Abstract
Purpose
To determine the effect of vitrectomy for center-involved diabetic macular edema (CI-DME).
Methods
This was a retrospective study of 53 eyes of 45 patients who had vitrectomy for CI-DME and were followed up for at least 12 months. Charts were reviewed for visual acuity (VA), central subfield mean thickness measured by optical coherence tomography, presurgical and postsurgical interventions for CI-DME, and number of office visits in the first 12 months after surgery. Preoperative spectral domain optical coherence tomography was performed on 38 patients, and they were graded for ellipsoid zone (EZ) intactness by three independent graders with assessment of agreement between graders using intraclass correlation coefficients and Bland–Altman analysis.
Results
The median VA improved from 20/100 (interquartile range [IQR], 20/63–20/200) at baseline to 20/63 (IQR, 20/32–20/125) at 12 months. The median central subfield mean thickness improved from 505 μm (IQR, 389–597 μm) at baseline to 279 μm (IQR, 246–339 μm) at 12 months. Intergrader agreement for EZ intactness was moderate (intraclass correlation coefficients 0.4294–0.6356). There was no relationship between preoperative intactness of the EZ and the 12-month change in VA.
Conclusion
Vitrectomy consistently thins the macula in CI-DME and, on average, leads to clinically significant improvement in VA comparable in size to that reported with serial intravitreal anti-vascular endothelial growth factor injections. A large, comparative, prospective, randomized clinical trial of these two treatments is needed to determine which is more effective and cost-effective.
Keywords: center-involved diabetic macular edema, diabetic macular edema, vitrectomy, spectral domain OCT
Introduction
Center-involved diabetic macular edema (CI-DME) is the most common cause of decreased visual acuity (VA) in patients with diabetic retinopathy.1 In the US, ~750,000 patients are affected.2 The standard of care for treatment of DME in developed countries is serial intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) drugs.3 However, this regimen is associated with residual edema in 25%–64% of eyes, is expensive, and entails a burdensome schedule of regular office visits with repeated injections that may last for 5 years, which is unfeasible in a large fraction of patients.3–5 In some countries with limited resources available for medical care with expensive pharmaceuticals, serial anti-VEGF therapy is not an option.
An alternate treatment approach to CI-DME is vitrectomy with internal limiting membrane (ILM) peeling, laser photocoagulation, and perioperative intraocular corticosteroid therapy.6,7 Compared to anti-VEGF therapy, vitrectomy is attractive because it is potentially less expensive and is associated with a smaller visit burden with longer lasting effects. Unlike serial anti-VEGF therapy, vitrectomy has never been examined in an adequately powered, prospective, randomized, controlled clinical trial. Many published retrospective case series, fewer prospective case series, and rare, small prospective randomized clinical trials using focal/grid laser as a control arm show that vitrectomy thins the edematous macula but inconsistently improves VA.8,9
We report a single-surgeon case series that adds to the evidence that, on average, VA and macular edema improve after vitrectomy. We also examine whether preoperative intactness of the ellipsoid zone (EZ) line on spectral domain optical coherence tomography (SD-OCT) predicts the VA outcome.
Methods
A retrospective chart review was performed on all patients who underwent vitrectomy for CI-DME between May 2005 and May 2014 by one surgeon (DJB) in a private retina practice setting. Presence of a cataract was not an exclusion criterion as long as the view to the fundus was adequate to perform vitreoretinal surgery. An exclusion criterion was follow-up for <1 year. The primary outcomes were change in corrected ETDRS VA with spectacles and pinhole at 12 months and change in central subfield thickness at 12 months. Secondary outcomes included the frequency of postoperative cataract surgery, interventions for refractory or recurrent CI-DME, and number of office visits in the first 12 months after surgery.
A subset of 38 eyes (69%) had SD-OCT studies performed preoperatively. For these patients, intactness of the EZ was independently graded by three retina specialists (DJB, MWS, and MBL) in a masked fashion. The grade assigned was the percentage of EZ that was intact within 500 μm of the fovea on a horizontal SD-OCT scan through the fovea obtained at the preoperative visit. Scans were performed on either a Zeiss Cirrus (Carl Zeiss Meditec AG, Jena, Germany) or a Heidelberg Spectralis (Heidelberg Engineering, Franklin, MA, USA) instrument. We converted all Spectralis measurements to their equivalent values with the Cirrus instrument by adding 19.3 μm to the Spectralis measurement, which was the average difference between Spectralis and Cirrus measurements found by Lammer et al.10 Intergrader agreement was calculated using intraclass correlation coefficients (ICCs) and Bland–Altman analyses yielding mean difference in grades between graders and the limits of agreement between graders. ICCs of 0–0.2 indicate poor agreement, 0.3–0.4 indicate fair agreement, 0.5–0.6 indicate moderate agreement, 0.7–0.8 indicate strong agreement, and >0.8 indicate almost perfect agreement. The relationship of preoperative EZ status and VA outcome was assessed by analysis of variance using the average of the three grades of EZ intactness as the independent variable.
