Abstract
Objective
To assess depression in children with chronic kidney disease (CKD) and to determine associations with patient characteristics, intellectual and educational levels, and health related quality of life (HRQoL).
Study design
Subjects aged 6–17 years from the Chronic Kidney Disease in Children cohort study completed the Children’s Depression Inventory (CDI), Wechsler’s Abbreviated Scales of Intelligence, Wechsler Individual Achievement Test-II-Abbreviated, and the Pediatric Inventory of Quality of Life Core Scales 4.0. Regression analyses determined associations of CDI score and depression status with subject characteristics, intellectual and educational levels, and HRQoL. A joint linear mixed model and Weibull model were used to determine the effects of CDI score on longitudinal changes in glomerular filtration rate (GFR) and time to renal replacement therapy.
Results
344 subjects completed the CDI. Eighteen (5%) had elevated depressive symptoms and another 7 (2%) were being treated for depression. In adjusted analyses, maternal education beyond high school was associated with 5% lower CDI scores (estimate 0.95; 95% CI 0.92, 0.99). Depression status was associated with lower IQ (99 versus 88, P= 0.053), lower achievement (95 versus 77.5, P<0.05), and lower HRQoL by parent and child reports (effect estimates −15.48; 95% CI −28.71, −2.24 and −18.39; 95% CI −27.81, −8.96, respectively). CDI score was not related to change in GFR.
Conclusion
Children with depression had lower psychoeducational skills and worse HRQoL. Identifying and treating depression should be evaluated as a means to improve the academic performance and HRQoL of children with CKD.
Keywords: Child Depression Inventory, adolescents, children, mental health, quality of life, chronic renal insufficiency
Depression is a common co-morbidity in children with chronic medical disease1–5 and has been associated with poor adherence to medication and worse outcomes.4–6 Similar to children with other chronic diseases, children with chronic kidney disease (CKD) often have growth restriction, multiple surgical scars, and frequently miss school and other childhood activities. Compared with prior decades, children with CKD are experiencing improved survival but the adversities related to their underlying condition predispose them to the development of depression. Compared with a 12 month prevalence of depression of 7.5% in adolescents in the general population,7 prior studies assessing depression in pediatric CKD6,8–12 have found a point prevalence of 10–35%, with variations according to the specific populations studied (transplant versus dialysis versus pre-end stage renal disease and young children versus adolescents) and methods used to define depression.
Although studies investigating the role of depression in pediatric CKD are limited, the role of depression in adults with CKD is better defined. Studies in adults with CKD indicate that the prevalence of depression is between 20 and 40%.13–16 For adult hemodialysis patients, major depression is associated with a three-to-four fold greater risk of death,15 and those patients with CKD but not on dialysis have an increased risk of poor outcomes including hospitalizations, death and initiation of dialysis.16 In pediatrics, adverse effects of depression are suggested by studies in adolescents in which depression is associated with neurocognitive impairment that improves after remission of depression17 and in studies of children with chronic illness that show depression to be associated with poor quality of life.18,19 These findings may be relevant in pediatric chronic kidney disease because prior studies have found that 21–40% of children with CKD fall at least 1 SD below the mean on IQ and academic achievement21 and that children with CKD suffer from lower health related quality of life (HRQOL) than healthy children. 22
The Chronic Kidney Disease in Children (CKiD) cohort study is a prospective observational study of pediatric CKD patients with mild to moderate renal dysfunction from 54 pediatric nephrology centers throughout North America20. A primary goal of the study is to determine how a decline in kidney function affects neurocognitive function and behavior. Because prior studies evaluating depression in pediatric CKD are limited by small sample sizes (ranging from 15–60 subjects) and none have attempted to correlate depression with intellectual impairment, quality of life, or progression of disease we used Children’s Depression Inventory (CDI )23 data collected from the CKiD cohort to determine (1) the prevalence of depression and elevated depressive symptoms in children with mild to moderate CKD; (2) the demographic and clinical factors associated with depression in pediatric CKD; (3) the relationship between depression and intellectual level, academic skills, and quality of life; and (4) the relationship between baseline depression and longitudinal changes in kidney function.
Methods
The CKiD study is a multicenter prospective, longitudinal, observational cohort study of children with mild-to-moderate CKD from 54 pediatric nephrology centers throughout North America.20 The CKiD study was initiated in 2005 and enrolled children, from a variety of pediatric nephrology practices, between the ages of 1–16 years with an estimated glomerular filtration rate (GFR) of 30–90 ml/min/1.73m2 (calculated by the original Schwartz equation24); exclusion criteria include prior malignancy, transplantation, or dialysis within the previous three months, and a limited number of other conditions. Children are seen initially at a baseline study visit and then again for a study visit 3–6 months later at which point neurocognitive and psychosocial data are collected. Thereafter, subjects are followed longitudinally on an annual basis until they are 21 years of age, undergo transplantation, initiate dialysis or are transferred to an adult center. This report includes subjects enrolled from 2005–2008 between the ages of 6 and 17 years since these are the years of the study during which the CDI was administered and only children at least 6 years of age completed the CDI. The CKiD study protocol was approved by the Institutional Review Boards of each participating center and informed consent was obtained from all participants. Further information regarding CKiD study design and objectives have been previously described.20
We performed a cross-sectional analysis of data collected at the baseline and 3–6 month study visit to determine the prevalence of depression and depressive symptoms and to determine the associations of depression with patient characteristics, intellectual function, overall academic skills, and quality of life. In a longitudinal analysis, we evaluated the relationship of CDI score to changes in GFR.
