumulated more proline than the rest (Fig. 3, bottom). Similarly, in leaves, JA-deficient/insensitive lines accumulated more proline than other lines. It must be noted that, in the aerial part, proline concentration increased in WT lines (Col-0, gl-1 and Ler) to lower concentrations than in JA-deficient/insensitive lines. Proline levels in roots of abi2 seedlings did not significantly increase with respect to well-watered plants whereas in shoots, proline accumulation after desiccation increased to a similar extent than in jai1 plants (around 25% increase). However, these levels were much lower than the accumulation found in jar1 and coi1-16 (186% and 144% increase, respectively, Fig. 3, top).
Figure 3.
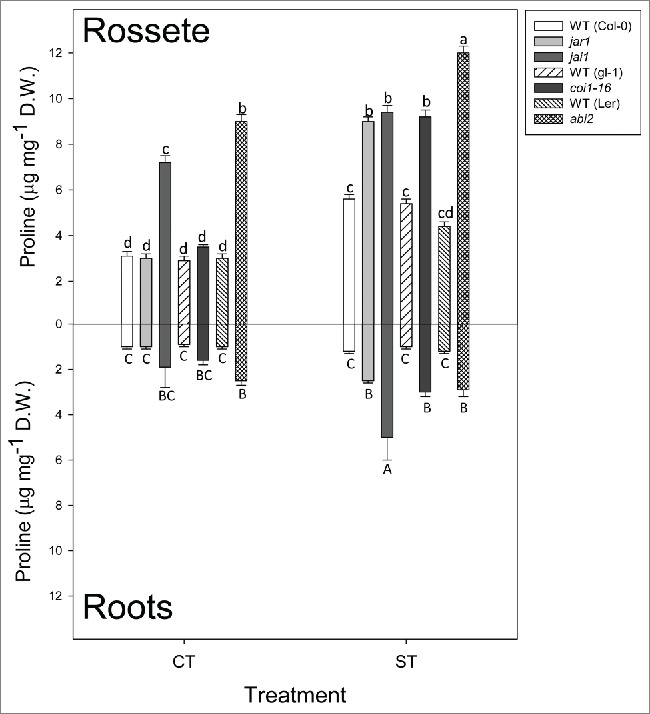
Proline in leaves (top) and roots (bottom) under control conditions (CT), and after 300 min of desiccation (ST). Lines under treatment: Columbia-0 (Col-0), WT background for jar1-1 and jai1, glabra (gl-1), WT background for coi1-16, Lansberg Erecta (Ler), WT background for abi2. Data are mean values ± standard deviation of 3 independent determinations. Letters denote statistical significance after Fisher´s LSD (p ≤ 0.05) between lines under the same conditions.
The pattern of proline accumulation is difficult to explain in terms of ABA accumulation/signaling: first, proline accumulation in jai1 seedlings parallels that of ABA but the fact that the JA-defective/insensitive seedlings are those that differentially accumulate more proline in roots and shoots despite the lower ABA accumulation in roots points to a non-ABA related phenotype.
Regarding the JA-ABA crosstalk in roots it is surprising that jai1 seedlings not only were able to accumulate ABA to the same extend than WT lines but levels of this hormone were even higher when exposed to the same dehydration conditions. The JA-insensitiveness in jai1 is caused by the loss of function of the MYC2 transcription factor (TF) that performs several functions such as controlling the activation of the PDF2.1/HEL branch of the JA-dependent signaling, antagonizing the VSP1/LOX2 branch, and organizing ABA dependent responses.12 Therefore, the promiscuity of MYC2 makes very difficult to assess its role in JA/ABA crosstalk. Nonetheless, the completely opposite behavior in terms of ABA accumulation in roots of jar1-1/coi1-16 (lower levels) and jai1 (higher levels) makes this crosstalk very interesting in terms of understanding the modulation of ABA synthesis and signaling in response to stress.