The operative procedure was not identical for all patients and varied according to retinal pathology and era during which surgery was performed. All patients underwent vitrectomy with removal of the posterior hyaloid and performance of panretinal photocoagulation of 1,000–1,500 spots. All surgeries were performed with 25 G instruments. Patients with macular epiretinal membranes (ERMs) underwent peeling of the membranes such that the foveal avascular zone was uninvolved after surgery. In the early years of the series, the ILM was not peeled. In the later years, the ILM was peeled after staining with indocyanine green (0.5 mg/mL, for 30 seconds). A circular area with a radius of 1–2 disc diameters from the fovea was peeled. Intravitreal injection of corticosteroid was not performed in earlier cases. In later cases, intravitreal corticosteroid, initially as triamcinolone acetonide, 4 mg, and later as a dexamethasone, 0.7 mg, slow release device, was used as an adjunct to surgery to decrease early postoperative inflammation.
Descriptive statistics were computed with JMP 4.0 software (SAS Institute Inc., Cary, NC, USA). ICCs and Bland–Altman analyses were carried out with Medcalc software 15.11.1 (Medcalc, Ostend, Belgium). Waiver of both informed consent and Health Insurance Portability and Accountability Act authorization for this retrospective study of de-identified patient data were approved by the Presbyterian Hospital Institutional Review Board.
Results
Fifty-three eyes of 45 patients who underwent vitrectomy for CI-DME between 2005 and 2014 were analyzed. The median age of the patients was 66 years (interquartile range [IQR], 55–72 years), 19 (42%) were females, and 39 (87%) had type 2 diabetes. Other demographic characteristics of the patients are given in Table 1.
Table 1.
Demographic data
| Characteristic | Value |
|---|---|
| Age (years) | Median 66, IQR (55–72) |
| Sex, F:M | 19:26 |
| Diabetes type, 1:2 | 6:39 |
| Dialysis, Y:N | 0:45 |
| HbA1c known, Y:N | 19:26 |
| HbA1c (%) when known | Median 7.2, IQR (6.6–8.0) |
| Ethnicity | |
| White | 37 (82.2%) |
| Black | 3 (6.7%) |
| South Asian | 2 (4.4%) |
| East Asian | 2 (4.4%) |
| Middle Eastern | 1 (2.2%) |
| Diastolic BP (mmHg) | Median 80, IQR (71–86) |
| Systolic BP (mmHg) | Median 138, IQR (124–150) |
| Mean arterial blood pressure (mmHg) | Median 98, IQR (92–107) |
Note: Mean arterial blood pressure = [systolic blood pressure + 2 (diastolic blood pressure)]/3.
Abbreviations: IQR, interquartile range; F, female; M, male; Y, yes; N, no; HbA1C, hemoglobin A1C; BP, blood pressure.
Previous treatments for DME and diabetic retinopathy were common, although 18 eyes had received no treatment for DME before the vitrectomy. Thirty-five eyes had previous treatments, which are detailed in Table 2.
Table 2.
Previous treatments for diabetic retinopathy
| Previous treatments for diabetic retinopathy | Yes | Number of indicated treatments
|
No | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Other* | |||
| Focal/grid | 35 | 11 | 17 | 5 | 1 | 0 | 6 (1) | 18 |
| PRP | 16 | 8 | 6 | 2 | 0 | 0 | 0 | 37 |
| Bevacizumab | 17 | 6 | 4 | 1 | 3 | 0 | 8 (1), 12 (1), 19 (1) | 36 |
| Ranibizumab | 3 | 2 | 0 | 0 | 0 | 0 | 10 (1) | 50 |
| Triamcinolone | 18 | 10 | 6 | 2 | 0 | 0 | 0 | 35 |
Notes:
These data are formatted as number of treatments (number of patients receiving that number of treatments).
Abbreviations: Focal, focal laser photocoagulation; grid, grid laser photocoagulation; bevacizumab, intravitreal bevacizumab; ranibizumab, intravitreal ranibizumab; triamcinolone, intravitreal triamcinolone; PRP, panretinal photocoagulation.
Preoperatively, 29 eyes (55%) were pseudophakic. As part of the vitrectomy (three eyes) or during the 12 months following vitrectomy (nine eyes), 12 eyes (23%) had uncomplicated phacoemulsification cataract extraction with posterior chamber intraocular lens implantation. Twelve eyes (23%) were phakic at the 12-month end point.