Depression
Participants completed the Children’s Depression Inventory (CDI) at the study visit that occurred 3–6 months after study entry. The CDI is a validated 27 question survey designed to assess depressive symptomatology and to offer information regarding likely diagnoses of depression in children 7–17 years of age.23 Children completed the CDI independently. For children who required help reading, the CDI was read to them by a study coordinator. The CDI assesses symptoms of depression in the domains of negative mood, interpersonal problems, ineffectiveness, anhedonia and negative self-esteem. The results from the CDI yield a raw score which is then converted into a T-score. The T-score is based on general population norms and standardized for age and sex, with a mean score and standard deviation of 50± 10 in the general child and adolescent population. A higher CDI T-score indicates the presence of more depressive symptoms; a CDI T-score ≥60 is considered to be high average and a T-score ≥65 is considered to be clinically significant. For this study, status as depressed was assigned based on a CDI T-score ≥60 or on a self-reported prior diagnosis of depression currently being treated with an antidepressive medication. Depression was evaluated as a dichotomous outcome variable and depressive symptoms were evaluated as a continuous outcome variable measured by the CDI T-score.
Intellectual Functioning, Academic Achievement, and Quality of life measures
All intellectual, academic, and quality of life measures were obtained concurrently with the CDI at the 3–6 month CKiD study visit. Intelligence was measured by the Wechsler Abbreviated Scales of Intelligence (WASI).25 Academic achievement was measured by the Wechsler Individual Achievement Test-II-Abbreviated (WIAT-II-A)26 and the need for additional school support was assessed by self-reported presence of an Individualized Education Plan (IEP) or 504 plan in the school setting. HRQoL was evaluated by completion of the Pediatric Inventory of Quality of Life Core Scales (PedsQL) 4.0.27,28 The participants’ caregivers completed the PedsQL parent proxy form and the participants ≥ 8 years completed the PedsQL child form.
Other Variables
Based on factors found to be associated with other neuropsychological measures in prior CKiD studies21,22,29 we also collected data regarding age, sex, race, maternal education, etiology of CKD, age at diagnosis, duration of CKD, GFR, urine protein:creatinine ratio, body mass index, height, casual blood pressure measurements, past history of psychiatric disease and current medication use as variables possibly related to depression. For the cross sectional analysis, demographic and medical history information was collected at the baseline study visit. For the longitudinal analysis, GFR was determined by plasma iohexol disappearance at the baseline visit, the first annual follow-up visit, and every two years thereafter.30 At intervening visits, GFR was estimated by validated equations.31 For this paper, GFR refers to estimated GFR or measured GFR when available.
Statistical Analyses
Univariate relationships between depression status and patient characteristics were computed using Wilcoxon rank-sum tests for continuous variables and chi-squared tests for categorical variables. To determine the relationships between CDI T-score and patient characteristics, we performed log-linear regression in a multi-variable model including variables found to be associated with depression in the univariate analysis or hypothesized a priori to be related to depression.
Intellectual assessment and HRQoL scores for subjects with and without depression were reported as median values with interquartile range (IQR) or frequencies and percentages. To determine the associations between depression and intellectual assessment and quality of life, we performed median regression analyses for continuous variables and logistic regression analyses for dichotomous variables. Median regression was used due to the HRQoL scales, which are bounded between 0 and 100 and are non-normally distributed. Each analysis controlled for height Z-score, maternal education, age, duration of CKD and incontinence (defined as not toilet trained or toilet trained with bed wetting) because these have been previously reported as factors associated with neurocognitive measures and/or HRQoL in prior studies from the CKiD cohort. 21,22,32–33
In order to determine if baseline CDI score was associated with progression of CKD, we jointly modeled the effect of baseline CDI on longitudinal GFR and time to renal replacement therapy (RRT, defined as initiation of dialysis or kidney transplantation), using a linear mixed model for the longitudinal component and a Weibull model for the time to RRT. The joint model accounts for informative dropout, as subjects with lower GFRs and/or faster progression tend to receive RRT earlier.34 GFR was log-transformed for normality and CDI was centered at 45. No other covariates were included in this model.
All data analyses were carried out using SAS 9.2 (SAS Institute, Cary, NC) except the joint models, which were fit with the stjm package in Stata 12 (StataCorp, College Station, TX). P values <.05 were considered to be statistically significant.
Results
The CDI was completed by 344 children aged 6–17 years. Table I shows the clinical and demographic characteristics of the study subjects. The median age was 13 [IQR 10–15]. Most were male (59%), 61% were white and one-half had congenital disorders of the kidneys and urinary tract. Median GFR was 41.6 ml/min per 1.73m2 [IQR 31.9–53.6]. Seven percent of the children (25) carried a prior diagnosis of depression and 31% (107) had a prior history of any psychiatric illness. Of those with a prior diagnosis of depression, 8 were receiving anti-depressant medications at the time of the study visit.
Prevalence of Depressive Symptomatology
Twenty-five children (7%) met study criteria for depression; 17 had an elevated CDI score alone (T-score >60), 7 were taking an anti-depressant medication for treatment of depression and 1 had an elevated CDI score and was taking an anti-depressant. Of the 319 children without depression, 14 had a self-identified history of depression but were not taking an anti-depressant and had a normal CDI score. The median overall CDI score of the cohort was 43 [IQR 39–49]; for the subjects with depression, the median was 62 [IQR 56–67] and for the subjects without depression the median score was 42 [IQR 39–48]. Table I shows the clinical and demographic characteristics of the subjects according to depression status as defined by the study criteria. Subjects classified as depressed were more likely to have a mother with high school or less education, shorter stature, lower blood pressure, and a history of a prior psychiatric diagnosis.
