Abstract
This study appraised the effects of dietary blend of 80% canola oil and 20% palm oil and postmortem ageing on oxidative stability, fatty acids and quality attributes of gluteus medius (GM) muscle in goats. Twenty-four Boer bucks were randomly allotted to diet supplemented with 0, 4 and 8% oil blend, fed for 100 days and slaughtered, and the GM muscle was subjected to a 7 d chill storage (4±1°C). Diet had no effect (P> 0.05) on the colour, drip loss, thiobarbituric acid-reactive substances (TBARS) value, free thiol, carbonyl, myoglobin and metmyoglobin contents, metmyoglobin reducing activity (MRA), antioxidant enzyme activities and abundance of myosin heavy chain (MHC) and actin in the GM muscle in goats. The meat from goats fed 4 and 8% oil blend had higher (P< 0.05) concentration of α and γ-tocopherol and abundance of troponin T compared with that from the control goats. The GM muscle from the oil-supplemented goats had lower (P< 0.05) concentration of C16:0 and greater (P< 0.05) concentration of C18:1n-9, C18:3n-3 and C20:5n-3 compared with that from the control goats. Nonetheless, diet did not affect (P< 0.05) the total fatty acid in the GM muscle in goats. Regardless of the diet, the free thiol and myoglobin contents, concentration of tocopherol and total carotenoids, MHC and MRA in the GM muscle decreased (P< 0.05) while carbonyl content, TBARS, drip loss and metmyoglobin content increased over storage. Dietary blend of 80% canola oil and 20% palm oil beneficially altered tissue lipids without hampering the oxidative stability of chevon.
Introduction
The increase in the consumers’ awareness of the relationship between the consumption of ruminant meat and the incidence of chronic diseases has created the impetus to develop healthier ruminant meat, which would confer potential health benefits to consumers whilst enhancing consumers’ confidence in ruminant meat [1, 2]. A case in point was the recent report of the World Health Organization, which classified the consumption of red meat as “probably carcinogenic to humans” [3].
The incorporation of unsaturated fats in the diet of ruminants is an effective strategy for modifying the fatty acid composition of ruminant meat in response to the demands of the consumers [2, 4]. However, this practice could have negative impact on the oxidative stability and physicochemical properties of ruminant meat [2, 4]. In addition, postmortem alterations in muscle and its conversion to meat during ageing could create conditions in which the balance between antioxidant and pro-oxidant capacity favours oxidative damage [5, 6]. Oxidative deterioration of meat lipids and proteins could imperil the nutritional and sensory qualities and the shelf life of meat, and their potential perils to humans is of current interest [4, 7, 8]. Thus, attenuating oxidative deteriorations to maintain meat quality and safety is essential.
Little attention has been paid to the role of dietary fat on protein oxidation as opposed to lipid oxidation in ruminant meat. In addition, there is dearth of information on the response of myoglobin and myofibrillar proteins to dietary lipids in ruminant. The antioxidant status of muscle is the major factor influencing oxidative spoilage in muscle foods [6–8]. The potency of synthetic antioxidants in curbing postmortem lipid oxidation has been documented [9, 10]. Nonetheless, the potential of synthetic antioxidants causing toxicological effects [11, 12] has stimulated interest in the utilization of natural antioxidants [9, 13, 14]. In addition, synthetic antioxidant are very scarce and expensive particularly in the developing countries [13, 14]. Canola oil can be utilized in animal nutrition to alter that fatty acid profile of animal products [2, 15]. In addition, palm oil [16] and canola oil [17] are rich sources of natural antioxidants. Thus, it was hypothesized that dietary supplementation of canola oil and palm oil blend would enhance the beneficial lipids and oxidative stability of myoglobin, lipids and myofibrillar proteins in goat meat. The objective of this study was to investigate the influence of blend of 80% canola oil and 20% palm oil and postmortem refrigerated storage on fatty acid composition, antioxidant status, oxidative stability of myoglobin, lipid and myofibrillar proteins, physicochemical properties and metmyoglobin reducing activity in gluteus medius muscle in goats.
Materials and Methods
Animal welfare
This study was conducted following the guidelines of the research policy of the Universiti Putra Malaysia on Animal welfare and ethics. The experimental protocol was approved by the Universiti Putra Malaysia Animal use and care committee. The care of the experimental goats was in accordance to Malaysian standards.