The median VA improved from baseline (20/100; IQR, 20/63–20/200) to 3 months, followed by stabilization to 12 months (20/63; IQR, 20/32–20/125) (Figure 1). The median central subfield mean thickness (CSMT) followed a similar course from baseline (505 μm; IQR, 389–597 μm) to 12 months (279 μm; IQR, 246–339 μm) (Figure 2). ERM peeling and ILM peeling were performed in 23 cases (43%) and 35 cases (66%), respectively. No intraocular corticosteroid was used in eight cases (15%), whereas an intraoperative intravitreal triamcinolone acetonide 4 mg and an intraoperative dexamethasone implant (0.7 mg) were used in 29 cases (55%) and 16 cases (30%), respectively. In univariate analysis of variance, peeling of the ILM was associated with a smaller 12-month improvement in the logarithm of the minimum angle of resolution (logMAR) VA (P=0.0070), peeling of an ERM was associated with a larger improvement in VA (P=0.0425), but the use of intraocular steroids had no effect (P=0.8282) (Table 3). In a multivariate analysis, none of these three variables had a significant effect on 12-month change in VA (Table 3).
Figure 1.
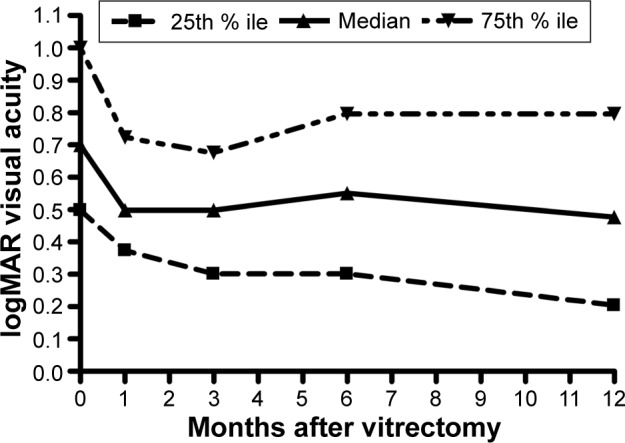
Time course of response of the logMAR VA after vitrectomy for diabetic macular edema.
Abbreviations: % ile, percentile; logMAR, logarithm of the minimum angle of resolution; VA, visual acuity.
Figure 2.
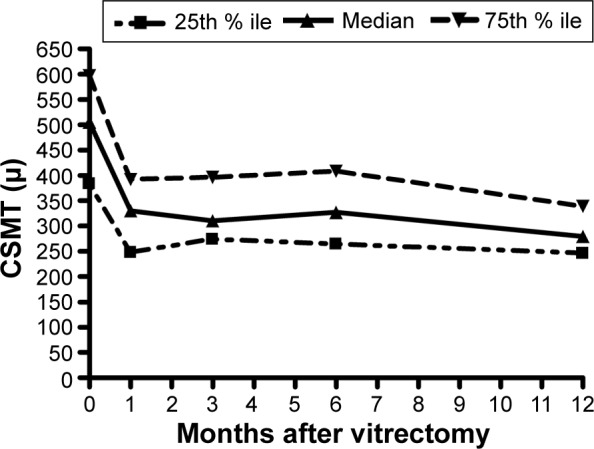
Time course of response of the CSMT after vitrectomy for diabetic macular edema.
Abbreviations: % ile, percentile; CSMT, central subfield mean thickness.
Table 3.
Effects of operative steps on 12-month change in logMAR VA
| Intraoperative step | Univariable analysis | Multivariable analysis |
|---|---|---|
| Peeling ILM | 0.0070 | 0.0752 |
| Peeling ERM | 0.0425 | 0.8684 |
| Intraocular steroid | 0.8282 | 0.7638 |
Notes: Intraocular steroid: intraocular triamcinolone or dexamethasone sustained release device. The cell entries are P-values of univariable (column 2) or multivariable (column 3) regression analyses of 12-month change in logMAR VA as a function of one or more of the steps shown in column 1.
Abbreviations: logMAR, logarithm of the minimum angle of resolution; VA, visual acuity; ILM, internal limiting membrane; ERM, epiretinal membrane.
There were no intraoperative complications in the 53 cases reviewed and no secondary vitreoretinal surgeries performed in the 12 months following the vitrectomy for DME. The median number of office visits for the 12 months following surgery was 7 (IQR, 5–9).
Table 4 shows the reproducibility of the three graders for EZ intactness. The ICCs ranged from 0.4294 to 0.6356 or moderate agreement among the three graders when assessed pairwise. The median grade of preoperative EZ intactness was 61% (IQR, 30%–93%). Figure 3 shows the relationship of the 12-month change in logMAR VA as a function of the preoperative intactness of EZ (mean of the three graders). No relationship was demonstrated either clinically or statistically (P=0.5641).
