Abstract
Rationale
Myocardial ischemia-reperfusion (I/R) results in the generation of oxygen-derived free radicals and the accumulation of lipid peroxidation-derived unsaturated aldehydes. However, the contribution of aldehydes to myocardial I/R injury has not been assessed.
Objective
We tested the hypothesis that removal of aldehydes by glutathione S-transferase P (GSTP) diminishes I/R injury.
Methods and Results
In adult male C57BL/6 mouse hearts, Gstp1/2 was the most abundant GST transcript followed by Gsta4 and Gstm4.1, and GSTP activity was a significant fraction of the total GST activity. mGstp1/2 deletion reduced total GST activity, but no compensatory increase in GSTA and GSTM or major antioxidant enzymes was observed. Genetic deficiency of GSTP did not alter cardiac function, but in comparison with hearts from wild-type (WT) mice, the hearts isolated from GSTP-null mice were more sensitive to I/R injury. Disruption of the GSTP gene also increased infarct size after coronary occlusion in situ. Ischemia significantly increased acrolein in hearts, and GSTP deficiency induced significant deficits in the metabolism of the unsaturated aldehyde, acrolein, but not in the metabolism 4-hydroxy-trans-2-nonenal (HNE) or trans-2-hexanal; and, upon ischemia, the GSTP-null hearts accumulated more acrolein-modified proteins than WT hearts. GSTP-deficiency did not affect I/R-induced free radical generation, JNK activation or depletion of reduced glutathione. Acrolein-exposure induced a hyperpolarizing shift in INa, and acrolein-induced cell death was delayed by SN-6, a Na+/Ca++ exchange inhibitor. Cardiomyocytes isolated from GSTP-null hearts were more sensitive than WT myocytes to acrolein-induced protein crosslinking and cell death.
Conclusions
GSTP protects the heart from I/R injury by facilitating the detoxification of cytotoxic aldehydes such as acrolein.
Keywords: Acrolein, aldehydes, glutathione S-transferase, glutathiolation, myocardial infarction, lipid peroxidation, protein adduction, oxidative stress, myocardial ischemia, reperfusion injury, cardioprotection, oxidized lipids
INTRODUCTION
Despite extensive investigations, the cellular and molecular mechanism that contribute to myocardial ischemia are not well understood and to-date no infarct sparing therapeutic interventions, other than early perfusion, have been developed. Previous studies show that during ischemia, inhibition of the mitochondrial respiratory chain perturbs electron transport, leading to an increase in the generation of free radicals.1–3 Free radical generation is further increased upon reperfusion. Therefore, even though early perfusion halts subsequent ischemic injury, free radicals generated during reperfusion attenuate tissue salvage and induce additional cell death. Increased production of free radicals and related reactive oxygen species (ROS) during reperfusion has been linked to apoptosis, calcium overload and reentrant arrhythmias.4 Nevertheless, attempts to attenuate I/R injury using enzymatic and non-enzymatic antioxidants have not been universally successful, and it remains unclear whether free radicals are a direct cause of myocardial injury or whether this injury is caused by other secondary species generated by the free radicals.
Free radicals generated during I/R are short-lived and their ability to induce tissue injury is paradoxically limited by their high reactivity. Because these radicals react readily with neighboring molecules, most are unable to diffuse away from their site of formation and cause only site-specific damage. It is, therefore, likely that tissue injury attributed to free radicals is caused by secondary, more-stable species that the radicals generate. While free radicals generate a range of secondary species, products of lipid peroxidation have received the most attention.5 Membrane lipids are the most vulnerable targets of free radicals and even transient increases in free radicals are associated with the accumulation of lipid peroxidation products. Therefore, increased production and accumulation of lipid oxidation products could account for much of the tissue injury attributed to free radicals.
Of the several products generated by oxidized lipids, unsaturated aldehydes are some of the most toxic species.6 These aldehydes are generated in high abundance and they avidly react with tissue nucleophiles leading to depletion of reduced glutathione and covalent modification of nucleophilic protein side chains and nucleotides. Moreover, unlike their radical progenitors, aldehydes can diffuse away from their site of origin and thereby prolong and amplify oxidative injury. However, tissue reactivity of aldehydes is regulated by processes involved in their metabolism and detoxification.7 In most tissues, aldehydes are either reduced or oxidized to less reactive products. In addition, unsaturated aldehydes are conjugated to reduced glutathione (GSH) and are excreted as mercapturic acids in the urine. While unsaturated aldehydes spontaneously react with GSH, this reaction is further accelerated by glutathione S-transferases (GSTs), which augments detoxification and decreases tissue accumulation of unsaturated aldehydes.
The GSTs represent a family of Phase II enzymes that are expressed to high abundance in most tissues. At least 16 genes encode GSTs in the cytosol and 6 GST gene products are localized in the membrane.8 These proteins catalyze glutathione conjugation with a range of endogenous and xenobiotic substrates.9 Several GSTs are involved in the metabolism and detoxification of unsaturated aldehydes: GST4-4 catalyzes the addition of glutathione to aldehydes such as 4-hydroxy-trans-2-nonenal (HNE)10, whereas GSTP supports glutathiolation of small chain aldehydes including acrolein and base propenals.11 GSTP is the major extra-hepatic GST isoform. Previous studies have shown that fibroblasts isolated from GSTP-null mice are more sensitive to various forms of oxidative stress including UV radiation and H2O2, and that deletion of GSTP increases tumor formation and growth.12 Nevertheless, the role of GSTP in myocardial ischemic injury has not been studied.
The present study was designed to test the hypothesis that myocardial ischemic injury is mediated, in part, by unsaturated aldehydes, and thus, the detoxification of these aldehydes by GSTP protects the heart from oxidative damage during I/R. The results of this study show that genetic deficiency of GSTP diminishes aldehyde detoxification in the heart and exacerbates myocardial I/R injury. These findings reveal a new mechanism of cardioprotection and support the notion that aldehydes generated in the ischemic heart are a significant cause of tissue injury and cell death.
METHODS
Detailed methodology is provided in the online supplement. WT and GSTP-null mice, generated on a MF1 background strain using homologous recombination12 were bred for >12 generations with C57BL/6J mice. The mice were treated in accordance with the Declaration of Helsinki and with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health. All treatment protocols were approved by the University of Louisville IACUC. The mice were genotyped using primers that amplified the region between exons 5 and 6 of GSTP1 and a region in the lacZ gene to identify a null allele. The mice were healthy and reproduced in a Mendelian fashion. Their cardiac function was measure by echocardiography after Avertin anesthesia at 12 to 16 weeks of age. In a subset of mice, LV mechanical function was assessed using a Millar pressure-conductance catheter as described previously.13
Cardiac myocyte isolation, electrophysiology and hypercontracture
Isolation
Calcium-tolerant cardiomyocytes were isolated from the left ventricle using collagenase digestion as described previously.14
Electrophysiology
Whole cell sodium currents were recorded by the patch clamp technique at room temperature (21–22°C) as described before.15, 16 The recording pipettes were filled with internal solution containing (in mM) CsCl 134, NaCl 10, MgCl2 1, Na2-ATP 1, HEPES 10, EGTA 10, adjusted to pH 7.2 with CsOH. Cells were superfused at 1 to 1.5 mL/min with bath solution containing (in mmol/L) NaCl 20, CsCl 129, CaCl2 1.8, MgCl2 1, HEPES 10, LaCl3 0.5, adjusted to pH 7.4 using CsOH. Cs2+ and La3+ were used to block K+- and Ca2+-dependent currents, respectively.
Hypercontracture
After isolation, myocytes were incubated overnight in laminin-coated plates, and then to measure oxidant sensitivity, myocytes were superfused in an oxygenated-HEPES buffer without or with acrolein or H2O2 for 60 min. As indicated, in some experiments the cells were preincubated with an NCX1 inhibitor17 SN-6 (10 μM, 30 min) before superfusion with acrolein (25 μM) for 60 min in HBSS. Digital images of myocytes (60–100/field) were used for quantification of the total number of rod-shaped cells as previously described.14, 18
Ischemia-reperfusion injury
Mouse hearts were subjected to ischemia reperfusion ex vivo in the Langendorff mode or to coronary ligation in situ. These models have been described in detail before.19, 20 For ex vivo perfusion the hearts were perfused at a constant pressure (~80 mm Hg) with Krebs-Henseleit buffer containing (in mM): NaCl 118, KCl 4.7, KH2PO4 1.25, MgCl2 1.25, CaCl2 2.5, EDTA 0.5, NaHCO3 25, glucose 10; (pH 7.4; 37 °C) for 30 min prior to ischemia. In select experiments, total flow and left ventricular pressure were recorded and the perfusate was collected for measuring CK and LDH activities.21
Electron Paramagnetic Resonance (EPR) spectroscopy
Free radical generation was measured in isolated perfused hearts. After ischemia, the spin trap 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) in buffer containing 100 μM diethylenetriaminepentaacetic acid (DPTA) was infused in the heart at a rate of 100 μl/min through a side arm located close to the heart. The effluent was collected at indicated times after reperfusion and each tube was frozen immediately in liquid nitrogen. EPR spectra were recorded and analyzed as previously described.20, 21
Acrolein measurement
For measuring free acrolein in perfused hearts subjected to ischemia, we used a carbonyl derivatizing reagent, N-[2-(aminooxy)ethyl]-N,N-dimethyl-1-dodecylammonium iodide (QDA; 13CD3-QDA internal standard)22, and UPLC-MS multiple reaction monitoring (MRM) with femtomole sensitivity.
Aldehyde metabolism
For measuring acrolein and HNE metabolism, cardiac lysates (2 mg/ml) were incubated with reduced glutathione (GSH, 100 μM) and indicated concentrations of either acrolein or HNE. The GS-conjugate formed in the reaction mixture was separated by HPLC and quantified by ESI+-mass spectrometry using either GS-13C-propanal or GS-13C-4HNE as internal standards.