Animals, husbandry conditions and diets
The goats were obtained and reared at Ar-Raudhah Biotech Farm, Pty Ltd. Kuang, Selangor, Malaysia following the approval by the farm management. Twenty-four Boer crossbred bucks (4–5 months old, average initial live-weight of 20.54±0.475 kg) were drenched against parasite and randomly allotted to diets containing 0, 4 and 8% oil blend on a DM basis and fed daily for 100 d following a two-week period of adaptation. Each goat was individually housed in a wooden slated floor pen equipped with feeding and drinking facilities. Dietary treatments were formulated to meet the nutritional requirements of growing goats following the recommendation of NRC [18]. Each diet consisted of 50% concentrate mixture and 50% forage (oil palm frond) on a DM basis [19]. The oil blend replaced the corn grain in the concentrate mixture and other components of the concentrate mixture were adjusted [19] to make the diets isocaloric and isonitrogenous (Table 1). The oil-based diets were prepared by manually incorporating the oil blend into the ground concentrate followed by a thorough mixing. The diets were prepared fresh twice a day and no antioxidant was added. The diets were offered as complete ration mix (forage and concentrate) in two equal meals at 0830 and 1430 hours. All goats had ad libitum access to water. Feed samples (300 g) were collected weekly and stored at -20°C until analysis. Feed samples were dried at 60°C for 48 h to determine the DM content, ground to pass a through a 1 mm screen and analysed for ash, ether extract and crude protein according to the protocol of AOAC [20]. The neutral detergent fibre and acid detergent fibre were analysed by the protocol of Van Soest et al. [21].
Table 1. Chemical and fatty acid composition and antioxidant content of dietary treatments.
| Levels of oil blend (%) | |||
|---|---|---|---|
| Chemical composition, % DM | 0 | 4 | 8 |
| Dry matter | 67.70 | 67.90 | 68.07 |
| Crude Protein | 14.27 | 14.37 | 14.39 |
| Ether extract | 2.30 | 6.35 | 11.11 |
| Organic matter | 93.16 | 93.42 | 93.55 |
| Nitrogen free extract | 16.56 | 13.97 | 12.45 |
| ADF | 35.04 | 33.28 | 32.52 |
| NDF | 63.52 | 62.67 | 62.06 |
| Metabolizable energy, MJ/Kg DM1 | 11.59 | 11.61 | 11.62 |
| Ca | 1.02 | 1.05 | 1.04 |
| P | 0.52 | 0.54 | 0.54 |
| Fatty acid (g/kg DM) | |||
| C12:0 | 0.01 | 0.03 | 0.04 |
| C14:0 | 0.53 | 0.51 | 0.51 |
| C16:0 | 2.79 | 5.98 | 7.78 |
| C16:1 | 0.08 | 0.11 | 0.15 |
| C18:0 | 0.56 | 1.12 | 1.43 |
| C18:1n-9 | 3.82 | 14.87 | 26.32 |
| C18:2ω-6 | 7.05 | 11.87 | 12.06 |
| C18:3ω-3 | 1.06 | 2.61 | 4.13 |
| n6/n3 | 6.65 | 4.55 | 2.92 |
| Total FA | 15.83 | 37.09 | 52.27 |
| Antioxidants (mg/kg) | |||
| Total carotenoid | 14.81 | 16.71 | 19.86 |
| α-tocopherol | 101.12 | 112.47 | 123.21 |
| γ-tocopherol | 10.22 | 34.55 | 49.17 |
| δ-tocopherol | 1.21 | 3.45 | 5.93 |
1Calculated
Slaughtering procedure and muscle sampling
The goats were fasted overnight with ad libitum access to water and slaughtered according to the halal procedure as outlined in MS1500:2009 [22]. After carcass dressing on day 0, 45 g of gluteus medius (GM) muscle, was dissected from the outer surface of the left pelvis, trimmed free of external fat and epimyseal connective tissue and divided into three equal parts. The remaining GM samples were left on the carcasses intact and removed after 1, 4 and 7 d of storage. The first part (15 g) was pulverized in liquid nitrogen to produce a homogenous powder and assigned for the determination of fatty acids, myoglobin, metmyoglobin reducing activity (MRA), antioxidants, lipid oxidation, protein oxidation, SDS-PAGE and metmyoglobin content. The second part (15 g) was vacuum packaged and stored in a chiller at 4±1°C for the determination of drip loss. The third part (15 g) was assigned for the determination of colour on d 0. Same measurements were conducted on samples taken at d 1, 4 and 7 postmortem from the muscle remaining on the carcasses.
Determination muscle glycogen and pH
The glycogen content was determined using EnzyChromTM Glycogen Assay kit (Cat# E2GN-100, BioAssays, USA) following the procedure of the manufacturer. The pH of muscles was determined following the method of AMSA [23] using a portable pH meter (Mettler Toledo, AG 8603, Switzerland). The pH meter was calibrated with a pH 4.0 buffer and then with a pH 7.0 buffer prior to use. Each pulverized sample (0.5 g) was homogenized with 10 mL ice-cold water in the presence of 5 mM sodium iodoacetate to prevent further glycolysis. The pH of the resultant homogenate was measured at 20±1°C.