Table 4.
Reproducibility of graders for EZ intactness
| Grader comparison | ICC | 95% CI | Mean difference | Limits of agreement |
|---|---|---|---|---|
| 1 versus 2 | 0.5803 | (0.3243, 0.7573) | 0.05 | (−0.68, 0.78) |
| 1 versus 3 | 0.6356 | (0.2464, 0.8219) | −0.21 | (−0.79, 0.37) |
| 2 versus 3 | 0.4294 | (0.0599, 0.6793) | −0.25 | (−0.95, 0.44) |
Notes: Mean difference represents the mean of the differences of the graders’ scores indicated by the row label over the 38 eyes with preoperative spectral domain optical coherence tomographs. Limits of agreement are the mean difference plus and minus 1.96 times the standard deviation of the differences of the graders’ scores indicated by the row label over the 38 eyes with preoperative spectral domain optical coherence tomographs.
Abbreviations: EZ, ellipsoid zone; ICC, intraclass correlation coefficient.
Figure 3.
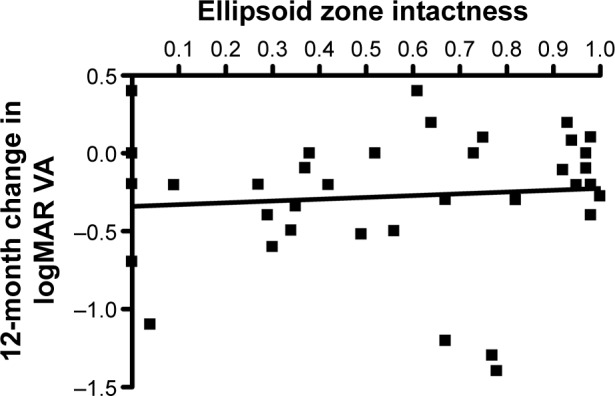
Graph of 12-month change in logMAR VA after vitrectomy for diabetic macular edema versus the intactness of the EZ assessed as the average of three scores by independent graders.
Notes: The black line is the least-squares regression line. The slope does not differ from zero to a statistically significant extent (P=0.5641).
Abbreviations: logMAR, logarithm of the minimum angle of resolution; VA, visual acuity; EZ, ellipsoid zone.
The median follow-up was 37.4 months (IQR, 15.6–58.8). During the postoperative follow-up, 35 eyes (66%) received additional treatment for persistent DME and 18 eyes (34%) received no additional treatment. Table 5 specifies the treatments given after vitrectomy for diabetic retinopathy.
Table 5.
Postvitrectomy treatments for diabetic retinopathy
| Postvitrectomy treatments for diabetic retinopathy | Yes | Number of indicated treatments
|
No | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Other* | |||
| Focal/grid | 12 | 6 | 5 | 0 | 1 | 0 | 41 |
| PRP | 6 | 6 | 0 | 0 | 0 | 0 | 47 |
| Bevacizumab | 11 | 6 | 3 | 1 | 1 | 0 | 42 |
| Ranibizumab | 2 | 1 | 0 | 0 | 0 | 6 (1) | 51 |
| Triamcinolone | 3 | 0 | 0 | 1 | 1 | 25 (1) | 50 |
| Dexamethasone sustained release device | 2 | 2 | 0 | 0 | 0 | 0 | 51 |
Notes:
These data are formatted as number of treatments (number of patients receiving that number of treatments).
Abbreviations: Focal, focal laser photocoagulation; grid, grid laser photocoagulation; bevacizumab, intravitreal bevacizumab; ranibizumab, intravitreal ranibizumab; triamcinolone, intravitreal triamcinolone; PRP, panretinal photocoagulation.
Case reports
Case 1
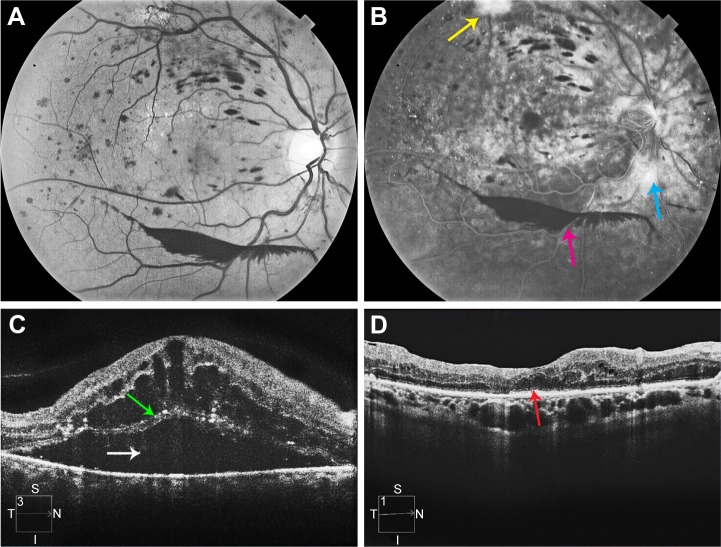
A 53-year-old woman with type 2 diabetes mellitus and systemic arterial hypertension had VA of 20/800 and CSMT of 1,183 μm in her right eye. The eye had neovascularization of the disk, preretinal hemorrhage, and severe macular edema with subretinal fluid (Figure 4). The eye had previously undergone focal laser photocoagulation and an intravitreal injection of bevacizumab with little response. The preoperative SD-OCT showed loss of the EZ (Figure 4). Vitrectomy, ILM peeling, panretinal laser photocoagulation, and intraocular injection of a dexamethasone implant (0.7 mg) were performed. At 12 months follow-up, her VA had improved to 20/70 and her CSMT had decreased to 246 μm. The 12-month SD-OCT showed persistent loss of the EZ (Figure 4).