Protein expression, abundance and enzyme activity
Western blots were developed using commercially available antibodies against GSTA, M and P. Western blots were scanned on a Typhoon 9600 and band density of interest was normalized to α-tubulin staining band density. Total GST conjugating activity toward substrates 1-chloro-2,4-dinitrobenzene (CDNB; 1 mM) and ethacrynic acid (EA; 200 μM) was measured in cardiac homogenates as reported previously.23, 24
Histology and immunohistochemistry
Formalin-fixed, paraffin-embedded tissue sections (4 μm) were stained with H&E, anti-GSTP1 antibody (1:1,500; Novocastra)25, IgG-purified polyclonal rabbit anti-protein-acrolein antibody (1:1,000)23 or murine anti-myeloperoxidase antibody (MPO, Ab-1; Thermo Fisher Scientific).23 Images of mid-heart cross sections were made using a digital Spot camera mounted on an Olympus microscope and analyzed using Metamorph (Molecular Devices).
Statistical analysis
Data are presented as mean ± SEM. For different treatment groups, data were compared using paired or unpaired t-test or One Way ANOVA with repeated measures and Dunnett or Bonferroni post-test where appropriate (SigmaStat, SPSS, Inc., Chicago, Il). Mean lifetime (μ; min) of cardiac myocytes superfused with oxidants was calculated using the Weibull distribution as described before.14 P<0.05 was considered statistically significant.
RESULTS
Cardiac distribution of GSTP1/P2 protein
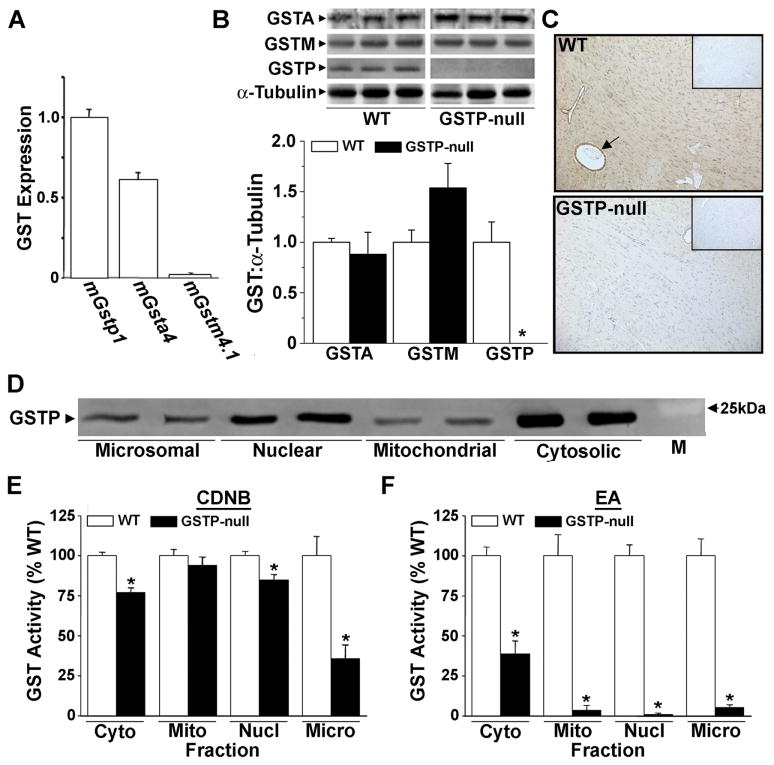
Most tissues express several GST isoforms. To determine the expression of GST isoforms in the heart, we measured the relative expression of the three major GST isoforms – GSTA (α), GSTM (μ) and GST P1/P2 (π) in the hearts of WT and GSTP-null mice. RT-PCR analysis showed that mGstp1/2 was the most abundant GST transcript followed by mGsta4 and mGstm4.1 (Fig. 1A). RT-PCR and Western blotting did not show a compensatory increase in the expression of GSTA and GSTM isoforms in GSTP-null mice (Fig. 1A,B). Immunohistochemical studies showed diffuse localization of GSTP in the myocardium with positive immunoreactivity associated with cardiac myocytes and coronary blood vessels. No positive immunohistochemical reactivity was observed in GSTP-null hearts (Fig. 1C). Subcellular fractionation showed that GSTP was localized mainly in the cytosolic (>50%) and the nuclear fractions, although low levels of the protein were also detected in the microsomal and mitochondrial fractions (Fig. 1D). GST activity measured with CDNB, which is conjugated by most GSTs, as well as ethacrynic acid, a GSTP-specific substrate, was significantly lower in subcellular fractions of GSTP-null hearts compared with WT hearts (Figs. 1E,F) indicating that GSTP contributes significantly to total GST conjugating activity in the heart. There were no differences, however, in protein abundance (Online Figure IA) or the activity (Online Table II) of major antioxidant enzymes, including SOD, glutathione reductase, glutathione peroxidase, aldose reductase (AR) and thioredoxin (TRX) between WT and GSTP-null hearts. A small (8%), but statistically significant, difference in catalase activity was observed, but this is unlikely to be physiologically significant. Similarly, the inducible proteins, iNOS and HO-1, were not detected in WT or GSTP-null hearts (Online Figure IB), and there was no difference in basal level of protein-HNE adducts (Online Figure IC). Collectively, these data indicate that GSTP is a major cardiac GST isoform and that GSTP deficiency does not result in compensatory changes in other GSTs or in persistent oxidative stress.
Figure 1. Distribution and activity of cardiac GSTs in WT and GSTP-null mice.
A, Real-time-PCR, B, Western blots and quantification (normalized to α-tubulin) of GST alpha (GSTA), mu (GSTM) and pi (GSTP) in WT and GSTP-null hearts. C, Mid-left ventricular (LV) sections from WT (arrow, endothelium staining) and GSTP-null mice stained with anti-GSTP antibody (Insets: IgG-negative controls). D, Western blots showing the relative abundance of GSTP in microsomal (9.2±1.7%), nuclear (34.8±8%), mitochondrial (3.8±2.8%) and cytosolic (52.2±3.6%) fractions of WT hearts. GST activity in subcellular fractions of WT and GSTP-null hearts measured with: E, 1-chloro-dinitrobenzene (CDNB) or F, ethacrynic acid (EA) as substrate. * P<0.05 (n=3–5 per group).
Cardiac function
Cardiac dimensions and function in GSTP-null mice were similar to aged-matched WT mice (Online Table III). No significant differences between LV or heart/body weight ratios or heart rate were observed. Although both LV end diastolic volume and ejection fraction were slightly higher in GSTP-null mice, these differences were slight; indicating that genetic deficiency of GSTP does not markedly alter cardiac dimension or function.
GSTP and ischemia-reperfusion injury
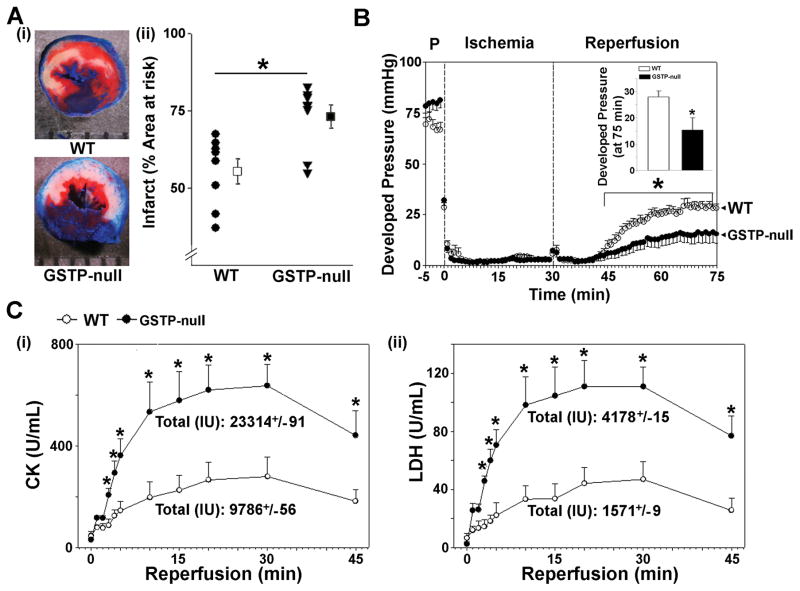
To determine whether GSTP prevents ischemic injury, WT and GSTP-null mice were subjected to 30 min of coronary occlusion followed by reperfusion. In WT mice, this procedure resulted in the large infarcts that were on average 55.6±3.9 % of the area-at-risk. In comparison, the GSTP-null mice were more sensitive to ischemia; the infarct size in these mice was 73.1±3.7% of the area-at-risk (Fig. 2Ai,ii). These observations suggest that genetic deficiency of GSTP results in greater myocardial injury induced by coronary ligation. Because GSTP deletion could affect a variety of systemic responses that could indirectly affect myocardial ischemic injury, we examined whether hearts isolated from GSTP-null mice were more sensitive to I/R injury. In isolated perfused WT hearts, global ischemia for 30 min significantly suppressed contractile function and after 45 min of reperfusion, the recovery of developed pressure was approximately 30% of its pre-ischemic value (Fig. 2B; Online Figure II). In contrast, in GSTP-null hearts, there was <15% recovery in developed pressure, indicating that GSTP-deficiency decreases post-ischemic recovery (Fig. 2B). In addition, the release of the myocardial enzymes - LDH and CK, which is indicative of cell death, was significantly higher in GSTP-null than WT hearts (Fig. 2Ci,ii). No significant difference in heart rate (at baseline or after reperfusion) was observed between WT and GSTP-null hearts. These data indicate that deletion of GSTP increases myocardial sensitivity of I/R injury, independent of any systemic factors, and that in both in situ and ex vivo models, the absence of GSTP exacerbates I/R injury.
Figure 2. Genetic deletion of GSTP exacerbates ischemia-reperfusion injury.
A, (i) Representative mid-LV cross-sections from WT or GSTP-null hearts subjected to 30 min of coronary occlusion, followed by 4h reperfusion. (ii) Infarct size as a percent of area-at-risk in WT and GSTP-null mice. B, Developed pressure in isolated, perfused WT and GSTP-null hearts subjected to 30 min of global ischemia followed by 45 min of reperfusion (Inset: Final developed LV pressure). C, Post-ischemic release of (i) CK and (ii) LDH in the perfusate during 45 min of reperfusion. * P<0.05 (n=7 per group).
Cardioprotective mechanism of GSTP
Because GSTP is a multifunctional protein, we considered several mechanisms by which it could affect I/R injury. These are described below.