Determination of Drip loss
Drip loss was measured as described by Sabow et al. [24]. The fresh meat samples from the GM muscle on d 0 were weighed and recorded as initial weight (W1). The weighed samples were placed into polyethylene plastic bags, labelled, vacuum packaged and stored at 4°C. After 1, 4 and 7 d postmortem, the samples were removed from the bags, gently blotted dried, weighed and recorded as W2. Drip loss was calculated and expressed as the percentage of the difference between the initial and the final weight of sample after storage divide by the initial weight of sample as shown in the equation below:
Determination of colour
Meat colour coordinates were determined using a Colour Flex spectrophotometer (Hunter Lab Reston, VA, USA) based on the International Commission on Illumination (CIE) Lab-values (also known as lightness (L*), redness (a*) and yellowness (b*) with D65 illuminant and 10° standard observer, tristimulus values (X,Y,Z) and reflectance at specific wavelength (400–700) nm [23]. The device was calibrated against black and white reference tiles prior to use. The samples were bloomed for 30 min and placed at the base of the colour flex cup. For each sample, three readings for each of L*, a* and b* values were recorded and averaged.
Determination of myoglobin, metmyoglobin content and metmyoglobin reducing activity
The extraction and quantification of myoglobin followed the method of Warriss [25]. The metmyoglobin content was determined as described by Krzywicki [26]. The extraction of myoglobin reductase and the determination of metmyoglobin reducing activity (MRA) in pulverized meat samples followed the procedure of Mikkelsen et al. [27].
Fatty acid analysis
Total lipids in feed and meat samples were extracted in chloroform:methanol (2:1, v/v) mixture according to the method of Folch et al. [28] modified by Rajion et al. [29]. The extracted lipids were transmethylated to their fatty acid methyl esters (FAME) using 2 mL 14% BF3 and 2 mL 0.66 N KOH in methanol according to the method of AOAC [20]. The FAME was separated in a gas liquid chromatograph (Agilent 7890A, Agilent Technologies, Inc., anta lara, CA) equipped with a flame ionization detector using a 100 m x 0.25mm ID (0.20 μm film thickness) Supelco SP-2560 capillary column. Helium was the carrier gas and the split ratio after the FAME injection was 10:1. The temperature of the injector and detector were programmed at 250°C and 300°C, respectively. The column temperature was set at 100°C, held for 2 min and warmed to 170°C at 0°C/min, held for 2 min, warmed to 230°C at 5°C/min and then held for 20 min. The fatty acid composition of sample was determined by comparing the relative retention times of FAME peaks from samples with those from standards.
Determination of lipid oxidation
Lipid oxidation was measured as 2-thiobarbituric acid reactive substances (TBARS) using QuantiChromTM TBARS Assay Kit (DTBA-100, BioAssay Systems, USA) following the description of the manufacturer.
Antioxidant Enzyme activity
Glutathione peroxidase (GPX) activity was measured with the aid of EnzyChromTM glutathione Peroxidase Assay Kit EGPX-100, (BioAssay Systems, USA) following the manufacturer’s protocol. Superoxide dismutase (SOD) activity was measured using Cayman SOD Assay kit 706002, (Cayman chemical) following the manufacturer’s protocol. Catalase (CAT) activity was measured using Cayman Catalase Assay Kit 707002, (Cayman chemical) following the manufacturer’s procedure.
Determination of total carotenoid
The carotenoid contents in feed and meat samples were extracted and quantified following the method described by Okonkwo [30]. Two gram of each sample was homogenized with 10 mL acetone. The contents were stirred for 30 min and two 5 mL aliquot of acetone was used to rinse the flask and re-extract the residue. The extracts were pooled and 1 mL of deionized water was added. The mixture was transferred into 5 mL n-hexane and centrifuged at 3000 g for 10 min. The absorbance of the hexane layer was read at 450 nm using a spectrophotometer (Secomam, Domont, France). The total carotenoid contents was estimated by the following formula:
Where A = absorbance
V = Volume of n-hexane (mL)
W = Sample weight
A1%/1cm = 2592 (absorption coefficient of carotene)
Determination of tocopherol
The extraction of tocopherol from feed and tissue samples followed the method of Kamal-Eldin et al. [31]. The tocopherol contents were quantified using Agilent 1200 series HPLC as described by Pegg and Amarowicz [32]. The column used was C30 YMCTM carotenoid (250 mm x 4.6 mm. i.d, 5 μm) (YMC, USA). An isocratic mobile phase made up of 99% n-hexane and 1% Isopropanol was used. The flow rate was 0.5 mL/min and the injection volume was 20 μL. UV detection was monitored at 295 nm. The isomers of tocopherol were quantified by comparing the peak area of samples with those of tocopherol standards in the HPLC controller software.