Figure 4.
Multimodal imaging of patient described in case 1.
Notes: (A) Monochromatic fundus photograph of the right eye of the patient described in case 1. (B) Late phase frame of the fluorescein angiogram of the right eye of the patient described in case 1. The yellow arrow indicates neovascularization elsewhere. The blue arrow indicates neovascularization of the disc. The pink arrow indicates preretinal hemorrhage. (C) Preoperative SD-OCT of the right eye of the patient described in case 1. White arrow indicates submacular fluid. Green arrow indicates the photoreceptor layer with an absent EZ line. (D) 12-month postvitrectomy SD-OCT. The red arrow indicates an absent EZ line.
Abbreviations: SD-OCT, spectral domain optical coherence tomography; EZ, ellipsoid zone.
Case 2
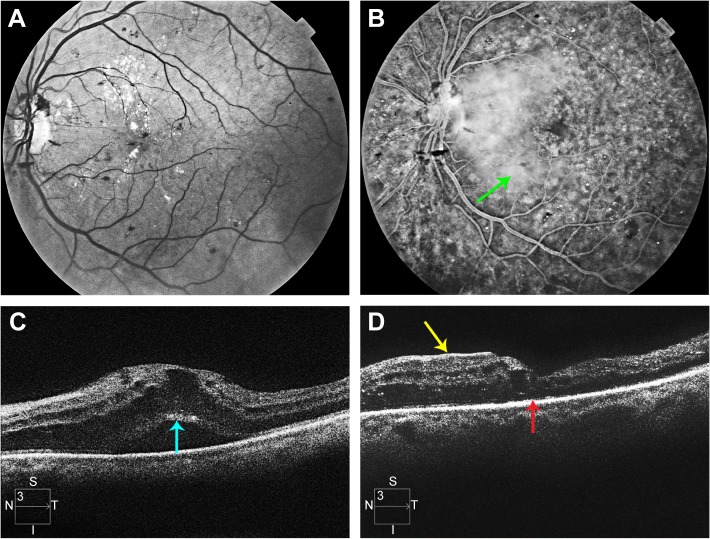
A 50-year-old woman with type 2 diabetes mellitus for 16 years had VA of 20/63 and CSMT of 712 μm in her left eye. She had neovascularization of the disc and severe macular edema with subretinal fluid (Figure 5). She had previously undergone an intravitreal injection of bevacizumab with little response. The preoperative SD-OCT showed loss of the EZ (Figure 5). Vitrectomy, ILM peeling, panretinal laser photocoagulation, and an intraocular injection of a dexamethasone implant (0.7 mg) were performed. Her 12-month postoperative VA remained 20/63, and her CSMT had decreased to 280 μm. The 12-month SD-OCT showed persistent loss of the EZ (Figure 5).
Figure 5.
Multimodal imaging of patient described in case 2.
Notes: (A) Monochromatic fundus photograph of the left eye of the patient described in case 2. (B) Late phase frame of the fluorescein angiogram. The green arrow indicates profuse leakage of fluorescein throughout the macula. (C) Preoperative SD-OCT scan of the left eye. The turquoise arrow indicates the photoreceptor layer with an absent EZ line. (D) 12-month postvitrectomy SD-OCT. The red arrow indicates an absent EZ line. The yellow arrow indicates a nasal ERM that does not cover the fovea.
Abbreviations: SD-OCT, spectral domain optical coherence tomography; EZ, ellipsoid zone; ERM, epiretinal membrane.