MYC2 has been defined as an ABA and drought responsive gene.12 Constitutive expression of MYC2 and gene disruption by knockout resulted in enhanced and reduced sensitivity to ABA,13,14 respectively. In addition, MYC2 activates the expression of RD22 suggesting a positive role on ABA signaling.13 However, according to,15 Arabidopsis myc2/jai1 seedlings showed increased drought tolerance (based on biomass reduction data). Therefore, myc2 phenotype under drought conditions is far from being completely characterized.
The fact that JA is involved in plant responses to drought also raises some interesting questions about the ABA-JA crosstalk. ABA seems to suppress the ERF1/PDF1.2 branch of the JA pathway but strongly induces the MYC2-VSP1 branch of JA signaling pathway. Interestingly, it has been demonstrated that TFs ANAC019 and ANAC055 (containing a NAC domain) also induce this pathway, being this effect reduced in myc2 mutants.16 These TFs are also activated by JA which suggest that ANAC019 and ANAC055 act downstream of both JA and ABA. A recent study has shown that ANAC019, ANAC055 and the homologous ANAC072 play synergistic and antagonistic roles in ABA signaling and osmotic stress.17
Recently CML37, a Ca2+ sensor involved in the regulation of herbivore-induced plant defenses mediated by jasmonates was also identified as a positive regulator of ABA in drought stress.18 The cml37-1 mutant displays a lower expression of JA related genes in response to wounding and, interestingly, this mutant also has a reduced ABA accumulation in response to drought stress,19 moreover, another member of this Ca 2+ sensor family, CML42 has antagonistic effects and Arabidopsis mutant defective in this protein is able to accumulate ABA in response to drought.
Results here presented and recent literature point to a complex crosstalk between JA and ABA. Both signaling pathways seem to act together modulating each other responses and hence other metabolic and signaling pathways of responses to biotic and abiotic stress. Although challenging, this complex interplay offers the opportunity to modulate plants responses to various types of stress.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
Hormonal profiles were performed at Instrumental central facilities (SCIC) of Universitat Jaume I.
Funding
This work was supported by the Spanish Ministerio de Economía y Competitividad (MINECO) through grant AGL2013-42038R.
References
- 1.Choudhary NL, Sairam RK, Tyagi A. Expression of Delta(1)-pyrroline-5-carboxylate synthetase gene during drought in rice (Oryza sativa L.). Indian J Biochem Biophys 2005; 42:366-70; PMID:16955737 [PubMed] [Google Scholar]
- 2.Yoshiba Y, kiyosue T, KatagirI T, Ueda H, Mizoguchi T, Yamaguchishinozaki K, Wada K, Harada Y, Shinozaki K. Correlation between the induction of a gene for delta(1)-pyrroline- 5-carboxylate synthetase and the accumulation of proline in Arabidopsis thaliana under osmotic stress. Plant J 1995; 7:751-60; PMID:7773306; http://dx.doi.org/ 10.1046/j.1365-313X.1995.07050751.x [DOI] [PubMed] [Google Scholar]
- 3.Kishor PBK, Hong ZL, Miao GH, Hu CAA, Verma DPS. Overexpression of delta-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol 1995; 108:1387-94; PMID:12228549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kishor PBK, Sangam S, Amrutha RN, Laxmi PS, Naidu KR, Rao K, Rao S, Reddy KJ, Theriappan P, Sreenivasulu N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci 2005; 88:424-38 [Google Scholar]
- 5.Szabados L, Savoure A. Proline: a multifunctional amino acid. Trends Plant Sci 2010; 15:89-97; PMID:20036181; http://dx.doi.org/ 10.1016/j.tplants.2009.11.009 [DOI] [PubMed] [Google Scholar]