Determination of carbonyl and free thiol contents
Protein thiol content was quantified according to the Elman’s method using 2,2-dithiobis (5-nitropyridine) DTNP [33]. The results were expressed as nmol/mg protein. The carbonyl content in muscles was determined using Cayman protein carbonyl colorimetric assay kit (10005020) following the manufacturer’s procedure. Carbonyl content was expressed as nmol/mg protein.
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting
The myofibrillar proteins were extracted with an extraction buffer containing 3 mM MgCl2, 25 mM KCl, 4 mM EDTA and 150 mM NaCl at a pH of 6.5 following the method of Morzel et al. [34]. The protein concentration of the samples was determined following the Bradford method [35] using Protein Assay Kit II 500–0002 (Bio-Rad, Hercules, CA, USA). Bovine serum albumen (BSA) was used to prepare the protein standards.
Myofibrils were incubated at 90°C for 10 min in a buffer containing 2.3% (w/v) SDS, 62.5 mM Tris–HCl (pH 6.8), 5% (v/v) mercaptoethanol, 0.05% (w/v) bromophenol blue and 30% (v:v) glycerol. One dimensional SDS-PAGE was performed according to the method of Laemmli [36] using polyacrylamide gels of 8 cm × 5.5 cm (length × width) and 0.8 mm thickness. The resolving gels were over-layered with 4% stacking gel solution. Samples (30 μg protein each) were separated in running buffer (0.025 mol/L Tris base, 0.192 mol/L glycine, 0.1 SDS, pH 8.3) using a mini PROTEAN® Tetra system (Bio-Rad) set at a constant voltage of 120 V and a current of 0.4 A for 90 min. Coomassie blue stain (0.05% Coomassie blue, 5.0% acetic acid, 15% methanol) was used to stain the gels for 60 min. Thereafter, the gels were destained with destaining solution (10% acetic acid and 30% methanol) for 45 min to remove excessive background. The bands of myofibrillar proteins were visualized using a GS-800 Calibrated Imaging Densitometer (Bio-Rad, USA).
The electrophoresed proteins were transferred from the gel onto polyvinylidene difluoride (PVDF) membranes using Trans-Blot® SD semi-dry transfer system cell (Bio-Rad, USA). Myosin heavy chain was transferred at constant amperage of 250 mA per gel, voltage limit of 25 V for 135 min while actin and troponin-T were transferred at the same amperage and voltage for 45 min. The membranes were immersed in ponceau staining solution (0.5% ponceau S and 5% trichloroacetic acid) for 5 min to visualize the proteins of interest and to verify the electrophoretic transfer. The membranes were washed with deionized water thrice and later washed with TBST buffer (100 mM Tris-HCl, 150 mM NaCl and 0.05% Tween 20) once. The membranes were blocked with blocking buffer (5% BSA in TBST buffer) for 3 h at room temperature (26°C) with constant shaking (Multi Shaker-FMS3-FINEPCR, Korea). Thereafter, the membranes were incubated overnight with 1: 500 dilution of primary antibody. The primary antibody used for myosin heavy chain (fast), myosin heavy chain (slow), actin and troponin-T were Monoclonal Anti-Myosin (Skeletal, Fast, produced in mouse; Cat # M4276, Sigma- Aldrich, USA), Monoclonal Anti-Myosin (Skeletal, Slow, produced in mouse; Cat # M842, Sigma- Aldrich, USA), monoclonal Anti-actin (produced in rabbit; Cat # A2066 227, Sigma- Aldrich, USA) and monoclonal anti-troponin T (produced in mouse; Cat # T6277, Sigma- Aldrich, USA) respectively. Subsequently, the membranes were washed three times in TBST buffer (5 min incubation at 26°C) with constant shaking). The membranes were further incubated at 26°C in 1:10000 dilution of secondary antibody [anti- mouse IgG (whole molecule)-peroxidase, antibody developed in rabbit; Cat # A9044, Sigma- Aldrich, USA] in 3% BSA in TBS-T buffer for 90 min. The membranes were washed thrice with TBST buffer. The blocked membranes were detected using a DAB substrate kit Code: E733, DAB SUBSTRATE SYSTEM (aMReSCO®, Ohio). The band intensity of myofibrillar proteins was measured by Quantity one® software on GS-800 Calibrated Imaging Densitometer (Bio-Rad, USA).