Associated Demographic and Clinical Factors
In the multiple linear regression model examining the relationship between CDI score and patient characteristics, only maternal education was found to be significantly associated with CDI score (Table II). After adjusting for other demographic and disease-related factors, CDI scores among children whose mothers had more than a high school education were 5% (95%CI: 1%,8%) lower than those whose mothers had a high school education or less. The association of CDI T-score with BMI Z-score tended towards significance (effect estimate 1.02; 95% CI 1.00–1.04). Duration of CKD, congenital etiology of CKD, GFR and height and BP were not associated with CDI T-score.
Intellectual Level, Academic Skills, HRQoL
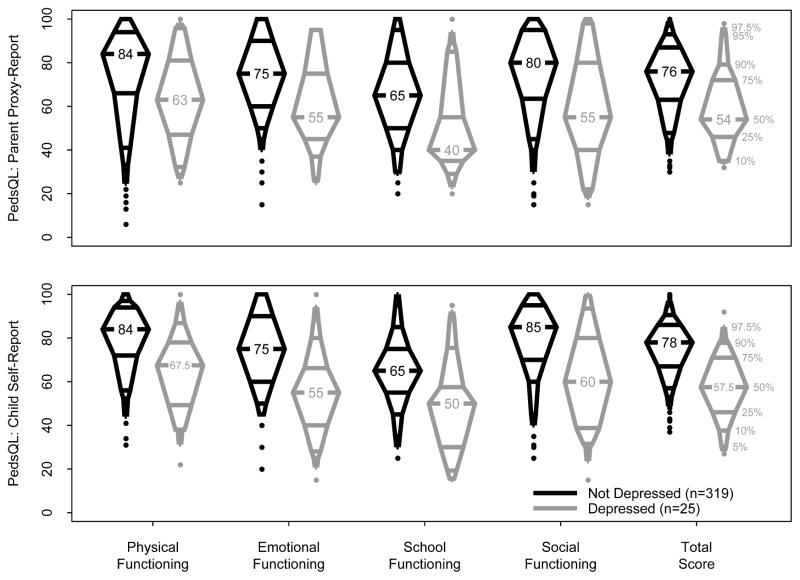
The Figure shows the unadjusted HRQoL parent and child report scores by depression status. Children with depression had median scores 15–35 points lower than the children without depression across all domains. The largest difference between children with and without depression was in social functioning. The lowest parent proxy HRQoL scores for subjects with and without depression were in school functioning (median 40 and 65, respectively) and the highest were in physical functioning (median 63 and 84, respectively). The lowest self-reported HRQoL scores for subjects with and without depression were in school functioning (median 59 and 65, respectively). The highest self-reported HRQoL score for subjects with depression was in physical functioning (median 67.5) and the highest in the subjects without depression was in social functioning (median 85).
Figure 1.
Unadjusted HRQoL Overall and Domain Median Scores by Parent Proxy and Self Report
Table III displays the the median HRQoL, intelligence and achievement scores and the results from the regression models for these scores after adjusting for maternal education, height Z-score, age, duration of CKD and urinary incontinence. Full scale IQ trended towards being lower in the subjects with depression than those without depression (median 88 versus 99; p=0.053). The median achievement scores for the children with depression were in the borderline range and were lower than the median scores in the group without depression (77.5 in the group with depression versus 95 in the group without depression). Except for the parent proxy social HRQoL score, the associations of depression with parent proxy and self HRQoL scores overall and in all domains persisted after adjustment.
Longitudinal changes in GFR
Median follow-up time for this analysis was 4.22 years with a range of 0–7.51 years. In order to determine if CDI score was associated with decline in GFR, baseline CDI score and time to RRT were jointly modeled with GFR. Although there was a significant association between GFR and time to RRT (p<0.0001), baseline CDI score did not have any relationship with baseline GFR (β = −0.003, p=0.27), GFR trajectory over time (β = 0.0005, p=0.43), or progression to RRT (β = −0.0003, p=0.98).
Discussion
In this study evaluating depression in children with mild-to-moderate CKD, we identified a strong relationship between depression and HRQoL. We found that the presence of depressive symptoms was more strongly associated with HRQoL than with any other factor and that the magnitude of the relationship exceeded any other relationships found in other CKiD studies examining HRQoL.22,32,33 Prior CKiD studies evaluating factors associated with HRQoL have found associations within only certain domains of HRQoL or within a particular domain per child report but not the parent report. For example, Gerson et al found older age to be associated with overall HRQoL, physical HRQoL, emotional HRQoL and social HRQoL on the child measures but not at all in the parent proxy measures.22 In the current study, even after adjustment, a strong association between depression and HRQoL was noted across all domains in the self-report measure and virtually all of the HRQoL domains in the parent proxy measure. The effect size of this relationship is also of a greater magnitude than that seen in prior studies. 22,32,33 In our study, the effect size seen was on the order of 15–20 points as opposed to the 1–5 point effect size seen in prior studies. Further demonstrating the importance of the relationship between depression and HRQoL, the quality of life items associated with depression were not limited to those related to mood but also included the mood-free items. These findings suggest that depression may represent a modifiable factor that influences all aspects of HRQoL in children with CKD and highlight the importance of assessing and addressing depression when evaluating and designing strategies to improve HRQoL. Studies of depression in other pediatric chronic disease have also found a unique and strong relationship between depression and HRQoL. A large study of pediatric epilepsy patients found that disease status, such as remission or complicated epilepsy, was not related to HRQoL, in contrast to the relationship that did exist between HRQoL and a history of an internalizing psychiatric disorder. 18 Likewise, a study of young adults with congenital heart disease found that even minor symptoms of depression had a larger impact on quality of life than exercise tolerance.19 Most closely related to our study, a Brazilian study evaluated the relationship between HRQoL and behavioral disorders in 135 children with CKD stages 2–5 including transplant34 and found that none of the CKD related factors were associated with HRQoL, but that there was a significant negative correlation between the presence of behavioral and emotional disorders and HRQoL scores.