Regulation of GSH levels
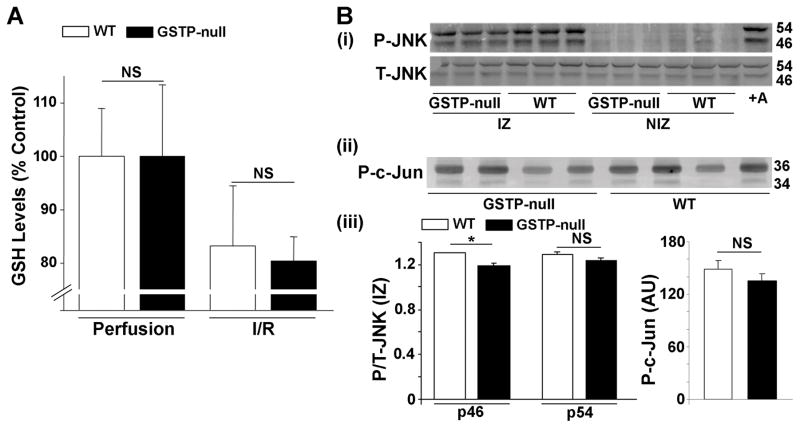
GSTP catalyzes the conjugation of glutathione with electrophilic substrates, therefore, the absence of the enzyme could increase tissue glutathione levels. However, our measurements showed that there was no difference in the levels of reduced glutathione (GSH) between perfused WT (GSH, 2.56±0.26 nmol/mg wet weight, n=7) and null (GSH, 3.27±0.42 nmol/mg wet weight, n=7) hearts. After 15 min of ischemia and 15 min of reperfusion, levels of reduced GSH were decreased by approximately 20% in both WT and GSTP-null mice when compared with hearts subjected to perfusion alone (Fig. 3A). These results indicate that GSTP-deficiency does not exacerbate ischemic depletion of GSH in the heart, and therefore, the effects of GSTP on myocardial I/R injury are unlikely to be mediated by changes in GSH levels.
Figure 3. GSTP-deficiency does not affect reduced glutathione (GSH) levels or JNK activation during cardiac I/R.
A, Cardiac levels of GSH in isolated, perfused WT and GSTP-null hearts with Krebs-Henseleit solution for 45 min (Perfusion only) or after 30 min perfusion, 15 min of ischemia and 15 min of reperfusion (I/R). B, Western blots of (i) phosphorylated JNK (p-JNK), (ii) phosphorylated c-Jun (P-c-Jun; measurement of JNK kinase activity) in lysates of ischemic (IZ) and non-ischemic zones (NIZ; posterior wall), and (iii) quantification of phosphorylated to total protein of WT and GSTP-null hearts subjected to 15 min of coronary ligation and 15 min of reperfusion in situ. * P<0.05 (n=3–6 per group).
JNK activation
Previous studies have shown that genomic deletion of mGstp1/p2 leads to constitutive activation of JNK in the liver and the lung.26 Hence GSTP could affect JNK activity, which in turn could alter myocardial sensitivity to I/R injury. However, we found that the basal levels of phospho-JNK and JNK activity (measured as phosphorylated c-jun) were similar between WT and GSTP-null hearts, suggesting that in the heart, unlike the lung or the liver26, the levels of constitutively active JNK are not elevated by GSTP deletion. Moreover, coronary occlusion for 15 min, followed by 15 min of reperfusion significantly increased phospho-JNK levels in both WT and GSTP-null hearts in the area-at-risk, but not in the non-ischemic zone (posterior wall LV)(Fig. 3Bi,iii). The level of phospho-JNK in the ischemic zone was not different in GSTP-null hearts compared with WT hearts, indicating that the absence of GSTP did not enhance JNK activation during I/R. Moreover, the levels of phosphorylated c-Jun were not different in the ischemic zone of WT and GSTP-null hearts (Fig. 3Bii,iii). These observations suggest that GSTP-mediated cardioprotection is unlikely to be related to changes in JNK activation.
Oxidative stress
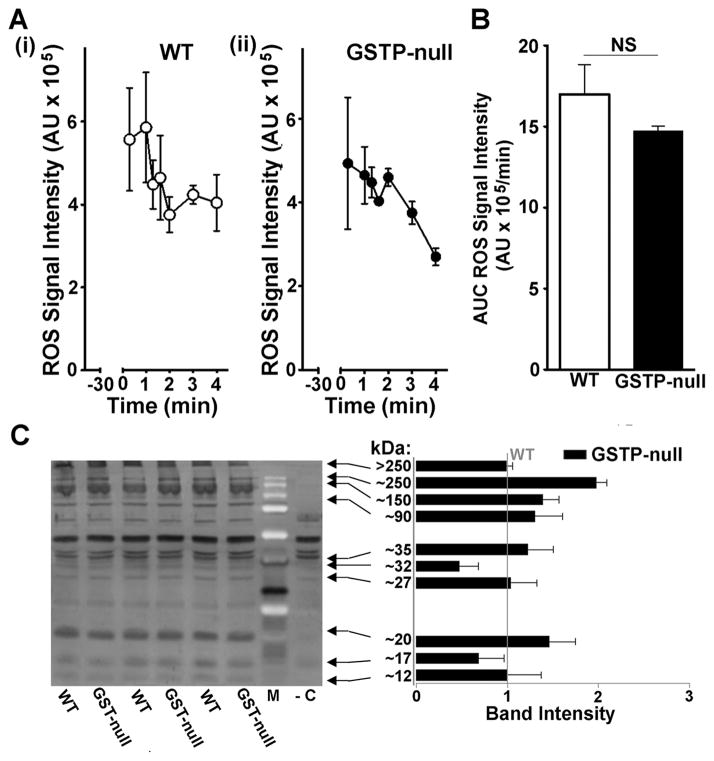
To examine whether GSTP regulates the production of free radicals upon reperfusion, we measured free radicals in the perfusates of perfused heart and lipid peroxidation-derived aldehyde in cardiac lysates. To measure free radicals, hearts were perfused with the radical trap DMPO and stable radical-DMPO adducts in the perfusate were measured by EPR.20 Under these conditions, peak radical signal was detected within 1 min of reperfusion and the EPR signal diminished over the next 4 min to baseline in both WT and GSTP-null hearts (Fig. 4A). There was no difference in peak signal intensity and ROS AUC (Fig. 4B) between WT and GSTP-null hearts. In addition, the abundance of DMPO-modified proteins was similar in WT and GSTP-null hearts (Fig. 4C), suggesting that disruption of GSTP does not increase the formation of protein-radicals in the hearts. Collectively, these observations indicate that both WT and GSTP-null hearts generate equal levels of ROS and that deletion of GSTP does not increase free radical production in hearts subjected to ischemia-reperfusion. To determine whether GSTP-deficiency affects the basal levels and ischemia-induced increase in lipid peroxidation-derived aldehydes, we measured myocardial levels of free malondialdehyde (MDA), hexanal or HNE in perfused hearts and in hearts subjected to I/R by GC/MS. We found no difference in the levels of these aldehydes in WT and GSTP-null hearts (Online Table IV), indicating that GSTP activity does not regulate MDA, hexanal or HNE levels. Similarly, the ischemia reduced:oxidized glutathione ratio (GSH:GSSG) was not different between WT and GSTP-null hearts (Online Table V). Collectively, these results suggest that GSTP-deficiency does not increase generalized oxidative stress in the heart during ischemia-reperfusion.
Figure 4. GSTP deficiency does not affect free radical production upon reperfusion.
A, Graphs showing the time course of electron paramagnetic resonance signal intensity (mean ± SEM; n=4,4) after administration of DMPO (1 mol/L; 0.1 mL/min) during first 5 min of reperfusion after 30 min of global ischemia in (i) WT and (ii) GSTP-null hearts. B, Integrated ROS signal intensity as area under the curve (AUC) from 0–5 min of reperfusion. C, Western blot staining and quantification of cardiac lysates for DMPO-modified proteins in WT and GSTP-null hearts at 5 min of reperfusion (-C, negative control). NS, no significant difference (n=4 per group).
Acrolein formation, metabolism and toxicity
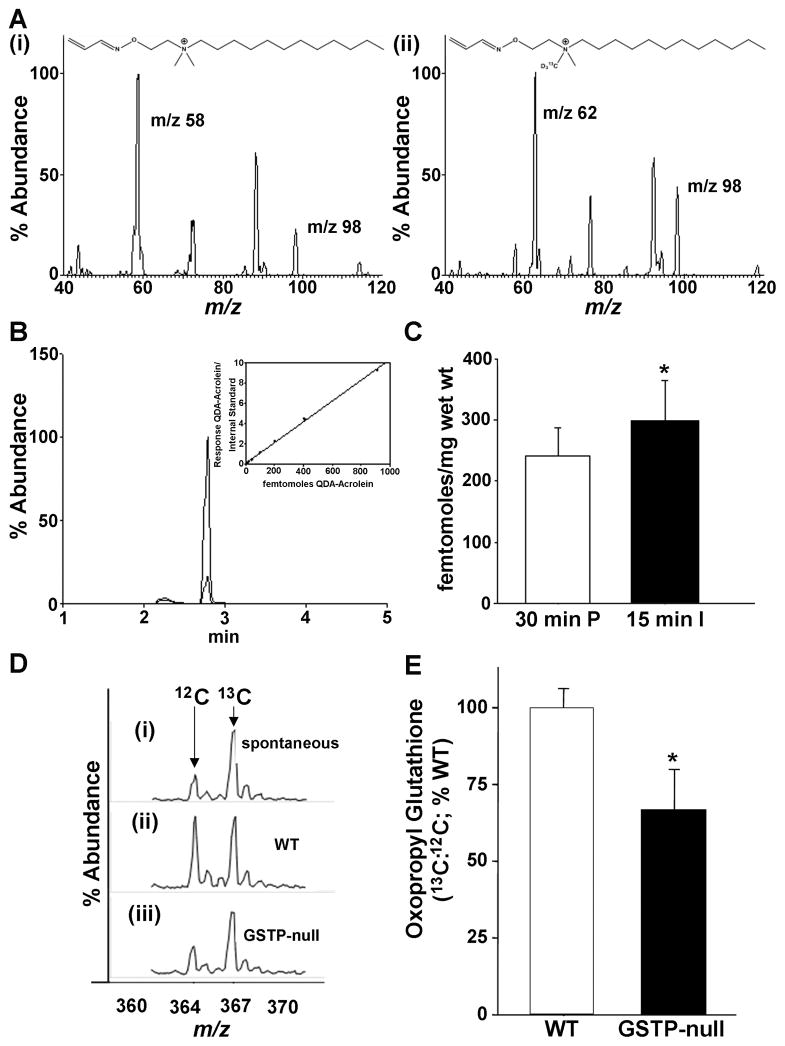
A previous study showed that both left ventricular pacing and heart failure generate lipid peroxidation products including unsaturated aldehydes such as HNE, however, free acrolein has not been detected in the ischemic heart.27 Hence, we measured free acrolein using an UPLC-MS and MRM method in perfused hearts subjected to global ischemia for 15 min with N-[2-(aminooxy)ethyl]-N,N-dimethyl-1-dodecylammonium iodide (QDA) that binds acrolein. The native acrolein-QDA adduct recovered from ischemic hearts had an elution and ion fragmentation profile identical to an isotopic internal standard 13CD3-QDA-acrolein (Fig. 5A,B). These measurements showed that in comparison with hearts perfused under aerobic conditions, hearts subjected to ischemia had significantly higher levels of free acrolein (Fig. 5C), indicating that myocardial ischemia results in acrolein formation.