Statistical analysis
The experiment followed a completely randomized design model. Data obtained were analysed using the PROC MIXED procedure of SAS [37] in which diet, postmortem ageing and interaction between diet and postmortem ageing were fitted as fixed effects in a repeated measure analysis of variance. Means were separated by Tukey HSD test at significant level of P< 0.05.
Results and Discussion
Muscle pH, glycogen and drip loss
Dietary oil blend did not affect (P> 0.05) the glycogen content and pH of GM muscle throughout the postmortem storage (Table 2). This observation could be due to the comparable available energy from the dietary treatments and the homogenous management and slaughter conditions employed during the trial [19]. Dietary energy and antemortem stress affect the concentration of muscle glycogen at the time of slaughter [38, 39].
Table 2. Mean physicochemical properties of gluteus medius muscle in goats as influenced by dietary oil blend and postmortem ageing.
| Level of oil blend (%) | Storage days | P value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | 0 | 4 | 8 | SEM | 0 | 1 | 4 | 7 | SEM | diet | days | diet x days |
| Glycogen mg/g | 1.07 | 1.07 | 1.07 | 0.12 | 1.30a | 0.58b | 0.56b | 0.57b | 0.01 | 0.312 | 0.031 | 0.213 |
| pH | 5.61 | 5.63 | 5.64 | 0.45 | 6.34a | 5.60b | 5.60b | 5.59b | 0.19 | 0.102 | 0.002 | 0.221 |
| Drip loss (%) | 6.71 | 6.67 | 6.60 | 0.44 | - | 5.89c | 6.91b | 7.60b | 0.11 | 0.234 | 0.001 | 0.237 |
| L* | 31.49 | 30.41 | 33.09 | 1.27 | 34.04b | 34.98b | 37.22a | 38.02a | 1.59 | 0.138 | 0.034 | 0.106 |
| a* | 12.04 | 12.38 | 12.79 | 0.90 | 11. 21a | 12.02a | 10.23b | 9.45c | 0.72 | 0.041 | 0.002 | 0.219 |
| b* | 12.77 | 13.75 | 12.60 | 0.94 | 13.14 | 12.98 | 12.47 | 12.33 | 0.31 | 0.512 | 0.128 | 0.106 |
| Myoglobin (mg/g) | 2.80 | 2.81 | 2.84 | 0.07 | 2.89a | 2.80a | 2.71b | 2.60b | 0.08 | 0.111 | 0.041 | 0.223 |
| Metmyoglobin (%) | 6.69 | 6.55 | 6.56 | 0.08 | 2.91d | 7.39c | 12.35b | 18.99a | 3.12 | 0.222 | < .0001 | 0.441 |
| MRA (nmol min-1g-1) | 212.4 | 210.2 | 212.7 | 14.0 | 212.7a | 200.5b | 190.2c | 181.9d | 10.22 | 0.516 | 0.021 | 0.354 |
a, b, c means having different superscripts along the same row for each factor are significantly different.
L* = lightness. a* = redness. b* = yellowness. MRA = metmyoglobin reducing activity. SEM = standard error of mean
Regardless of the diet, the muscle glycogen content and pH observed on d 0 were greater (P< 0.05) than those observed on other storage days. The finding could be due to postmortem glycolysis. After slaughter, the dwindling oxygen supply shifts muscle metabolism from aerobic to anaerobic, which breaks down available muscle glycogen via the glycolytic pathway to form lactic acid that lowers the muscle pH [39, 40]. The stability of the muscle pH and glycogen on d 1, 4 and 7 postmortem indicates that postmortem glycolysis was completed at 24 h postmortem. The breakdown of glycogen to lactic acid continues until a pH is reached when the enzymes effecting the conversion of glycogen to lactic acid become inactivated [38–40].
Diet had no effect (P< 0.05) on the drip loss of GM muscle in goats (Table 2). The current finding agrees with that of Mir et al. [41] who observed that the drip loss in beef from steers fed sunflower oil with or without vitamin E was similar to that from the control steers. Drip loss increased (P< 0.05) over storage. This observation could be due to the decrease in the available space (steric effects) for water to reside in the muscle due to the formation of cross-bridges between the thick and thin myofibrillar filaments during rigor development [39, 42]. The current observation is consistent with that of Sabow et al. [24].