The relationship between depressive symptoms and IQ and overall academic skills was not as strong as that to HRQoL, although the median WIAT-II-A achievement score for children with depression was more than 1 standard deviation below the mean. From this it is unclear if lower IQ and achievement skills contribute to increased depressive symptoms or if depression causes impaired psychoeducational functions. Literature from the general adolescent population suggests that depression may lead to impairment of neurocognitive functioning.17 Knowing that children with CKD are at risk for intellectual impairment,21 it is important to determine the contribution of depression to this risk as we seek to improve school functioning and transition into productive adulthood.
The prevalence of depression in the CKiD cohort was lower than anticipated based on prior literature. Seven percent of children met study criteria for depression and 5% of the children demonstrated elevated depressive symptoms (T-score >60) as assessed by the CDI. This does not differ from the prevalence cited in the general adolescent population (7.5%)7 and is substantially less than that identified in other pediatric CKD studies in which up to 35% of subjects were reported to suffer from depression.6,8–12 One explanation for this unexpected finding may be that because CKiD is a large, rigorous, multicenter study requiring long term yearly follow-up, the children in this study come from a select group of patients with families that have the means to take the extra time to participate in the protracted time commitment of the study. Such a committed and organized family unit is likely protective against depression and may be the reason that depression was less prevalent in CKiD than in other smaller studies evaluating depression in CKD.
In the adjusted analysis, the only demographic or clinical factor determined to be associated with depressive symptoms was maternal education, with higher maternal education being protective. Prior studies have likewise failed to find many demographic or clinical factors to be associated with depression in pediatric CKD. A study done in Peru assessing depressive symptoms in children on dialysis did not find an association between depressive symptoms and demographic factors.8 A study of pre-dialysis and dialysis pediatric CKD patients in Egypt also failed to find any associations with demographic or clinical factors.10 Unfortunately, given their single center designs and small sample sizes, these findings may be reflective of inadequate power as opposed to a true lack of association and the possibility that there are other predictors of depression remains.
Our study of depression, although representing a larger sample of pediatric CKD patients than prior studies, is limited by the small number of children meeting criteria for depression which may, in part, be due to characteristics unique to study participants, as mentioned above. This reduces the power of our study to determine if there are specific clinical or demographic factors that relate depression to pediatric CKD. We are also limited in our ability to identify the true prevalence of depression due to the lack of a diagnostic interview, which is the gold standard to diagnose depression, and instead relied on the CDI and patient reported history. The utility of the CDI should not be discounted though because it has adequate sensitivity and specificity, 83% and 84% respectively.36 Using the CDI may have introduced some misclassification into this study, but as opposed to identifying spurious associations, this would have biased our results towards the null.
Given that previous HRQoL findings in children with CKD have not been consistent across parent and child measures or across all HRQoL domains22,32,33 depressive symptoms may be mediating the role that different factors have on HRQoL and should be considered as a potential modifying factor in future studies evaluating HRQoL. As we seek to develop new methods of reducing and eliminating the physical burdens of disease for our patients, we should simultaneously seek to eliminate the mental anguish and the diminished quality of life for them as well.
Table 1.
Descriptive statistics of the 344 children with CDI at 3–6 month visit stratified by depression status
| Characteristic – Median [IQR] or N (%) | Depressed (n=25) | Non-Depressed (n=319) | p-value |
|---|---|---|---|
| Age, years | 14 [12, 16] | 13 [10, 15] | 0.15 |
| Male sex | 14 (56%) | 188 (59%) | 0.77 |
| Race | 0.68 | ||
| Caucasian | 14 (56%) | 195 (61%) | |
| Black | 3 (12%) | 54 (17%) | |
| Hispanic | 5 (20%) | 42 (13%) | |
| Other | 3 (12%) | 28 (9%) | |
| Maternal Education | 0.02 | ||
| High school or less | 16 (64%) | 126 (41%) | |
| More than high school | 9 (36%) | 184 (59%) | |
| Etiology of CKD | 0.15 | ||
| Non-congenital | 9 (36%) | 163 (51%) | |