Figure 5. Ischemia increases free acrolein and GSTP-deficiency diminishes glutathione conjugation of acrolein in the heart.
A, Free acrolein was measured using UPLC-MS and multiple reaction monitoring (MRM) in perfused (P, 30 min) and ischemic (I, 15 min) hearts with N-[2-(aminooxy)ethyl]-N,N-dimethyl-1-dodecylammonium iodide probe (QDA) (i) that binds acrolein and results in MRMs identical (m/z: 58, 98) to (ii) internal standard 13CD3-QDA-acrolein (*QDA-acrolein; m/z: 62, 98). B, Representative UPLC elution profile of parent QDA-acrolein (m/z 311.2) at 2.9 min is identical to *QDA-acrolein (m/z 315.2). Inset: Standard curve indicating limit of detection (LOD) was in the sub-femtomolar range. C, Group data showing free acrolein in WT hearts perfused with aerobic buffer for 30 min or subjected to 15 min of ischemia (WT, n=7, 7; P=0.05). D, Representative electrospray ionization-mass spectrometry (ESI-MS) traces of oxopropyl glutathione formed (i) spontaneously or catalyzed in cardiac lysates prepared from either (ii) WT or (iii) GSTP-null hearts. E, The percentage of oxopropyl glutathione (relative to WT) generated in cardiac homogenates from WT and GSTP-null hearts incubated with acrolein (10 μM) and GSH (100 μM) for 15 min. The concentration of oxopropyl glutathione generated in the lysates (m/z 364) was quantified by ESI+-MS using 13C-oxopropyl glutathione (m/z 367) as an internal standard. * P<0.05 (n=6 per group).
Previous work has shown that GSTP catalyzes the glutathione conjugation of short-chain aldehydes such as acrolein. Therefore, GSTP deficiency could increase I/R injury by preventing the metabolism and detoxification of aldehydes such as acrolein generated in the ischemic heart. Acrolein is a reactive and toxic aldehyde that is generated during peroxidation of membrane lipids28 and as a product of myeloperoxidase-catalyzed reactions.29 Hence, we tested whether GSTP catalyzes the conjugation of acrolein in the heart and whether acrolein accumulation could account for the greater I/R injury in GSTP-null hearts. We found that incubation of acrolein with GSH led to the spontaneous formation of the glutathione-acrolein conjugate (oxopropyl glutathione), which could be detected by mass spectrometry using reagent 13C-oxopropyl glutathione as an internal standard (Fig. 5D). The extent of formation of the glutathione conjugate was significantly increased when acrolein and GSH were co-incubated with cardiac lysates from WT mice, indicating that the reaction is catalyzed by cardiac GSTs. Moreover, the extent of oxopropyl glutathione formation was consistently lower in GSTP-null than in WT hearts (-33±13 % of WT activity)(Fig. 5E). Under similar experimental conditions, there was no significant difference in GS-HNE formation between GSTP-null and WT hearts (-17±13 % of WT activity, n=3). Taken together, these data indicate that genetic deletion of GSTP decreases the rate of conjugation of acrolein with glutathione, whereas the formation of the glutathione conjugation of HNE is not affected. Therefore, the deficiency of GSTP could diminish acrolein metabolism and thereby increase the toxicity of acrolein generated during I/R.
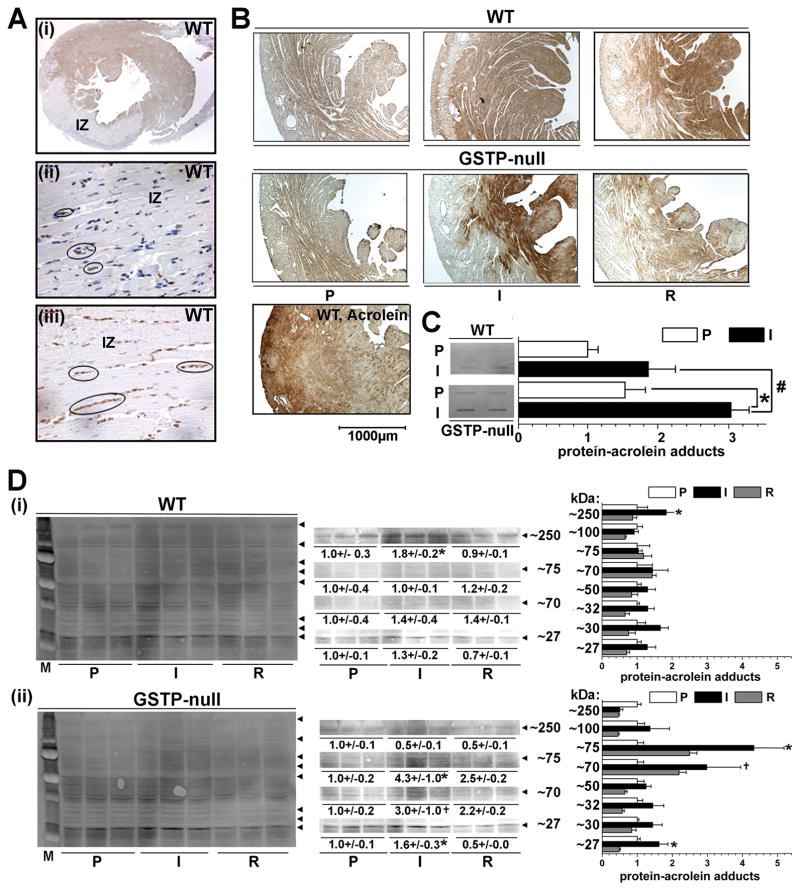
To determine whether GSTP deficiency affects the fates of acrolein generated in the heart, we measured changes in the formation of protein-acrolein adducts in WT and GSTP-null hearts subjected to I/R. The formation of protein-acrolein adducts was used as an indirect index of acrolein fate in the heart, because acrolein is highly reactive, and therefore, the levels of free acrolein are likely only a fraction of what reacts with nucleophilic cell constituents. Immunohistochemical analysis showed extensive positive reactivity with polyclonal anti-acrolein-protein antibodies in the peri-infarct region of hearts subjected to coronary ligation in situ (Fig. 6Ai). Significantly, a near absence of anti-protein acrolein staining was observed within the infarct zone, indicating that the increased protein modification by acrolein is not due to cell death and that these adducts are not associated with the dead tissue within the infarct. Indeed, what little staining that was present in the infarct zone (Fig. 6Aii) was co-localized with inflammatory cells stained for myeloperoxidase protein (MPO; Fig. 6Aiii). Similarly, in isolated perfused hearts, we found that 15 min of ischemia led to a significant increase in the formation of protein-acrolein adducts in WT hearts (Fig. 6B; I), which generally decreased upon reperfusion (Fig. 6B; R). Moreover, under each experimental condition, the intensity of staining seemed higher in GSTP-null than in WT hearts (Fig. 6B; I). To quantify total protein-acrolein adducts generated in the heart, slot blots were developed using lysates of WT and GSTP-null hearts subjected to 15 min of ischemia. As shown in Fig. 6C, there was an increase in protein-acrolein adduct formation in WT upon ischemia. The basal abundance of protein-acrolein adducts in perfused GSTP-null hearts was greater than WT hearts; upon ischemia there was an increase in adduct formation, which was significantly higher in GSTP-null than WT hearts. The results of Western blot analysis revealed that the intensity of several proteins bands (Mr ≈75, ≈70, ≈25) was higher in GSTP-null than in WT hearts (Fig. 6Di,ii). Moreover, incubation of isolated cardiac myocytes with acrolein led to the modification of several proteins and prominent increase in reactivity with anti-protein-acrolein antibody was observed with a protein band corresponding to 250 kDa, likely formed by cross-linked protein (Online Figure IVA). Removal of acrolein from the medium, led to time-dependent decrease in the intensity of this band in WT, but not in GSTP-null, cardiac myocytes, in which the intensity of the band continued to increase for the next 60 min (Online Figure IVB). Taken together these results suggest that I/R increases the formation of acrolein-modified proteins and that GSTP-deficiency results in a greater accumulation of protein-acrolein adducts in both ischemic and reperfused hearts. Similar bands of modified proteins could be detected also in isolated cardiac myocytes incubated with acrolein and that the formation and the persistence of these bands was higher in GSTP-null than in WT myocytes, is consistent with the key role of GSTP in preventing acrolein-induced protein modification.
Figure 6. Ischemia-induced formation of protein-acrolein adducts is increased in GSTP-null hearts.
A, Photomicrographs of mid-LV infarct zone (IZ) in WT hearts subjected to 30 min of coronary ligation followed by 24 h of reperfusion and immunohistochemically-stained with (i–ii) anti-protein-acrolein antibody (ii, IZ; 100x) or (iii) myeloperoxidase (MPO, IZ; 100x). Black ovals indicate localization of positive (ii) anti-protein-acrolein and (iii) MPO staining (in neutrophils) in IZ. B, Photomicrographs of LV cross-sections from isolated, perfused WT and GSTP-null hearts after 45 min of perfusion (P), 15 min of global ischemia (I) or 15 min I followed by 15 min of reperfusion (R) as indicated, or from WT heart perfused with 10 μM acrolein for 10 min (WT, Acrolein). C, Slot-blot analysis of total protein-acrolein adduct formation in WT and GSTP-null hearts after 15 min of global ischemia, and D, Western blots and band-density quantification of lysates prepared from (i) WT and (ii) GSTP-null hearts subjected to 45 min P, 15 min I or 15 min I and 15 min R (I/R). *, # P<0.05 between P and I; + 0.05<P<0.10 between P and I (n=3 per group).