Colour, myoglobin, metmyoglobin and metmyoglobin reducing activity
The colour coordinates, myoglobin and metmyoglobin contents and metmyoglobin reducing activity of GM muscle in goats are shown in Table 2. Colour is an important meat quality attribute because it is the first tool used by consumers for the identification and selection of meat [38, 39]. Diet had no effect (P> 0.05) on the redness, yellowness and lightness of GM muscle in goats. This finding could be attributed to the similar muscle myoglobin and metmyoglobin contents and metmyoglobin reducing activity in the meat of goats fed different diets. This observation is in agreement with that of Haak et al. [7] who observed that dietary supplementation of oxidized linseed oil with or without antioxidant had no effect on the colour coordinates of pork. In contrast, Jensen et al. [43] observed that dietary rapeseed oil enhanced pork redness.
The meat redness decreased (P< 0.05) while the meat lightness increased (P< 0.05) over storage. This finding could be due to the decrease in myoglobin concentration and metmyoglobin reducing activity (MRA) and the increase in metmyoglobin content over storage. Oxidation of muscle myoglobin to metmyoglobin can lead to discoloration of red meat [39, 44]. Previous studies have demonstrated that meat lightness increased while meat redness decreased during postmortem ageing of mutton [44], chevon [24] and beef [45].
Myoglobin is the major determinant of meat colour [39]. Diet did not affect the myoglobin concentration in GM muscle in goats. The decrease in the concentration of myoglobin could be responsible for the increase in the metmyoglobin content over storage. Similarly, the metmyoglobin content in beef patties subjected to a 10 d [9] refrigerated storage increased over storage. The MRA decreased (P< 0.05) over storage. This observation is in agreement with that of Madhavi and Carpenter [46] who observed a reduction in the MRA of beef during chill storage. In contrast, there was a significant increase in the MRA of beef patties during a 10 d chill storage [9]. In addition, the MRA of ovine longissimus [44] and beef patties [47] was stable during a 10 d and 9 d postmortem ageing respectively.
Fatty acid composition
The fatty acid (FA) composition of gluteus medius muscle in goats is shown in Table 3. Regardless of the diet, the three most abundant FA were C16:0, C18:0 and C18:1n-9. Similar trends were reported for chevon [2] and mutton [48]. The meat from goats fed 4 and 8% oil blend had lower (P< 0.05) concentration of C16:0 and C16:1n-7 compared with that from the control goats. This observation could be due to the displacement or dilution effects of other fatty acids, the decrease in the activity of lipogenic enzymes responsible for the synthesis of medium chain FA or the preferential incorporation of long chain FA from diet and/or adipose tissues [15, 49, 50]. Despite the clear differences between tissue FA deposition and milk FA secretion, the basic characteristics of ruminant lipid metabolism could be invoked in order to utilize information obtained in dairy cows for interpreting the meat composition in lambs, cattle and goats [49]. This finding is consistent with that of Nicole et al. [15] who found that the supplementation of 10.4% canola oil or flaxseed oil reduced the concentration of C16:0 and C16:1n-7 in cow milk. Similarly, Otto et al. [50] observed that the supplementation of crude degummed canola oil reduced the concentration of C16:0 in cow milk.