| Congenital disorder of kidneys and urinary tract | 16 (64%) | 156 (49%) | |
| Age at diagnosis of CKD, years | 6.5 [0.0, 10.5] | 2.5 [0.0, 8.5] | 0.21 |
| Duration of CKD, years | 8.1 [3.8, 13.2] | 8.3 [4.0, 11.9] | 0.77 |
| GFR, ml/min/1.73m2 | 44.4 [31.0, 53.0] | 41.6 [32.0, 53.6] | 0.90 |
| Urine Protein/Creatinine | 0.6 [0.2, 2.0] | 0.5 [0.2, 1.2] | 0.33 |
| BMI percentile | 52 [19, 94] | 62 [34, 88] | 0.73 |
| Height z-score | −1.3 [−1.9, −0.5] | −0.8 [−1.4, 0.1] | 0.03 |
| Systolic BP z-score | −0.2 [−0.8, 0.4] | 0.3 [−0.5, 1.0] | 0.03 |
| Hypertension status | 0.03 | ||
| No Hypertension | 10 (42%) | 120 (39%) | |
| Controlled Hypertension | 12 (50%) | 91 (29%) | |
| Uncontrolled Hypertension | 2 (8%) | 99 (32%) | |
| Use of Anti-hypertensive Medication | 20 (80%) | 216 (68%) | 0.20 |
| History of psychiatric diagnosis | |||
| ADD/ADHD | 7 (28%) | 34 (11%) | 0.01 |
| Depression | 11 (44%) | 14 (5%) | <0.001 |
| Learning disability | 9 (36%) | 40 (13%) | 0.002 |
| Anxiety disorder | 3 (12%) | 13 (4%) | 0.08 |
| Other | 3 (14%) | 18 (6%) | 0.18 |
| Any of the above | 18 (72%) | 89 (28%) | <0.001 |
| Medication | |||
| Immunosuppressive, Corticosteroid | 3 (12%) | 25 (8%) | 0.46 |
| Antidepressants | 8 (32%) | 2 (1%) | <0.001 |
| CNS stimulants | 4 (16%) | 14 (4%) | 0.01 |
| Antipsychotics | 0 (0%) | 2 (1%) | 0.69 |
| Sleep medications | 0 (0%) | 1 (<1%) | 0.78 |
| Mood/behavior medication, other | 0 (0%) | 0 (0%) | 1.00 |
| Any of the above | 10 (40%) | 41 (13%) | <0.001 |
| Patients with IEP and/or 504, % | 13 (52%) | 106 (33%) | 0.06 |
Depressed subjects defined as CDI ≥ 60 or self-reported diagnosis of depression plus current antidepressive medication. P-values from Wilcoxon rank-sum test (continuous) or chi-squared test (categorical).
Table 2.
Multivariate Association of Covariates with CDI score
| Covariate | Estimate | 95% CI | p-value |
|---|---|---|---|
| Age, per year increase | 1.00 | (0.99, 1.00) | 0.20 |
| Male sex | 1.01 | (0.97, 1.05) | 0.74 |
| Maternal Education | |||
| High school or less | 1 | (ref) | - |
| More than high school | 0.95 | (0.92, 0.99) | 0.02 |
| Duration of CKD | 1.00 | (0.99, 1.00) | 0.74 |
| Etiology of CKD | |||
| Non-congenital | 1 | (ref) | - |
| Congenital disorder of kidneys and urinary tract | 1.02 | (0.98, 1.06) | 0.32 |
| GFR, ml/min/1.73m2 | 1.00 | (0.98, 1.01) | 0.65 |
| Height z-score, per 1 unit increase | 0.99 | (0.97, 1.01) | 0.42 |
| BMI z-score, per 1 unit increase | 1.02 | (1.00, 1.04) | 0.05 |
| Hypertension status | |||
| None | 1 | (ref) | - |
| Controlled | 1.02 | (0.98, 1.07) | 0.31 |
| Uncontrolled | 1.01 | (0.97, 1.06) | 0.58 |
| Corticosteroid use | 1.02 | (0.94, 1.10) | 0.63 |
CDI log-transformed for normality. Estimates and 95% CIs are exponentiated so that interpretation is multiplicative, i.e., 0.95 indicates a CDI score 5% lower than reference.
Table 3.
PedsQL median scores and adjusted linear or logistic regression results for associations of depression status with intelligence, achievement, and quality of life measures
| Outcome | Depressed (n=25) | Non-Depressed (n=319) | Effect estimate (95% CI) | |
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| Full Scale IQ | 88 [79, 97] | 99 [86, 109] | −11.00* (−17.57, −4.43) | −3.70 (−17.55, 10.16) |
| Achievement | 77.5 [72, 97] | 95 [83, 108] | −17.00* (−27.90, −6.10) | −11.24 (−22.50, 0.03) |
| Child Overall HRQoL | 57.5 [46, 72] | 78 [67, 86] | −20.00** (−31.07, −8.93) | −18.39** (−27.81, −8.96) |
| Child Physical HRQoL | 67.5 [48.5, 78] | 84 [72, 94] | −15.00** (−23.01, −6.99) | −15.46* (−25.13, −5.79) |
| Child Emotional HRQoL | 55 [40, 67.5] | 75 [60, 90] | −20.00*** (−27.86, −12.14) | −18.43*** (−27.31, −9.56) |
| Child Social HRQoL | 60 [37.5, 80] | 85 [70, 95] | −20.00 (−43.47, 3.47) | −14.23 (−32.53, 4.06) |
| Child School HRQoL | 50 [30, 60] | 65 [55, 75] | −15.00** (−23.17, −6.83) | −15.00* (−25.53, −4.47) |
| Parent Overall HRQoL | 54 [46, 72] | 76 [63, 87] | −22.00*** (−32.37, −11.63) | −15.48* (−28.71, −2.24) |
| Parent Physical HRQoL | 63 [47, 81] | 84 [66, 94] | −21.00* (−33.52, −8.48) | −16.97* (−30.94, −3.00) |
| Parent Emotional HRQoL | 55 [45, 75] | 75 [60, 90] | −20.00*** (−29.70, −10.30) | −17.76* (−31.77, −3.76) |
| Parent Social HRQoL | 55 [40, 80] | 80 [63, 95] | −25.00* (−42.45, −7.55) | −19.66 (−40.36, 1.03) |
| Parent School HRQoL | 40 [35, 55] | 65 [50, 80] | −25.00*** (−31.87, −18.13) | −21.35*** (−30.60, −12.11) |
| Odds ratio (95% CI) | ||||
| IEP/504 Plan Usage | 13 (52%) | 106 (33%) | 2.18 (0.96, 4.94) | 1.78 (0.74, 4.30) |
Median regression used for continuous variables, logistic used for IEP/504 Plan Usage. Adjusted Model controls for height z-score, maternal education, age, duration of CKD, and incontinence (defined as either day/night urine leakage or bedwetting after toilet training).
p<0.05;
p<0.001;
p<0.0001
Appendix.