Discussion
The major results of this study were that vitrectomy consistently improves center-involving DME and that the median 12-month postvitrectomy VA improves by −0.20 logMAR (equivalent to ten ETDRS letters or two lines of VA on an ETDRS chart). The anatomic results agree with almost all previous reports, which have consistently shown thinning of the macula after vitrectomy surgery. The VA results agree with some of the previously published reports11–15 but not others.7,9,16–18 A meta-analysis of vitrectomy for DME concluded that vitrectomy offers neither structural nor functional benefits compared to focal/grid laser at 12 months follow-up, although only eleven of the hundreds of studies published on the topic were deemed suitable for review.19
Explanations for the inconsistent results in the literature include the heterogeneity of patients operated upon, the heterogeneity of the procedures performed, and the stage in the disease when vitrectomy was used as a treatment.20 It is possible that studies showing no VA benefits applied vitrectomy too late in the disease, after many previous treatments had failed, and the neuroretina was incapable of functional improvement.21
Which steps are critical in vitrectomy for CI-DME and which are not are unknown. We chose to apply panretinal photocoagulation in all cases to reduce the level of intraocular vascular endothelial growth factor, which has been linked to DME.22 We also peeled all ERMs to reduce traction on retinal vessels, theoretically helping reduce vascular permeability.20 In most cases, we peeled the ILM and injected an intraocular corticosteroid at the conclusion of the case. The purpose of ILM peeling is to reduce tangential traction that may exacerbate retinal vessel permeability and to provide easier egress of cytokines that increase vascular permeability.23 The purpose of intraocular corticosteroid injection at the conclusion of the operation is to reduce the concentration of postoperative inflammatory cytokines that could worsen vascular permeability.20 Although reports of toxicity of indocyanine green staining of the ILM in vitrectomy for DME exist,24 we used a low concentration that has been widely used without toxicity and did not observe any toxicity in this series. Intraocular corticosteroids are associated with accelerated cataract formation and intraocular pressure elevation in a proportion of cases, and the risks of these side effects must be balanced against the potential benefits.25
Factors that might predict a good VA outcome after vitrectomy for DME would be clinically important and have been examined previously. Unfortunately, the results are inconsistent. Many groups have reported no improvement in VA outcomes after ILM peeling compared to no ILM peeling; a meta-analysis concluded that there was no VA outcome benefit to ILM peeling.4,7,26–28 Some have reported that DME with associated vitreomacular traction responds better to vitrectomy than DME without traction,29 but others have not found this to be true.28,30 Preoperative intactness of the external limiting membrane and EZ was reported to be associated with improved VA outcomes in one study.31 In our multivariate analysis of ERM peeling, ILM peeling, and use of intraocular steroids with vitrectomy, we found no statistically significant association of improved VA outcomes with any of these factors.
As is true with all other treatments for CI-DME, not all eyes experience resolution of edema after vitrectomy. In our series, 34% of eyes did not require any further treatment over a median follow-up of 3 years. This decreased the number of necessary follow-up visits, thereby reducing the overall cost of care.
Eyes with diabetic retinopathy that undergo vitrectomy are at risk of complications such as retinal detachment and neovascular glaucoma. However, no surgery-related complications were observed in our cohort. This is consistent with recent reports of very low surgical complication rates following modern, small-gauge vitrectomy. The long-term complication rates due to vitrectomy for CI-DME may not be substantially different from the 3-year risk of exogenous endophthalmitis (approximately 1%) in eyes treated primarily with anti-VEGF injections.32,33
One advantage of vitrectomy for DME is a reduced burden of office visits afterward. In this study, the median of seven office visits (IQR, 5–9) found for the 12 months following surgery compares favorably with the median number of office visits (12) for the 12 months following the initial injection in regimens treating CI-DME with serial anti-VEGF injections.32,33
A theoretical disadvantage of vitrectomy for DME is that it would undercut the effectiveness of subsequent intravitreal injections of anti-VEGF drugs by shortening their intravitreal half-lives. This was studied in a subset of 25 eyes that had previously had vitrectomy and were enrolled in the Diabetic Retinopathy Clinical Research (DRCR) network protocol I.34 There was little evidence of any adverse effect of vitrectomy on the beneficial VA or macular thinning effects of serial intravitreal ranibizumab injections.
This study has limitations, and its results must be applied with caution. The retrospective design implies that standardized methods of data collection were not used. VA determinations were not obtained after protocol refractions. Not all patients who underwent vitrectomy for DME during the study period were followed for 12 months; these patients were excluded from the study, potentially biasing the outcomes. The components of the vitrectomy operation evolved over the 9 years of patient accrual, making analysis of the importance of each component problematic. Not all patients had SD-OCT available preoperatively, making EZ grading impossible for a fraction of the patients studied. Patient selection was at the discretion of the treating surgeon and was not based upon formalized a priori criteria. No control group against which the results of vitrectomy could be critically compared was available.