Table 3. Mean fatty acid composition (mg/ 100 g meat) of gluteus medius muscle in goats as influenced by dietary oil blend and postmortem ageing.
| Fatty acids | Levels of oil blend (%) | Storage time (days) | P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 4 | 8 | SEM | 0 | 4 | 7 | SEM | Diet | storage | dietxstorage | |
| C14:0 | 88.96 | 89.75 | 80.82 | 7.29 | 89.36a | 92.47b | 97.33c | 6.90 | 0.736 | 0.031 | 0.312 |
| C16:0 | 635.66a | 600.95b | 580.55c | 14.18 | 617.21a | 640.22b | 667.11c | 12.00 | 0.022 | 0.003 | 0.583 |
| C16:1n-7 | 89.28a | 70.31b | 67.30c | 6.21 | 84.22 | 80.23 | 78.19 | 5.12 | 0.017 | 0.082 | 0.783 |
| C18:0 | 573.78 | 565.78 | 495.97 | 20.11 | 560.21 | 578.12 | 590.20 | 15.87 | 0.375 | 0.213 | 0.091 |
| C18:1n-9 | 908.19b | 974.93a | 995.81a | 31.34 | 950.41a | 908.22b | 900.34c | 27.45 | 0.647 | 0.040 | 0.777 |
| C18:1trans-11 | 48.64 | 56.57 | 41.89 | 3.43 | 50.12 | 52.31 | 51.00 | 4.44 | 0.078 | 0.134 | 0.778 |
| cis-9 trans-11 CLA | 30.72 | 38.23 | 33.49 | 2.16 | 35.23 | 38.11 | 36.16 | 2.19 | 0.526 | 0.219 | 0.605 |
| trans-10 cis-12 CLA | 37.76 | 41.71 | 41.88 | 4.17 | 39.21 | 40.11 | 39.54 | 3.00 | 0.113 | 0.214 | 0.203 |
| C18:2n-6 | 399.53 | 397.25 | 397.93 | 14.11 | 380.21a | 350.11b | 341.97c | 12.10 | 0.167 | 0.001 | 0.479 |
| C18:3n-3 | 59.12a | 68.23b | 87.32c | 2.00 | 38.21a | 32.15b | 28.22c | 1.98 | 0.030 | 0.021 | 0.239 |
| C20:4n-6 | 199.13 | 153.81 | 156.71 | 14.22 | 200.10a | 189.21b | 170.45c | 10.23 | 0.109 | 0.003 | 0.196 |
| C20:5n-3 | 48.56a | 61.28b | 92.40c | 5.45 | 70.15a | 61.34b | 53.32c | 6.12 | 0.038 | 0.028 | 0.144 |
| C22:5n-3 | 30.64 | 57.07 | 76.62 | 6.16 | 56.15a | 41.23b | 34.22c | 7.80 | 0.072 | 0.045 | 0.347 |
| C22:6n-3 | 50.16 | 60.66 | 68.57 | 6.11 | 70.22a | 65.23b | 60.09c | 5.28 | 0.061 | 0.011 | 0.104 |
| Total FA | 3200.13 | 3236.54 | 3217.26 | 25.61 | 3060.81 | 3169.06 | 3113.92 | 23.23 | 0.218 | 0.567 | 0.284 |
| Fatty acid sum and ratios | |||||||||||
| ∑SFA | 1298.35a | 1256.48b | 1157.34b | 30.22 | 1266.78 | 1310.81 | 1354.64 | 32.11 | 0.041 | 0.001 | 0.327 |
| ∑MUFA | 1046.11 | 1101.81 | 1105.00 | 22.16 | 1084.75 | 1040.76 | 1029.53 | 21.45 | 0.586 | 0.056 | 0.682 |
| ∑PUFA | 855.62b | 878.24b | 954.92a | 21.07 | 889.48a | 817.49b | 763.97c | 20.00 | 0.001 | 0.001 | 0.174 |
| ∑ω-3 | 188.48a | 247.24b | 324.91c | 10.13 | 234.73a | 199.95b | 175.85c | 10.13 | 0.028 | 0.001 | 0.778 |
| ∑ω-6 | 598.66 | 551.06 | 554.64 | 17.12 | 580.31a | 539.32b | 512.42c | 15.78 | 0.004 | 0.011 | 0.788 |
| ω-6:ω-3 | 3.18a | 2.23b | 1.71c | 0.26 | 2.48 | 2.69 | 2.91 | 0.17 | 0.015 | 0.217 | 0.725 |
| UFA:SFA | 1.46 | 1.57 | 1.78 | 0.08 | 1.56 | 1.42 | 1.32 | 0.03 | 0.022 | 0.116 | 0.299 |
| PUFA:SFA | 0.65a | 0.69b | 0.83c | 0.05 | 0.70 | 0.62 | 0.79 | 0.04 | 0.038 | 0.291 | 0.243 |
a, b, c means having different superscripts along the same row for each factor are significantly different.
SEM = standard error of mean
The concentration of C18:1n-9 was greater (P< 0.05) in the meat from the oil-fed goats compared with that from the control goats. This observation could be due to the increase in the intake of C18:1n-9 as the level of the oil blend increased in diet [19]. In addition, the increase in C18:1n9 could be due to the delta-9 desaturation of 18:0 in tissues [15, 49, 50]. This observation is in agreement with the report of a companion in vitro study [51], which showed that the concentration of C18:1n-9 after 24 h incubation increased in response to incremental level of oil blend in the substrate. Similarly, dietary canola oil [15] and crude degummed canola oil [50] increased the concentration of C18:1n-9 in bovine milk.