List of CKiD sites and principal investigators by clinical coordinating center
| Sites | Principal Investigator |
|---|---|
| Midwest Clinical Coordinating Center Kansas City, MO |
Bradley Warady, MD |
| Children’s Hospital of Winnipeg Winnipeg, CA |
Allison Dart, MD, MSc, FRCPC |
| Egleston Children’s Hospital, Emory University Atlanta, GA |
Larry Greenbaum, MD, PhD |
| Children’s Mercy Hospital Kansas City, MO |
Bradley Warady, MD |
| Cincinnati Children’s Hospital and Medical Center Cincinnati, OH |
Jens Goebel, MD, Mark Mitsnefes, MD |
| Seattle Children’s Hospital Seattle, WA |
Joseph Flynn, MD |
| University of New Mexico Children’s Hospital Albuquerque, NM |
Craig Wong, MD |
| Children’s Hospital of Alabama Birmingham, AL |
Sahar Fathallah, MD |
| University of California, Los Angeles Los Angeles, CA |
Isidro Salusky, MD; Ora Yadin, MD |
| Case Western Reserve University Cleveland, OH |
Katherine Dell, MD |
| Phoenix Children’s Hospital Phoenix, AZ |
Bruce Morgenstern, MD |
| British Columbia Children’s Hospital Vancouver, WA |
Tom Blydt-Hansen, MD, FRCPC |
| Medical College of Wisconsin Milwaukee, WI |
Cynthia Pan, MD |
| St. Louis Children’s Hospital St. Louis, MO |
Keefe Davis, MD |
| Oregon Health and Science University Portland, OR |
Amira Al-Uzri, MD; Randall Jenkins, MD |
| UCSF Children’s Hospital San Francisco, CA |
Anthony Portale, MD |
| University of Texas Southwestern Medical Center Dallas, TX |
Mouin Seikaly, MD |
| Oklahoma University Health Sciences Center Oklahoma City, OK |
Martin Turman, MD, PhD |
| Stanford University Medical Center Standford, CA |
Cynthia Wong, MD; Steven Alexander, MD |
| LeBonheur Children’s Medical Center Memphis, TN |
Colleen Hastings, MD |
| Northwest Pediatric Kidney Specialist Portland, OR |
Randall Jenkins, MD |
| Children’s Hospital of Boston Boston, MA |
Nancy Rodig, MD; William Harmon, MD |
| University of Wisconsin Madison, WI |
Sharon Bartosh, MD |
| University of California, San Diego La Jolla, CA |
Nadine Benador, MD; Robert Mak, MD, PhD |
| Cardinal Glennon Hospital St. Louis, MO |
Ellen Wood, MD |
| Children’s Kidney Specialists, Idaho Boise, ID |
Randall Jenkins, MD |
| Children’s Hospital of Los Angeles Los Angeles, CA |
Gary Lerner, MD |
| East Coast Clinical Coordinating Center Philadelphia, PA |
Susan Furth, MD, PhD |
| Children’s Hospital of Philadelphia Philadelphia, PA |
Susan Furth, MD, PhD |
| Carolinas Medical Center Charlotte, NC |
Susan Massengill, MD |
| East Carolina Universit Greenville, NC |
Guillermo Hidalgo, MD |
| Johns Hopkins Children’s Center Baltimore, MD |
Meredith Atkinson, MD |
| University of Michigan, Mott Hospital Ann Arbor, MI |
Debbie Gipson, MD |
| Texas Children’s Hospital, Baylor Houston, TX |
Poyyapakkam Srivaths, MD |
| University of Texas, Houston Houston, TX |
Joshua Samuels, MD |
| Children’s Hospital at Montefiore Bronx, NY |
Frederick Kaskel, MD, PhD |
| Children’s Hospital at Dartmouth Dartmouth, NH |
Debora Mattosian, MD |
| DeVos Children’s Hospital at Spectrum Grand Rapids, MI |
Yi Cai, MD |
| Riley Hospital for Children at Indiana Univ. Health Indianapolis, IN |
Sharon Andreoli, MD, PhD |
| Icahn School of Medicine at Mount Sinai New York, NY |
Jeffrey Saland, MD |
| Nationwide Children’s Hospital, Ohio State Univ. Columbus, OH |
Amy Kogon, MD |
| University of Virginia Charlottesville, VA |
Victoria Norwood, MD |
| Hospital for Sick Children (Sick Kids) Toronto, CA |
Rulan Parekh, MD; Lisa Robinson, MD |
| University of Maryland Baltimore, MD |
Susan Mendley, MD |
| University of Rochester Medical Center, Golisano Children’s Hospital at Strong Rochester, NY |
Marc Lande, MD, George Schwartz, MD |
| University of Iowa Iowa City, IO |
Patrick Brophy, MD |
| University of Illinois, Chicago Chicago, IL |
Eunice John, MD |
| University of Florida Gainesville, FL |
Kiran Upadhyay, MD |
| University of North Carolina, Chapel Hill Chapel Hill, NC |
Maria Ferris, MD |
| Children’s Hospital of Michigan Detroit, MI |
Tej Matoo, MD |
| Maimonides Medical Center Brooklyn, NY |
Juan Kupferman, MD |
| RBHS - Robert Wood Johnson Medical School New Brunswick, NJ |
Lynne Weiss, MD |
| Ann & Robert H. Lurie Children’s Hospital of Chicago Chicago, IL |
Craig Langman, MD |
| INOVA Fairfax Hospital for Children Falls Church, VA |
Patricia Seo-Mayer, MD |
| Children’s National Medical Center Washington DC |
Kanwal Kher, MD |
| Maria Fareri Children’s Hospital at Westchester Valhalla, NY |
Dmitry Samsonov, MD |
Acknowledgments
CKiD is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurologic Disorders and Stroke, the National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U01-DK-82194, and U01-DK-66116).