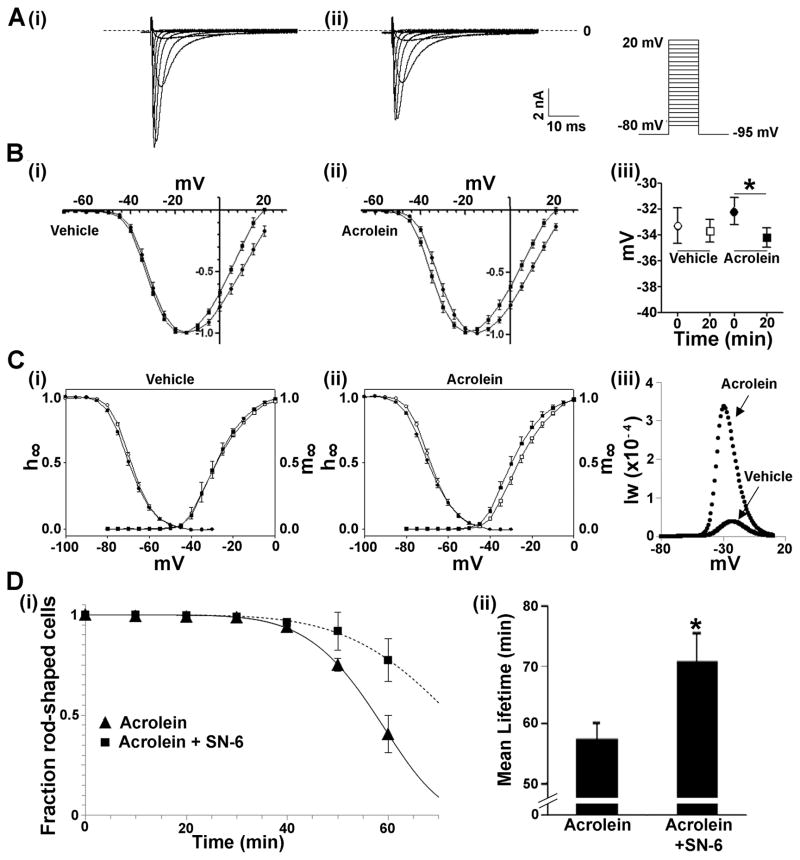
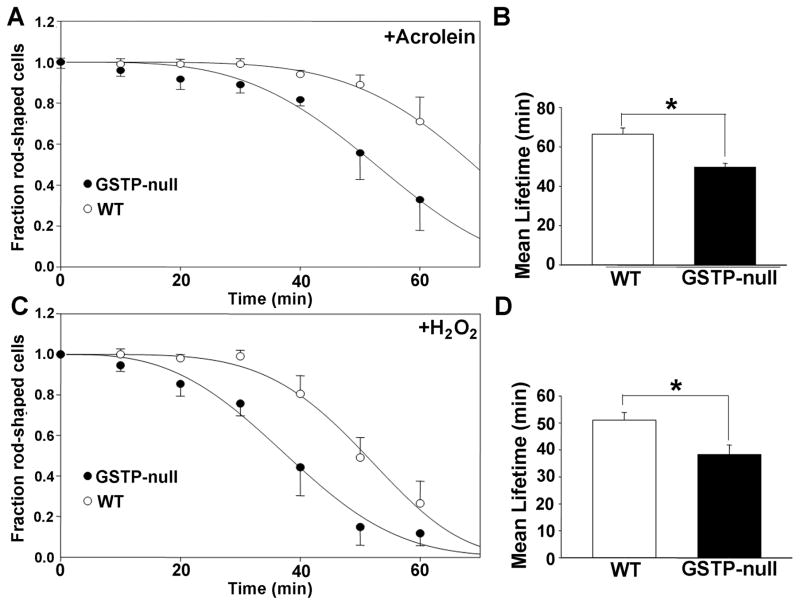
Given our observations suggesting that GSTP-deficiency increases the formation of protein-acrolein adducts, we tested the possibility that acrolein by itself could induce cardiac myocyte cell death, and determined its underlying mechanism. Previous studies have shown that INa is a critical target of reactive products generated by lipid peroxidation18, 30 and that perturbations in INa lead to an increase in [Na]i, which exchanges for extracellular calcium, via Na+-Ca2+ exchange, leading to calcium overload, myocyte hypercontracture and necrotic cell death.14 To determine whether acrolein induces cell death via a similar mechanism, we examined acrolein-induced changes in INa. Superfusion of isolated WT cardiac myocytes with acrolein led to a slight increase in the reversal potential of the current (Fig. 7A–C); and a time-dependent shift in the voltage-dependence of activation towards more negative potentials (Fig. 7Bi,ii) and a corresponding increase in the window current (Fig. 7Ciii); effects not seen in myocytes superfused with Ringer’s solution alone. To determine whether this was related to cell death, the myocytes were either left untreated or preincubated with SN-6 (an NCX1 inhibitor17), and then superfused with acrolein. Superfusion with acrolein led to a time-dependent hypercontracture of myocytes in 57.3 ± 2.9 min, however, in the presence of SN-6, the mean lifetime of acrolein-treated myocytes was significantly (P<0.01) increased to 70.7 ± 4.7 min (Fig. 7Di,ii), consistent with the notion that acrolein-induced hypercontracture is mediated by calcium-overload secondary to an increase in [Na]i via acrolein-induced changes in INa. Finally, to determine whether GSTP levels affect acrolein-induced cell death, cardiac myocytes isolated from WT and GSTP-null hearts were superfused with acrolein. As before, superfusion with 10 μM acrolein induced hypercontracture in most cells within 66.3 ± 3.3 min (Fig. 8A,B). In comparison, myocytes isolated from GSTP-null hearts were more sensitive to acrolein (mean lifetime = 49.6 ± 2.0 min; P<0.05 versus WT; Fig. 8A,B). Similarly, GSTP-null myocytes were also more sensitive to hydrogen peroxide than myocytes isolated from WT hearts (Fig. 8C,D). These results suggest that the absence of GSTP increases the sensitivity of these myocytes to peroxide- and acrolein-induced cell death and support the view that GSTP protects the heart from I/R injury by removing toxic lipid peroxidation products (see Online Figure V).
Figure 7. Acrolein alters sodium channel gating and induces cardiac myocyte hypercontracture.
A, Representative voltage clamp recordings of sodium current in a cardiac myocyte (i) before and (ii) after acrolein (50 μmol/L) superfusion (Inset, far right: voltage-clamp stimulus protocol). B, Current-voltage relationship of cardiac myocyte sodium current before (●) and after (■) 20 min superfusion with either (i) vehicle or (ii) acrolein. Biii, Acrolein induced a significant left-shift of the half-activation voltage of INa (P<0.05). C, Voltage dependence of steady state activation (m∞) and inactivation (h∞) of the cardiac Na+ channel before and after 20 min of (i) vehicle or (ii) acrolein exposure. Activation and inactivation parameters were calculated as described in Methods. Ciii, Window current (Iw) for each cardiac myocyte was estimated from the overlap of the activation (m∞) and inactivation (h∞) parameters from before and after acrolein superfusion calculated at 1 mV intervals. D, (i) Survival plots of cardiac myocytes superfused with acrolein without (▲) and after pre-incubation with SN-6 (■) for 60 min. Data are shown as discrete points and the curves are best fits of a Weibull distribution to the data. (ii) Graph showing the mean lifetime (min) of cardiac myocytes in the presence of acrolein without and with SN-6 pretreatment. * P<0.05 (n=4 per group).
Figure 8. GSTP-deficiency increases the sensitivity of cardiac myocytes to acrolein and H2O2.
Cardiac myocytes isolated from WT and GSTP-null hearts were plated overnight, then superfused with either acrolein (1–10 μM) or H2O2 (1–10 μM) and hypercontraction was monitored at 10 min intervals for 60 min. Survival plots of cardiac myocyte hypercontracture in response to A, acrolein (10 μM) or C, H2O2 (10 μM) exposure, and the mean lifetime (min) of myocytes in the presence of B, acrolein and D, H2O2. * P<0.05 (n=3 per group).
DISCUSSION
The results of this study show that GSTP is a cardioprotective protein that decreases the sensitivity of the heart to ischemic injury. Our results indicate that GSTP protects the heart by facilitating the metabolism and detoxification of small chain aldehydes such as acrolein. Removal of these aldehydes by GSTP decreases tissue injury and prevents post-translational modification of myocardial proteins. The observation that genetic disruption of GSTP exacerbates myocardial injury without affecting free radical generation suggests that cardiac injury during ischemia and reperfusion is not due to free radicals themselves, but instead, could be attributed to secondary oxidants such as aldehydes. These findings uncover a novel mechanism of cardioprotection and add a new facet to our understanding of the role of oxidative stress in myocardial I/R injury.
Proteins of the GST family are phase II detoxification enzymes that catalyze the conjugation of glutathione with reactive metabolites or xenobiotics. These conjugative reactions neutralize toxic chemicals and facilitate their removal from the body. The GSTs have been widely studied for their role in imparting chemoresistance to tumor cells; however, their cardiovascular roles are less well understood. In several cell types, GSTs protect against oxidative injury by removing electrophilic oxidants. In particular, some GST isoforms, such as GSTA4-4 and GSTP1, catalyze the conjugation of glutathione with unsaturated aldehydes derived from lipid peroxidation; and therefore, it is currently believed that they are important antioxidant defense enzymes. We found that GSTP is the major isoform expressed in the heart, although we detected both GSTA and GSTM as well, which is similar to the pattern in human heart.31, 32 Previous studies have shown that the human heart has less abundance of the major GST isoforms compared with the liver.32 Consistent with its major role in xenobiotic detoxification, the liver possesses high GST activity, whereas heart GST activity is significantly less.32 This difference suggests that in the heart, GSTs are involved in processes other than xenobiotic detoxification, although to date their role remains unclear. Our observation that deletion of GSTP does not affect the development of the heart or its contractile function at baseline suggests that GSTP may not be an important regulator of normal cardiac development and physiology, but that it might be required under conditions of injury or stress.