Conclusion
Vitrectomy for CI-DME not only thins the macula but also on an average produces similar VA improvements to those obtained with anti-VEGF injection regimens.32,33,35 A prospective, randomized clinical trial comparing vitrectomy to serial anti-VEGF injections is needed to determine the relative efficacy and cost-effectiveness of these two treatments for CI-DME. Such an idea has been submitted to the DRCR network, which has the infrastructure enabling execution of such a trial. Relevant evidence for this protocol idea can be obtained from the International Consortium Investigating Early Vitrectomy in Diabetic Macular Edema Patients trial (clinical trials identifier #NCT02639507), a prospective, international, multicenter trial with standardized collection of VA and OCT data that is currently enrolling patients.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Klein R, Klein BE, Moss SE, Cruikshanks KJ. The Wisconsin epidemiologic study of diabetic retinopathy. XV. The long-term incidence of macular edema. Ophthalmology. 1995;102:7–16. doi: 10.1016/s0161-6420(95)31052-4. [DOI] [PubMed] [Google Scholar]
- 2.Varma R, Bressler NM, Doan QV, et al. Prevalence and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132:1334–1340. doi: 10.1001/jamaophthalmol.2014.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diabetic Retinopathy Clinical Research Network. Elman MJ, Qin H, Aiello LP, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment. Three year randomized trial results. Ophthalmology. 2012;119:2312–2318. doi: 10.1016/j.ophtha.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakajima T, Roggia MF, Noda Y, Ueta T. Effect of internal limiting membrane peeling during vitrectomy for diabetic macular edema, systematic review and meta-analysis. Retina. 2015;35:1719–1725. doi: 10.1097/IAE.0000000000000622. [DOI] [PubMed] [Google Scholar]
- 5.The Diabetic Retinopathy Clinical Research Network. Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–1203. doi: 10.1056/NEJMoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel JI, Hykin PG, Schadt M, Luong VY, Fitzke F, Gregor ZJ. Pars plana vitrectomy with and without peeling of the inner limiting membrane for diabetic macular edema. Retina. 2006;26:5–13. doi: 10.1097/00006982-200601000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Mochizuki Y, Hata Y, Enaida H, et al. Evaluating adjunctive surgical procedures during vitrectomy for diabetic macular edema. Retina. 2006;26:143–148. doi: 10.1097/00006982-200602000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Laidlaw DA. Vitrectomy for diabetic macular edema. Eye. 2008;22:1337–1341. doi: 10.1038/eye.2008.84. [DOI] [PubMed] [Google Scholar]
- 9.Diabetic Retinopathy Clinical Research Network. Haller JA, Qin H, Apte RS, et al. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology. 2010;117:1087–1093. doi: 10.1016/j.ophtha.2009.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lammer J, Scholda C, Prunte C, Benesch T, Schmidt-Erfurth U, Bolz M. Retinal thickness and volume measurements in diabetic macular edema: a comparison of four optical coherence tomography systems. Retina. 2011;31:48–55. doi: 10.1097/IAE.0b013e3181e095a4. [DOI] [PubMed] [Google Scholar]
- 11.Terasaki H, Kojima T, Niwa H, et al. Changes in focal macular electroretinograms and foveal thickness after vitrectomy for diabetic macular edema. Invest Ophthalmol Vis Sci. 2003;44:4465–4472. doi: 10.1167/iovs.02-1313. [DOI] [PubMed] [Google Scholar]
- 12.Tachi N, Ogino N. Vitrectomy for diffuse macular edema in cases of diabetic retinopathy. Am J Ophthalmol. 1996;122:258–260. doi: 10.1016/s0002-9394(14)72018-5. [DOI] [PubMed] [Google Scholar]
- 13.Recchia FM, Ruby AJ, Carvalho Recchia CA. Pars plana vitrectomy with removal of the internal limiting membrane in the treatment of persistent diabetic macular edema. Am J Ophthalmol. 2005;139:447–454. doi: 10.1016/j.ajo.2004.09.076. [DOI] [PubMed] [Google Scholar]
- 14.Otani T, Kishi S. Tomographic assessment of vitreous surgery for diabetic macular edema. Am J Ophthalmol. 2000;129:487–494. doi: 10.1016/s0002-9394(99)00409-2. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto T, Akabane N, Takeuchi S. Vitrectomy for diabetic macular edema: the role of posterior vitreous detachment and epimacular membrane. Am J Ophthalmol. 2001;132:369–377. doi: 10.1016/s0002-9394(01)01050-9. [DOI] [PubMed] [Google Scholar]
- 16.Rosenblatt BJ, Shah GK, Sharma S, Bakal J. Pars plana vitrectomy with internal limiting membranectomy for refractory diabetic macular edema without a taut posterior hyaloid. Graefes Arch Clin Exp Ophthalmol. 2005;243:20–25. doi: 10.1007/s00417-004-0958-z. [DOI] [PubMed] [Google Scholar]