Abbreviations and Acronyms
- CKD
chronic kidney disease
- HRQoL
health related quality of life
- CKiD
Chronic Kidney Disease in Children cohort study
- CDI
Children’s Depression Inventory
- GFR
glomerular filtration rate
- PedsQL
Pediatric Inventory of Quality of Life Core Scales 4.0
- IQR
interquartile range
Footnotes
No request for reprints
Portions of the study were presented as a poster at the meeting of the American Society of Nephrology, November <days>, 2013, Atlanta, GA.
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pinquart M, Yuhui Shen. Depressive Symptoms in Children and Adolescents with Chronic Physical Illness: An Updated Meta-Analysis. J Pediatr Psychol. 2011;36:375–384. doi: 10.1093/jpepsy/jsq104. [DOI] [PubMed] [Google Scholar]
- 2.Burke P, Meyer V, Kocoshis S, Orenstein DM, Chandra R, Nord DJ, et al. Depression and anxiety in pediatric inflammatory bowel disease and cystic fibrosis. J Am Acad Child Adolesc Psychiatry. 1989;28:948–951. doi: 10.1097/00004583-198911000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Ettinger AB, Weisbrot DM, Nolan EE, Gadow KD, Vitale SA, Andriola MR, Lenn NJ, Novak GP, Hermann BP. Symptoms of depression and anxiety in pediatric epilepsy patients. Epilepsia. 1998;39:595–599. doi: 10.1111/j.1528-1157.1998.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 4.Hood KK, Huestis S, Maher A, Butler D, Volkening L, Laffel LM. Depressive symptoms in children and adolescents with type 1 diabetes: Association with diabetes-specific characteristics. Diabetes Care. 2006;29:1389–1391. doi: 10.2337/dc06-0087. [DOI] [PubMed] [Google Scholar]
- 5.Hood KK, Rausch JR, Dolan LM. Depressive symptoms predict change in glycemic control in adolescents with type 1 diabetes: rates, magnitude, and moderators of change. Pediatr Diabetes. 2011;12:718–723. doi: 10.1111/j.1399-5448.2011.00771.x. [DOI] [PubMed] [Google Scholar]
- 6.Maikranz JM, Steele RG, Dreyer ML, Stratman AC, Bovaird JA. The Relationship of Hope and illness-Related Uncertainty to Emotional Adjustment and Adherence Among Pediatric Renal and Liver Transplant Recipients. J Pediatr Psychol. 2007;32:571–581. doi: 10.1093/jpepsy/jsl046. [DOI] [PubMed] [Google Scholar]
- 7.Avenevoli S, Swendsen J, Jian-Ping He, Bustein M, Ries Merikangas K. Major Depression in the National Comorbitidy Survey-Adolescent Supplement: Prevalence, Correlates, and Treatment. J Am Acad of Child Adolesc Psychiatry. 54:37–44. doi: 10.1016/j.jaac.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez EG, Loza R, Vargas H, Jara MF. Depressive Symptomatology in Children and Adolescents with Chronic Renal Insufficiency Undergoing Chronic Dialysis. Int J Nephrol. 2011:798692. doi: 10.4061/2011/798692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garralda ME, Jameson RA, Reynolds JM, Postlethwaite RJ. Psychiatric adjustment in children with chronic renal failure. J Child Psychol Psychiatry. 1988;29:79–90. doi: 10.1111/j.1469-7610.1988.tb00691.x. [DOI] [PubMed] [Google Scholar]
- 10.Bakr A, Amr M, Sarhan A, Hammad A, Ragab M, El-Refaey A, El-Mougy A. Psychiatric disorders in children with chronic renal failure. Pediatr Nephrol. 2007;22:128–131. doi: 10.1007/s00467-006-0298-9. [DOI] [PubMed] [Google Scholar]
- 11.Berney-Martinet S, Key F, Bell L, Lepine S, Clermont MJ, Fombonne E. Psychological profile of adolescents with a kidney transplant. Pediatr Transplant. 2009;13:701–710. doi: 10.1111/j.1399-3046.2008.01053.x. [DOI] [PubMed] [Google Scholar]
- 12.Kogon AJ, Vander Stoep A, Weiss NS, Smith J, Flynn JT, McCauley E. Depression and its associated factors in pediatric chronic kidney disease. Pediatr Nephrol. 2013;28:1855–1861. doi: 10.1007/s00467-013-2497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimmel PL, Peterson RA. Depression in end-stage renal disease patients treated with hemodialysis: Tools, correlates, outcomes, and needs. Semin Dial. 2005;18:91–97. doi: 10.1111/j.1525-139X.2005.18209.x. [DOI] [PubMed] [Google Scholar]