Given the extensive evidence supporting the antioxidant role of GSTs, we tested whether GSTP prevents oxidative injury induced by free radicals generated during I/R. We found that in both isolated perfused hearts and in hearts subjected to coronary ligation in situ, the absence of GSTP led to a consistent and robust increase in I/R injury. At baseline, deletion of GSTP did not induce a state of generalized oxidative stress and there were no compensatory changes in glutathione levels, other GSTs or antioxidant enzymes, suggesting that GSTP plays a unique, non-redundant role in protecting the heart from oxidants produced during ischemia and upon reperfusion. Our results suggest that this protective role of GSTP relates to the detoxification of aldehydes such as acrolein. Although GSTP is a multifunctional enzyme, it displays high catalytic efficiency with small aldehydes such as acrolein and base propenals, but not other bulkier aldehydes such as HNE.11 In agreement with this selectivity, we found that disruption of mGstp1/2 did not increase myocardial levels of HNE and hexanal, suggesting that changes in the levels of these aldehydes are unlikely to account for the cardioprotective effects of GSTP. Nevertheless, deletion of GSTP was accompanied by a significant decrease in the capacity of the heart to synthesize glutathione conjugates of acrolein and to prevent post-translational modification of proteins by acrolein. In isolated cardiac myocytes, deletion of GSTP also increased the sensitivity to acrolein toxicity. Collectively, these observations support that view that the GSTP-null hearts are inefficient at metabolizing acrolein, and therefore, when generated during I/R, this aldehyde accumulates in the heart and contributes to tissue injury to a larger extent than in WT hearts.
Acrolein is a highly reactive, three-carbon unsaturated aldehyde. It reacts readily with cellular nucleophiles, including reduced glutathione, histidine, lysine and cysteine side chains of proteins leading to the formation of covalent adducts that can modify protein function. Acrolein and acrolein-modified proteins have been detected in human atherosclerotic lesions28 and in mouse hearts after MI, where it has been suggested that acrolein is derived mainly from the oxidation of unsaturated lipids.6, 33 Extensive accumulation of acrolein-modified proteins in the ischemic heart, observed in this study, is consistent with lipid peroxidation as the major source of acrolein in the heart. However, acrolein could also be generated by the catalytic oxidation of amino acids by myeloperoxidase (MPO) or as a product of polyamine oxidase.29, 34 MPO is highly expressed in neutrophils and in infarcted mouse hearts, we found co-localization of MPO and protein-acrolein adducts (see Fig. 6Ai–iii). Nevertheless, deletion of MPO did not decrease infarct size in mice35 and we found that GSTP-deletion increased I/R injury in buffer-perfused heart, even in the absence of blood and blood-borne cells. Thus, it appears unlikely that MPO is the major source of acrolein in the heart or that the cardioprotective effects of GSTP are related to an increase in MPO-derived products. Previous studies suggest that oxidation of polyamines by polyamine oxidase could be another source of tissue acrolein production.34, 36 Indeed, polyamine oxidase is stimulated during cerebral ischemia and inhibition of this enzyme or neutralization of its products diminishes neuronal cell damage during focal cerebral ischemia.37 However, the role of polyamine oxidase in myocardial ischemia has not been well studied and further studies are required to determine its contribution to myocardial I/R injury and to the formation of acrolein in the ischemic heart.
A significant role of acrolein in I/R injury is indicated by our results showing that there is extensive accumulation of acrolein-modified proteins in the ischemic heart and that a decrease in acrolein metabolism via GSTP exacerbates I/R injury. This view is supported by the observation that disruption of GSTP decreased cardiac metabolism of acrolein and increased the sensitivity of cardiac myocytes to acrolein-induced protein crosslinking and cell death, suggesting that increased acrolein removal is an important requirement in protecting the heart from oxidative stress (see Online Figure V). However, we also found that in addition to acrolein, GSTP-null cardiac myocytes are also more sensitive to H2O2. Because GSTs have significant peroxidase activity38, this might mean that GSTP protects the heart from I/R injury by removing peroxides. Yet, peroxides cause cell injury by generating free radicals, which were not increased in GSTP-null hearts. Moreover, peroxides increase the formation of aldehydes such as HNE and cause GSH depletion; both of which were also not affected in GSTP-null hearts. These observations suggest that increased ischemic injury in the GSTP-null hearts is not due to greater accumulation of peroxides, but an accumulation of specific aldehydes (such as acrolein) appears to be the most likely mechanism both of increased I/R injury in GSTP-null hearts and the increase in the sensitivity of the GSTP-null myocytes to H2O2.
Our investigation into the mechanism of acrolein-induced cell death revealed that acrolein treatment caused changes in sodium current. These included a shift in the voltage-dependence of inactivation to more negative potential leading to a significant increase in the window current. These changes are similar to those previously reported for oxidants such as tert-butyl hydroperoxide,14 and lipid peroxidation products such as HNE,18 and isoketal.39 In cells exposed to HNE18 and peroxide,40 the increase in the window current was accompanied by prolongation of the action potential, increased arrhythmogenesis and elevation in intracellular sodium. This increase in intracellular sodium has been suggested to increase [Ca2+]i, presumably via Na+-Ca2+ exchange, and could lead to Ca2+-induced rigor and hypercontracture.14 Our results with acrolein suggest a similar mechanism of cell death that is further supported by the observation that treatment with SN-6, an NCX1 Na+/Ca2+ exchange inhibitor17, delayed acrolein-induced myocyte hypercontracture, implicating calcium overload as a major trigger of cell death, as suggested before.41 Both dysregulation of sodium current and calcium overload are also important features of myocardial ischemic injury, which leads to contractile filament hypercontracture, myocyte necrosis42 and arrhythmogenesis. Thus taken together, our observations imply that by altering sodium current and increasing calcium overload, lipid peroxidation products such as acrolein could contribute, at least in part to I/R-induced myocardial necrosis and sudden cardiac death (see Online Figure V). GSTP could protect against such injury by removing acrolein and related toxicants, which is consistent with our observation that GSTP-null myocytes were more sensitive to both peroxide and acrolein-induced cell death.
The lack of an increase in free radicals in GSTP-null hearts (compared with I/R WT hearts) suggests that even with the same levels of free radical generation, ischemic injury could be significantly attenuated by increasing aldehyde removal. Numerous studies show that overexpression of antioxidant enzymes such as CuZn-SOD43, MnSOD44, catalase45, ecSOD21 and glutathione peroxidase46 prevent I/R injury, and mice with deficiency of MnSOD47, CuZn-SOD48 or glutathione peroxidase49 are more sensitive to ischemic injury. Collectively, these studies provide compelling evidence that free radicals generated during I/R are an important cause of tissue injury. Our observations do not contradict this view, but extend our understanding of the nature of the oxidative injury by showing that it is not necessarily the free radicals themselves but the secondary products they generate that are the proximal cause of injury. We support this view by showing that the levels of free acrolein are significantly increased in the ischemic heart (Fig. 5C). Thus, in addition to quenching free radicals, removing aldehydes such as acrolein may be another therapeutically useful approach for preventing myocardial I/R injury. Interestingly, intravenous GSTP1 administration appears to reduce the deleterious remodeling after myocardial infarction.50 GSTP is a highly inducible enzyme and is robustly upregulated by dietary components such as cruciferous vegetables.51 Therefore, the cardioprotective effects of increased vegetable consumption could be in part due to GSTP induction, and therefore, dietary or pharmacological induction of GSTP may be an important therapeutic strategy for preventing and limiting myocardial ischemic injury.
The observation that disruption of GSTP increases myocardial I/R injury in mice raises the possibility that variations in GST activity due to induction, diet, xenobiotic exposure or genetic polymorphism could also affect ischemic injury in humans. In humans, several polymorphic forms of GSTs have been identified; however, meta-analyses of the link between GST polymorphisms and heart disease have led to discordant results.52, 53 In Caucasian males, the GSTM1-null phenotype is very common (50%) and it has been associated with increases in cardiovascular disease risk associated with air pollution by decreasing heart rate variability54, although it has been associated as well with a decreased risk of acute myocardial infarction55. Similarly, polymorphic forms of GSTT1 are also common and are associated with increased risk of cardiovascular disease in diabetics who smoke cigarettes.56 Like other GSTs, the human GSTP1 gene is also highly polymorphic (42 known polymorphisms) and a single nucleotide polymorphism (SNP) A313G (I104V) increases the relative risk of congestive heart failure in adults who were treated for childhood leukemia with anthracyclines.57 Protein variants of human GSTP differ in their catalytic efficiency with substrates such as acrolein.58 Therefore, polymorphic forms of human GSTP might vary in their ability to remove acrolein and related aldehydes from the heart, and thereby significantly modify the outcomes of ischemic episodes. Thus, our studies showing that GSTP is a cardioprotective gene could form the basis of future studies to investigate the role of human GSTP1 polymorphisms in clinical outcomes of acute myocardial infarction and heart failure.59
Supplementary Material
Novelty and Significance.
What Is Known?
Ischemia-reperfusion (I/R) induces oxidative stress that is associated with myocardial injury.
Enhanced antioxidant capacity protects the heart against I/R-induced oxidative stress and injury.
Oxidative stress increases lipid oxidation, which generates toxic aldehydes.
What New Information Does This Article Contribute?
Myocardial ischemia results in lipid peroxidation and generation of the toxic aldehyde called acrolein.
Acrolein impairs sodium conductance leading to calcium overload via sodium calcium exchange, which results in myocyte hypercontracture and cell death.
Glutathione S-transferase P (GSTP) metabolizes and detoxifies acrolein. Genetic deficiency of GSTP exacerbates acrolein toxicity.
GSTP-deficiency increase myocardial sensitivity to ischemia/reperfusion injury.