- 17.Hartley KL, Smiddy WE, Flynn HW, Jr, Murray TG. Pars plana vitrectomy with internal limiting membrane peeling for diabetic macular edema. Retina. 2008;28:410–419. doi: 10.1097/IAE.0b013e31816102f2. [DOI] [PubMed] [Google Scholar]
- 18.Hoerauf H, Brueggemann A, Muecke M, et al. Pars plana vitrectomy for diabetic macular edema. Internal limiting membrane delamination vs posterior hyaloid removal. A prospective randomized trial. Graefes Arch Clin Exp Ophthalmol. 2011;249:997–1008. doi: 10.1007/s00417-010-1610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simunovic M, Hunyor AP, Ho IV. Vitrectomy for diabetic macular edema: a systematic review and meta-analysis. Can J Ophthalmol. 2014;49:188–195. doi: 10.1016/j.jcjo.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Browning DJ. Diabetic macular edema. In: Browning DJ, editor. Diabetic Retinopathy. Evidence-Based Management. New York: Springer; 2010. pp. 141–202. [Google Scholar]
- 21.Adelman R, Parnes A, Michalewska Z, Patrolini B, Boscher C, Ducournea D. Strategy for the management of diabetic macular edema: the European Vitreo-retinal Society Macular Edema Study. Biomed Res Int. 2015;2015:1–9. doi: 10.1155/2015/352487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funatsu H, Yamashita H, Nakamura S, et al. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2006;113:294–301. doi: 10.1016/j.ophtha.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 23.Lam RF, Lai WW, Chan WM, Liu DT, Lam DS. Vitrectomy for diabetic macular edema with and without internal limiting membrane removal. Ophthalmologica. 2006;220:206. doi: 10.1159/000091768. [DOI] [PubMed] [Google Scholar]
- 24.Ando F, Yasui O, Hirose H, Ohba N. Optic nerve atrophy after vitrectomy with indocyanine green-assisted internal limiting membrane peeling in diffuse diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2004;242:995–999. doi: 10.1007/s00417-004-0864-4. [DOI] [PubMed] [Google Scholar]
- 25.Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002;109:920–927. doi: 10.1016/s0161-6420(02)00975-2. [DOI] [PubMed] [Google Scholar]
- 26.Figueroa MS, Contreras I, Noval S. Surgical and anatomical outcomes of pars plana vitrectomy for diffuse nontractional diabetic macular edema. Retina. 2008;28:420–426. doi: 10.1097/IAE.0b013e318159e7d2. [DOI] [PubMed] [Google Scholar]
- 27.Kumagai K, Hangai M, Ogino N, Larson E. Effect of internal limiting membrane peeling on long-term visual outcomes for diabetic macular edema. Retina. 2015;35:1422–1428. doi: 10.1097/IAE.0000000000000497. [DOI] [PubMed] [Google Scholar]
- 28.Flaxel CJ, Edwards AR, Aiello LP, et al. Factors associated with visual acuity outcomes after vitrectomy for diabetic macular edema: diabetic retinopathy clinical research network. Retina. 2010;30:1488–1495. doi: 10.1097/IAE.0b013e3181e7974f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah SP, Patel M, Thomas D, Aldington S, Laidlaw DA. Factors predicting outcome of vitrectomy for diabetic macular edema: results of a prospective study. Br J Ophthalmol. 2006;90:33–36. doi: 10.1136/bjo.2005.072934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonnin S, Sandali O, Bonnel S, Monin C, El Sanharawi M. Vitrectomy with internal limiting membrane peeling for tractional and nontractional diabetic macular edema: long term results of a comparative study. Retina. 2015;35:921–928. doi: 10.1097/IAE.0000000000000433. [DOI] [PubMed] [Google Scholar]
- 31.Chhlabani JK, Kim JS, Cheng L, Kozak I, Freeman W. External limiting membrane as a predictor of visual improvement in diabetic macular edema after pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2012;250:1415–1420. doi: 10.1007/s00417-012-1968-x. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE Study Ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–625. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 33.The Diabetic Retinopathy Clinical Research Network Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–1077. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bressler SB, Melia M, Glassman AR, et al. Diabetic Retinopathy Clinical Research Network Ranibizumab plus prompt or deferred laser for diabetic macular edema in eyes with vitrectomy before anti-vascular endothelial growth factor therapy. Retina. 2015;35:2516–2528. doi: 10.1097/IAE.0000000000000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT Study) 12 month data: report 2. Ophthalmology. 2010;117:1078–1086. doi: 10.1016/j.ophtha.2010.03.045. [DOI] [PubMed] [Google Scholar]