- 6.Hare PD, Cress WA. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul 1997; 21:79-102; http://dx.doi.org/ 10.1023/A:1005703923347 [DOI] [Google Scholar]
- 7.Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front Plant Sci 2014; 5:170; PMID:24904597; http://dx.doi.org/ 10.3389/fpls.2014.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Ollas C, Arbona V, Gómez-Cadenas A. Jasmonoyl isoleucine accumulation is needed for abscisic acid build-up in roots of Arabidopsis under water stress conditions. Plant Cell Environ 2015; 38:2157-2170 In press; PMID:25789569 [DOI] [PubMed] [Google Scholar]
- 9.Brossa R, Lopez-Carbonell M, Jubany-Mari T, Alegre L. Interplay between abscisic acid and jasmonic acid and its tole in water-oxidative stress in wild-type, ABA-deficient, JA-deficient, and ascorbate-deficient Arabidopsis plants. J Plant Growth Regul 2011; 30:322-33; http://dx.doi.org/ 10.1007/s00344-011-9194-z [DOI] [Google Scholar]
- 10.Sánchez-Romera B, Ruiz-Lozano JM, Liu G, Luu DT, Martínez-Ballesta MC, Carvajal M, Zamarreño AM, Garcia-Mina JM, Maurel C, Aroca R. Enhancement of root hydraulic conductivity by methyl jasmonate and the role of calcium and abscisic acid in this process. Plant Cell Environ 2014; 37:995-1008; http://dx.doi.org/ 10.1111/pce.12214 [DOI] [PubMed] [Google Scholar]
- 11.De Ollas C, Hernando B, Arbona V, Gómez-Cadenas A. Jasmonic acid transient accumulation is needed for abscisic acid increase in citrus roots under drought stress conditions. Physiol Plant 2013; 147:296-306; PMID:22671923; http://dx.doi.org/ 10.1111/j.1399-3054.2012.01659.x [DOI] [PubMed] [Google Scholar]
- 12.Kazan K, Manners JM. MYC2: the master in action. Mol Plant 2013; 6:686-703; PMID:23142764; http://dx.doi.org/ 10.1093/mp/sss128 [DOI] [PubMed] [Google Scholar]
- 13.Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003; 15:63-78; PMID:12509522; http://dx.doi.org/ 10.1105/tpc.006130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorenzo O, Chico JM, Sánchez-Serrano JJ, Solano R. JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 2004; 16:1938-50; PMID:15208388; http://dx.doi.org/ 10.1105/tpc.022319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harb A, Krishnan A, Ambavaram MMR, Pereira A. Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol 2010; 154:1254-1271; PMID:20807999; http://dx.doi.org/ 10.1104/pp.110.161752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bu Q, Jiang H, Li CB, Zhai Q, Zhang J, Wu X, Sun J, Xie Q, Li C. Role of the Arabidopsis thaliana NAC transcription factors ANAC019 and ANAC055 in regulating jasmonic acid-signaled defense responses. Cell Res 2008; 18:756-67; PMID:18427573; http://dx.doi.org/ 10.1038/cr.2008.53 [DOI] [PubMed] [Google Scholar]
- 17.Li XY, Li L, Liu X, Zhang B, Zheng WL, Ma WL. Analysis of physiological characteristics of abscisic acid sensitivity and salt resistance in Arabidopsis anac mutants (ANAC019, ANAC072 AND ANAC055). Biotechnol Biotechnol Equip 2012; 26:2966-70; http://dx.doi.org/ 10.5504/BBEQ.2012.0039 [DOI] [Google Scholar]
- 18.Scholz SS, Vadassery J, Heyer M, Reichelt M, Bender KW, Snedden WA, Boland W, Mithöfer A. Mutation of the Arabidopsis calmodulin-like protein CML37 deregulates the jasmonate pathway and enhances susceptibility to herbivory. Mol Plant 2014; 7:1712-26; PMID:25267731; http://dx.doi.org/ 10.1093/mp/ssu102 [DOI] [PubMed] [Google Scholar]
- 19.Scholz SS, Reichelt M, Vadassery J, Mithöfer A. Calmodulin-like protein CML37 is a positive regulator of ABA during drought stress in Arabidopsis. Plant Signal Behav 2015; 10(6):e1011951, In press; PMID:26176898 [DOI] [PMC free article] [PubMed] [Google Scholar]