Myocardial ischemia and its sequelae remain the leading cause of mortality in the U.S. Although increased generation of free radicals and calcium overload have been recognized to be key features of ischemia that trigger cell death, a better understanding of the underlying mechanisms is needed to develop cardioprotective approaches to limit ischemic injury. In this study, we tested the hypothesis that increased removal of lipid peroxidation products such as acrolein protects against myocardial I/R injury. We found that in mice, myocardial ischemia results in the formation of acrolein and acrolein-modified proteins and that GSTP prevents acrolein toxicity by removing the aldehyde via glutathione conjugation. Genetic deficiency of GSTP promotes acrolein accumulation and increases myocardial sensitivity to I/R injury. Also, our results show that acrolein alters sodium channel function, leading to calcium overload via sodium/calcium exchange; effects that are worsened in GSTP-deficient mice. Collectively, these findings establish GSTP as an important cardioprotective protein. We speculate that variation in GSTP activity, due to genetic polymorphisms or dietary induction by cruciferous vegetables may be an important determinant of inter-individual variability in susceptibility to I/R injury
Acknowledgments
We thank B. Bishop, D. Bolanowski, E. Cardwell, V. LaRussa, Jr., J. Luo, J. Marshall, D. Mitchell, D. Mosley, M. Peak, M. Singh, E. Steinmetz, E. Werkman and D. Young for excellent technical assistance. We thank Dr. Michael Nantz, Univ. of Louisville, for donation of QDA probes. We thank Drs. C. Henderson and R. Wolf, University of Dundee, for donation of breeding pairs of GSTP1/P2 wild-type and null mice.
SOURCES OF FUNDING
This work supported by American Health Assistance Fund/National Heart Foundation Grant #2007-202 (DJC) and NIH grants: GM103492 (AB), HL55477 (AB), HL89380 (DJC), ES14559 (T35; RAP), HL59378 (SDP), HL78825 (RB) and a Veterans Affairs Merit Award (SDP).
Nonstandard Abbreviations and Acronyms
- AR
aldose reductase
- DMPO
dimethyl propyloxide
- GSH
reduced glutathione
- GST
glutathione S-transferase
- 4HNE
4-hydroxy-trans-2-nonenal
- I/R
ischemia-reperfusion
- JNK
c-jun N-terminal kinase
- MPO
myeloperoxidase
- MRM
multiple reaction monitoring
- QDA
N-[2-(aminooxy)ethyl]-N,N-dimethyl-1-dodecylammonium iodide
Footnotes
DISCLOSURES
None.
In June, the average time from submission to first decision for all original research papers submitted to Circulation Research was 12.31 days.
References
- 1.Zweier JL, Flaherty JT, Weisfeldt ML. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci US A. 1987;84:1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolli R, Patel BS, Jeroudi MO, Lai EK, McCay PB. Demonstration of free radical generation in “stunned” myocardium of intact dogs with the use of the spin trap alpha-phenyl n-tert-butyl nitrone. J Clin Invest. 1988;82:476–485. doi: 10.1172/JCI113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Downey JM. Free radicals and their involvement during long-term myocardial ischemia and reperfusion. Annu Rev Physiol. 1990;52:487–504. doi: 10.1146/annurev.ph.52.030190.002415. [DOI] [PubMed] [Google Scholar]
- 4.Kloner RA, Przyklenk K, Whittaker P. Deleterious effects of oxygen radicals in ischemia/reperfusion. Resolved and unresolved issues. Circulation. 1989;80:1115–1127. doi: 10.1161/01.cir.80.5.1115. [DOI] [PubMed] [Google Scholar]
- 5.Hill BG, Bhatnagar A. Beyond reactive oxygen species: Aldehydes as arbitrators of alarm and adaptation. Circ Res. 2009;105:1044–1046. doi: 10.1161/CIRCRESAHA.109.209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takabe W, Niki E, Uchida K, Yamada S, Satoh K, Noguchi N. Oxidative stress promotes the development of transformation: Involvement of a potent mutagenic lipid peroxidation product, acrolein. Carcinogenesis. 2001;22:935–941. doi: 10.1093/carcin/22.6.935. [DOI] [PubMed] [Google Scholar]
- 7.Conklin D, Prough R, Bhatanagar A. Aldehyde metabolism in the cardiovascular system. Mol Biosyst. 2007;3:136–150. doi: 10.1039/b612702a. [DOI] [PubMed] [Google Scholar]
- 8.Awasthi YC, Sharma R, Singhal SS. Human glutathione s-transferases. Int J Biochem. 1994;26:295–308. doi: 10.1016/0020-711x(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 9.Sharma R, Ansari GAS, Awasthi YC. Toxicology of glutathione transferases. Taylor & Francis CRC Press; 2006. Physiological substrates of glutathione s-transferases; pp. 179–203. [Google Scholar]
- 10.Engle MR, Singh SP, Czernik PJ, Gaddy D, Montague DC, Ceci JD, Yang Y, Awasthi S, Awasthi YC, Zimniak P. Physiological role of mgsta4-4, a glutathione s-transferase metabolizing 4-hydroxynonenal: Generation and analysis of mgsta4 null mouse. Toxicology and Applied Pharmacology. 2004;194:296–308. doi: 10.1016/j.taap.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Berhane K, Widersten M, Engstrom A, Kozarich JW, Mannervik B. Detoxication of base propenals and other alpha, beta-unsaturated aldehyde products of radical reactions and lipid peroxidation by human glutathione transferases. Proc Natl Acad Sci US A. 1994;91:1480–1484. doi: 10.1073/pnas.91.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henderson CJ, Smith AG, Ure J, Brown K, Bacon EJ, Wolf CR. Increased skin tumorigenesis in mice lacking pi class glutathione s-transferases. Proc Natl Acad Sci US A. 1998;95:5275–5280. doi: 10.1073/pnas.95.9.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ismahil MA, Hamid T, Haberzettl P, Gu Y, Chandrasekar B, Srivastava S, Bhatnagar A, Prabhu SD. Chronic oral exposure to the aldehyde pollutant acrolein induces dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2011;301:H2050–2060. doi: 10.1152/ajpheart.00120.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castro GJ, Bhatnagar A. Effect of extracellular ions and modulators of calcium transport on survival of tert-butyl hydroperoxide exposed cardiac myocytes. Cardiovasc Res. 1993;27:1873–1881. doi: 10.1093/cvr/27.10.1873. [DOI] [PubMed] [Google Scholar]
- 15.Tipparaju SM, Saxena N, Liu SQ, Kumar R, Bhatnagar A. Differential regulation of voltage-gated k+ channels by oxidized and reduced pyridine nucleotide coenzymes. Am J Physiol Cell Physiol. 2005;288:C366–376. doi: 10.1152/ajpcell.00354.2004. [DOI] [PubMed] [Google Scholar]
- 16.Tipparaju SM, Li XP, Kilfoil PJ, Xue B, Uversky VN, Bhatnagar A, Barski OA. Interactions between the c-terminus of kv1.5 and kvbeta regulate pyridine nucleotide-dependent changes in channel gating. Pflugers Arch. 2012;463:799–818. doi: 10.1007/s00424-012-1093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niu CF, Watanabe Y, Ono K, Iwamoto T, Yamashita K, Satoh H, Urushida T, Hayashi H, Kimura J. Characterization of sn-6, a novel na+/ca2+ exchange inhibitor in guinea pig cardiac ventricular myocytes. Eur J Pharmacol. 2007;573:161–169. doi: 10.1016/j.ejphar.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 18.Bhatnagar A. Electrophysiological effects of 4-hydroxynonenal, an aldehydic product of lipid peroxidation, on isolated rat ventricular myocytes 16. Circulation Research. 1995;76:293–304. doi: 10.1161/01.res.76.2.293. [DOI] [PubMed] [Google Scholar]
- 19.Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, Guo Y, Bolli R, Cardwell EM, Ping P. Protein kinase cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circulation Research. 2003;92:873–880. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.West MB, Rokosh G, Obal D, Velayutham M, Xuan YT, Hill BG, Keith RJ, Schrader J, Guo Y, Conklin DJ, Prabhu SD, Zweier JL, Bolli R, Bhatnagar A. Cardiac myocyte-specific expression of inducible nitric oxide synthase protects against ischemia/reperfusion injury by preventing mitochondrial permeability transition. Circulation. 2008;118:1970–1978. doi: 10.1161/CIRCULATIONAHA.108.791533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obal D, Dai S, Keith R, Dimova N, Kingery J, Zheng YT, Zweier J, Velayutham M, Prabhu SD, Li Q, Conklin D, Yang D, Bhatnagar A, Bolli R, Rokosh G. Cardiomyocyte-restricted overexpression of extracellular superoxide dismutase increases nitric oxide bioavailability and reduces infarct size after ischemia/reperfusion. Basic Res Cardiol. 2012;107:305. doi: 10.1007/s00395-012-0305-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattingly SJ, Xu T, Nantz MH, Higashi RM, Fan TW. A carbonyl capture approach for profiling oxidized metabolites in cell extracts. Metabolomics. 2012;8:989–996. doi: 10.1007/s11306-011-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conklin DJ, Haberzettl P, Lesgards JF, Prough RA, Srivastava S, Bhatnagar A. Increased sensitivity of glutathione s-transferase p-null mice to cyclophosphamide-induced urinary bladder toxicity. J Pharmacol Exp Ther. 2009;331:456–469. doi: 10.1124/jpet.109.156513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conklin DJ, Haberzettl P, Prough RA, Bhatnagar A. Glutathione-s-transferase p protects against endothelial dysfunction induced by exposure to tobacco smoke. Am J Physiol Heart Circ Physiol. 2009;296:H1586–H1597. doi: 10.1152/ajpheart.00867.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green N, Weech M, Walters E. Localization and characterization of glutathione-s-transferase isozymes alpha, mu, and pi within the mouse vomeronasal organ. Neurosci Lett. 2005;375:198–202. doi: 10.1016/j.neulet.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 26.Elsby R, Kitteringham NR, Goldring CE, Lovatt CA, Chamberlain M, Henderson CJ, Wolf CR, Park BK. Increased constitutive c-jun n-terminal kinase signaling in mice lacking glutathione s-transferase pi. Journal of Biological Chemistry. 2003;278:22243–22249. doi: 10.1074/jbc.M301211200. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava S, Chandrasekar B, Bhatnagar A, Prabhu SD. Lipid peroxidation-derived aldehydes and oxidative stress in the failing heart: Role of aldose reductase. Am J Physiol Heart Circ Physiol. 2002;283:H2612–H2619. doi: 10.1152/ajpheart.00592.2002. [DOI] [PubMed] [Google Scholar]