- 14.Hedayati SS, Minhajuddin AT, Toto RD, Morris DW, Rush AJ. Prevalence of major depressive episode in CKD. Am J Kidney Dis. 2009;54:424–432. doi: 10.1053/j.ajkd.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young BA, Von Korff M, Heckbert SR, Ludman EJ, Rutter C, Lin EHB, et al. Association of major depression and mortality in stage 5 diabetic chronic kidney disease. Gen Hosp Psychiatry. 2010;32:119–124. doi: 10.1016/j.genhosppsych.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedayati SS, Minhajuddin AT, Afshar M, Toto RD, Trivedi MH, Rush AJ. Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA. 2010;303:1946–1953. doi: 10.1001/jama.2010.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maalouf FT, Brent D, Clark C, Tavitian L, Munnell McHugh R, Sahakian BJ, Phillips ML. Neurocognitive impairment in adolescent major depressive disorder: State vs. trait illness markers. J Affect Disorders. 2011;133:625–632. doi: 10.1016/j.jad.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bacca CB, Vickrey BG, Caplan R, Vassar SD, Berg AT. Psychiatric and Medical Comorbidity and Quality of Life Outcomes in Childhood-Onset Epilepsy. Pediatrics. 2011;126:e1532–e1543. doi: 10.1542/peds.2011-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller J, Hess J, Ager A. Minor symptoms of depression in patients with congenital heart disease have a larger impact on quality of life than limited exercise capacity. Int J Cardiol. 2012;154:265–269. doi: 10.1016/j.ijcard.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 20.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, et al. Design and methods of the chronic kidney disease in children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1:1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hooper SR, Gerson AC, Butler RW, Gipson DS, Mendley SR, Lande MB, et al. Neurocognitive Functioning of Children and Adolescents with Mild-to-Moderate Chronic Kidney Disease. Clin J Am Soc Nephrol. 2011;6:1824–1830. doi: 10.2215/CJN.09751110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerson AC, Wentz A, Abraham AG, Mendley SR, Hooper SR, Butler RW, Gipson DS, Lande MB, Shinnar S, Moxey-Mims MM, Warady BA, Furth SL. Health-Related Quality of Life of Children With Mild to Moderate Chronic Kidney Disease. Pediatrics. 2010;152(2):e349–e357. doi: 10.1542/peds.2009-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovacs M. Children’s Depression Inventory (CDI) New York: Multi-Health Systems, Inc; 1992. A copyrighted instrument. [Google Scholar]
- 24.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 25.Wechsler D. Wechsler abbreviated scale of intelligence. NCS Pearson, Inc; San Antonio: 1999. [Google Scholar]
- 26.Wechsler D. Wechsler individual achievement test-II-abbreviated. NCS Pearson, Inc; San Antonio: 2001. [Google Scholar]
- 27.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3:329–341. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory Version 4.0 Generic Core Scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Mendley SR, Matheson MB, Shinnar S, Lande MB, Gerson AC, Butler RW, et al. Duration of chronic kidney disease reduces attention and executive function in pediatric patients. Kidney Int. 2015;87:800–6. doi: 10.1038/ki.2014.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz GJ, Furth S, Cole SR, Waradu B, Munoz A. Glomerular filtration rate via plasma iohexol disappearance: Pilot study for chronic kidney disease in children. Kidney Int. 2006;69:2070–2077. doi: 10.1038/sj.ki.5000385. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady B. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82:445–453. doi: 10.1038/ki.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Uzri A, Matheson M, Gipson DS, Mendley SR, Hooper SR, Yadin O, Rozansky DJ, Mims-Moxey M, Furth SL, Warady BA, Gerson AC on behalf of the Chronic Kidney Disease in Children (CkiD) Study Group. The Impact of Short Stature on Health-Related Quality of Life in Children with Chronic Kidney Disease. J Pediatr. 2013;163:736–41. doi: 10.1016/j.jpeds.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dodson JL, Cohn SE, Cox C, Hmiel PS, Wood E, Mattoo TK, Warady BA, Furth SL. Urinary Incontinence in the CkiD Cohort and Health Related Quality of Life. J Urol. 2009;182:2007–2014. doi: 10.1016/j.juro.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albert PS, Follman DA. Shared Parameters. In: Fitzmaurice G, Davidian M, Verbeke G, Molenberghs G, editors. Longitudinal Data Analysis. Boca Raton, FL: Chapman & Hall/CRC Press; 2009. pp. 433–445. [Google Scholar]
- 35.Marciano RC, Soares CM, Diniz JS, Lima EM, Silva JM, Canhestro MR, et al. Behavioral disorders and low quality of life in children and adolescents with chronic kidney disease. Pediatr Nephrol. 2011;26:281–290. doi: 10.1007/s00467-010-1683-y. [DOI] [PubMed] [Google Scholar]
- 36.Stockings E, Degenhardt L, Yi Lee Y, Mihalopoulos C, Liu A, Hobbs M, Patton G. Symptom screening scales for detecting major depressive disorder in children and adolescents: A systematic review and meta-analysis of reliability, validity and diagnostic utility. J Affect Disorders. 2015;174:447–463. doi: 10.1016/j.jad.2014.11.061. [DOI] [PubMed] [Google Scholar]