- 28.Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y, Suzuki D, Miyata T, Noguchi N, Niki E, Osawa T. Protein-bound acrolein: Potential markers for oxidative stress. Proc Natl Acad Sci US A. 1998;95:4882–4887. doi: 10.1073/pnas.95.9.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson MM, Hazen SL, Hsu FF, Heinecke JW. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha,beta-unsaturated aldehydes by phagocytes at sites of inflammation. J Clin Invest. 1997;99:424–432. doi: 10.1172/JCI119176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uchida T, Nishimura M, Saeki T, Watanabe Y. Effects of membrane lipid peroxidation by tert butyl hydroperoxide on the sodium current in isolated feline ventricular myocytes. Heart Vessels. 1994;9:227–234. doi: 10.1007/BF01745102. [DOI] [PubMed] [Google Scholar]
- 31.Tsuchida S, Maki T, Sato K. Purification and characterization of glutathione transferases with an activity toward nitroglycerin from human aorta and heart. Multiplicity of the human class mu forms. J Biol Chem. 1990;265:7150–7157. [PubMed] [Google Scholar]
- 32.Dhanani SaA YC. Glutathione s-transferase isozyme composition of human tissues. In: Awasthi YC, editor. Toxicology of glutathione transferases. Boca Raton, FL: CRC Press; 2007. pp. 321–338. [Google Scholar]
- 33.Uchida K. Current status of acrolein as a lipid peroxidation product. Trends Cardiovasc Med. 1999;9:109–113. doi: 10.1016/s1050-1738(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 34.Sakata K, Kashiwagi K, Sharmin S, Ueda S, Igarashi K. Acrolein produced from polyamines as one of the uraemic toxins. Biochem Soc Trans. 2003;31:371–374. doi: 10.1042/bst0310371. [DOI] [PubMed] [Google Scholar]
- 35.Vasilyev N, Williams T, Brennan ML, Unzek S, Zhou X, Heinecke JW, Spitz DR, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation. 2005;112:2812–2820. doi: 10.1161/CIRCULATIONAHA.105.542340. [DOI] [PubMed] [Google Scholar]
- 36.Sakata K, Kashiwagi K, Sharmin S, Ueda S, Irie Y, Murotani N, Igarashi K. Increase in putrescine, amine oxidase, and acrolein in plasma of renal failure patients. Biochemical and Biophysical Research Communications. 2003;305:143–149. doi: 10.1016/s0006-291x(03)00716-2. [DOI] [PubMed] [Google Scholar]
- 37.Tomitori H, Usui T, Saeki N, Ueda S, Kase H, Nishimura K, Kashiwagi K, Igarashi K. Polyamine oxidase and acrolein as novel biochemical markers for diagnosis of cerebral stroke. Stroke. 2005;36:2609–2613. doi: 10.1161/01.STR.0000190004.36793.2d. [DOI] [PubMed] [Google Scholar]
- 38.Zimniak L, Awasthi S, Srivastava SK, Zimniak P. Increased resistance to oxidative stress in transfected cultured cells overexpressing glutathione s-transferase mgsta4-4. Toxicology and Applied Pharmacology. 1997;143:221–229. doi: 10.1006/taap.1996.8070. [DOI] [PubMed] [Google Scholar]
- 39.Fukuda K, Davies SS, Nakajima T, Ong BH, Kupershmidt S, Fessel J, Amarnath V, Anderson ME, Boyden PA, Viswanathan PC, Roberts LJ, 2nd, Balser JR. Oxidative mediated lipid peroxidation recapitulates proarrhythmic effects on cardiac sodium channels. Circ Res. 2005;97:1262–1269. doi: 10.1161/01.RES.0000195844.31466.e9. [DOI] [PubMed] [Google Scholar]
- 40.Beresewicz A, Horackova M. Alterations in electrical and contractile behavior of isolated cardiomyocytes by hydrogen peroxide: Possible ionic mechanisms. J Mol Cell Cardiol. 1991;23:899–918. doi: 10.1016/0022-2828(91)90133-7. [DOI] [PubMed] [Google Scholar]
- 41.Wang L, Sun Y, Asahi M, Otsu K. Acrolein, an environmental toxin, induces cardiomyocyte apoptosis via elevated intracellular calcium and free radicals. Cell Biochem Biophys. 2011;61:131–136. doi: 10.1007/s12013-011-9169-5. [DOI] [PubMed] [Google Scholar]
- 42.Hale SL, Leeka JA, Kloner RA. Improved left ventricular function and reduced necrosis after myocardial ischemia/reperfusion in rabbits treated with ranolazine, an inhibitor of the late sodium channel. J Pharmacol Exp Ther. 2006;318:418–423. doi: 10.1124/jpet.106.103242. [DOI] [PubMed] [Google Scholar]
- 43.Chen Z, Oberley TD, Ho Y, Chua CC, Siu B, Hamdy RC, Epstein CJ, Chua BH. Overexpression of cuznsod in coronary vascular cells attenuates myocardial ischemia/reperfusion injury. Free Radical Biology and Medicine. 2000;29:589–596. doi: 10.1016/s0891-5849(00)00363-4. [DOI] [PubMed] [Google Scholar]
- 44.Chen Z, Siu B, Ho YS, Vincent R, Chua CC, Hamdy RC, Chua BH. Overexpression of mnsod protects against myocardial ischemia/reperfusion injury in transgenic mice. J Mol Cell Cardiol. 1998;30:2281–2289. doi: 10.1006/jmcc.1998.0789. [DOI] [PubMed] [Google Scholar]
- 45.Li G, Chen Y, Saari JT, Kang YJ. Catalase-overexpressing transgenic mouse heart is resistant to ischemia-reperfusion injury. Am J Physiol. 1997;273:H1090–H1095. doi: 10.1152/ajpheart.1997.273.3.H1090. [DOI] [PubMed] [Google Scholar]
- 46.Yoshida T, Watanabe M, Engelman DT, Engelman RM, Schley JA, Maulik N, Ho YS, Oberley TD, Das DK. Transgenic mice overexpressing glutathione peroxidase are resistant to myocardial ischemia reperfusion injury. J Mol Cell Cardiol. 1996;28:1759–1767. doi: 10.1006/jmcc.1996.0165. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton KL, Quindry JC, French JP, Staib J, Hughes J, Mehta JL, Powers SK. Mnsod antisense treatment and exercise-induced protection against arrhythmias. Free Radic Biol Med. 2004;37:1360–1368. doi: 10.1016/j.freeradbiomed.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 48.Asimakis GK, Lick S, Patterson C. Postischemic recovery of contractile function is impaired in sod2(+/−) but not sod1(+/−) mouse hearts. Circulation. 2002;105:981–986. doi: 10.1161/hc0802.104502. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida T, Maulik N, Engelman RM, Ho YS, Magnenat JL, Rousou JA, Flack JE, III, Deaton D, Das DK. Glutathione peroxidase knockout mice are susceptible to myocardial ischemia reperfusion injury. Circulation. 1997;96:II–20. [PubMed] [Google Scholar]
- 50.Andrukhova O, Salama M, Krssak M, Wiedemann D, El-Housseiny L, Hacker M, Gildehaus FJ, Andrukhov O, Mirzaei S, Kocher A, Zuckermann A, Aharinejad S. Single-dose gstp1 prevents infarction-induced heart failure. J Card Fail. 2014;20:135–145. doi: 10.1016/j.cardfail.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 51.Lee SA, Fowke JH, Lu W, Ye C, Zheng Y, Cai Q, Gu K, Gao YT, Shu XO, Zheng W. Cruciferous vegetables, the gstp1 ile105val genetic polymorphism, and breast cancer risk. Am J Clin Nutr. 2008;87:753–760. doi: 10.1093/ajcn/87.3.753. [DOI] [PubMed] [Google Scholar]
- 52.Norskov MS, Frikke-Schmidt R, Loft S, Sillesen H, Grande P, Nordestgaard BG, Tybjaerg-Hansen A. Copy number variation in glutathione s-transferases m1 and t1 and ischemic vascular disease: Four studies and meta-analyses. Circ Cardiovasc Genet. 2011 doi: 10.1161/CIRCGENETICS.111.959809. [DOI] [PubMed] [Google Scholar]
- 53.Conklin DJ, Bhatnagar A. Are glutathione s-transferase null genotypes “null and void” of risk for ischemic vascular disease? Circ Cardiovasc Genet. 2011;4:339–341. doi: 10.1161/CIRCGENETICS.111.960526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwartz J, Park SK, O’Neill MS, Vokonas PS, Sparrow D, Weiss S, Kelsey K. Glutathione-s-transferase m1, obesity, statins, and autonomic effects of particles: Gene-by-drug-by-environment interaction. Am J Respir Crit Care Med. 2005;172:1529–1533. doi: 10.1164/rccm.200412-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson MH, Grant PJ, Hardie LJ, Wild CP. Glutathione s-transferase m1 null genotype is associated with a decreased risk of myocardial infarction. FASEB Journal. 2000;14:791–796. doi: 10.1096/fasebj.14.5.791. [DOI] [PubMed] [Google Scholar]
- 56.Doney AS, Lee S, Leese GP, Morris AD, Palmer CN. Increased cardiovascular morbidity and mortality in type 2 diabetes is associated with the glutathione s transferase theta-null genotype: A go-darts study. Circulation. 2005;111:2927–2934. doi: 10.1161/CIRCULATIONAHA.104.509224. [DOI] [PubMed] [Google Scholar]
- 57.Aplenc R, Blanco J, Leisenring W, Davies S, Relling M, Robison L, Sklar C, Stovall M, Bhatia S. Polymorphisms in candidate genes in patients with congestive heart failure (chf) after childhood cancer: A report from the childhood cancer survivor study (ccss) American Society of Clinical Oncology. 2006 [Google Scholar]
- 58.van Iersel ML, Ploemen JP, Lo BM, Federici G, van Bladeren PJ. Interactions of alpha, beta-unsaturated aldehydes and ketones with human glutathione s-transferase p1-1. Chemico-Biological Interactions. 1997;108:67–78. doi: 10.1016/s0009-2797(97)00096-3. [DOI] [PubMed] [Google Scholar]
- 59.Andrukhova O, Salama M, Rosenhek R, Gmeiner M, Perkmann T, Steindl J, Aharinejad S. Serum glutathione s-transferase p1 1 in prediction of cardiac function. J Card Fail. 2012;18:253–261. doi: 10.1016/j.cardfail.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.