The concentrations of 14:0, C18:0, C18:1trans11, C18:2n-6, CLA cis-9 trans-11 and CLA cis-12 trans-10 and C20:4n-6, C22:5n-3, C22:6n-3 and total FA in the GM muscle were not influenced (P> 0.05) by diet. The concentration of C18:3n-3 and C20:5n-3 was greater (P< 0.05) in the meat from the oil-supplemented goats compared with that from the control goats. The increase in the concentration of C18:3n-3 could be due to the increase in the dietary intake of C18:3n-3 [19] in response to the incremental level of the oil blend in the diet. The increase in the concentration of C20:5n-3 could be due to the elongation of C18:3n-3. Similarly, dietary C18:3n-3 enhanced the concentration of C18:3n-3 and its long chain derivatives in lambs [49]. The PUFA/SFA increased (P< 0.05) while the n6/n3 decreased (P< 0.05) as the level of the oil blend increased in diet. Similarly, Karami et al. [2] observed that goats fed canola oil had greater concentration of C18:3n-3 and lower n6/n3 in longissimus lumborum muscle in goats compared with those fed palm oil.
Postmortem ageing influenced (P< 0.05) the FA composition of GM muscle in goats. The concentration of C18:1n-9, n-3 and n-6 FA and total polyunsaturated fatty acids (PUFA) decreased (P< 0.05) while the concentration of C14:0 and C16:0 increased (P< 0.05) over storage. This observation could be attributed to the decrease in the concentration of tocopherols and total carotenoids (Table 4) and the increase in lipid oxidation over storage. Similarly, Muíño et al. [4] observed a decrease in the concentration of n-3 and n-6 PUFA in mutton subjected to chill storage for 12 d.
Table 4. Mean antioxidant contents, lipid and protein oxidation in gluteus medius muscle from goats as influenced by dietary oil blend and postmortem ageing.
| Level of oil blend (%) | Storage days | P value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | 0 | 4 | 8 | SEM | 0 | 4 | 7 | SEM | diet | storage | diet x storage |
| α-tocopherol (mg/ kg) | 2.46b | 3.38a | 4.02a | 0.26 | 3.24a | 2.92b | 2.90c | 0.30 | 0.001 | 0.023 | 0.101 |
| γ-tocopherol (mg/kg) | 0.47b | 0.77a | 0.98a | 0.08 | 0.93a | 0.85b | 0.80b | 0.06 | 0.001 | 0.008 | 0.243 |
| δ-tocopherol (mg/kg) | 0.06 | 0.08 | 0.08 | 0.01 | 0.07a | 0.05b | 0.03c | 0.01 | 0.196 | 0.010 | 0.311 |
| Carotenoid (mg/kg) | 0.24 | 0.26 | 0.29 | 0.07 | 0.25a | 0.20b | 0.18b | 0.08 | 0.142 | 0.014 | 0.099 |
| TBARS (mg MDA/kg) | 0.21 | 0.23 | 0.20 | 0.02 | 0.15a | 0.19b | 0.38c | 0.03 | 0.418 | <0.001 | 0.173 |
| Free thiol (nmol/mg protein) | 54.73 | 54.51 | 54.62 | 0.89 | 54.62a | 43.06b | 40.34c | 1.00 | 0.691 | 0.037 | 0.534 |
| Carbonyl (nmol/mg protein) | 3.16 | 3.09 | 3.10 | 0.04 | 1.23c | 3.45b | 5.87a | 0.67 | 0.584 | < .001 | 0.125 |
| Myosin heavy chain fast (density/mm2) | 41.45 | 43.76 | 45.50 | 3.22 | 46.78 | 42.33 | 37.38 | 1.92 | 0.502 | 0.023 | 0.564 |
| Myosin heavy chain slow (density/mm2) | 70.10 | 74.14 | 76.13 | 2.13 | 76.12a | 71.03b | 64.20c | 3.00 | 0.234 | 0.016 | 0.213 |
| Actin (density/mm2) | 16.27 | 16.34 | 16.61 | 0.21 | 16.67 | 16.33 | 16.15 | 0.67 | 0.127 | 0.124 | 0.145 |
| Troponin T (density/mm2) | 13.22b | 15.26a | 15.30a | 0.76 | 15.99a | 14.00b | 12.82c | 0.17 | 0.012 | 0.001 | 0.219 |
a, b, c means having different superscripts along the same row for each factor are significantly different. SEM = standard error of mean
Antioxidant status, lipid and protein oxidation
The antioxidant status and oxidative stability of chevon in response to dietary oil blend and postmortem ageing are presented in Table 4. Diet did not affect (P> 0.05) the catalase, superoxide dismutase and glutathione peroxidase activities in the GM muscle in goats. This observation is an indication that the oil blend did not instigate oxidative stress in the goats. It has been demonstrated that animals exposed to dietary oxidative stress respond with compensatory induction of antioxidant enzymes [52, 53]. The current observation contradicts that of Renerre et al. [53] who observed that dietary rapeseed oil increased the antioxidant enzyme activities in turkey meat. Postmortem ageing did not affect (P> 0.05) the antioxidant enzyme activities in the GM muscle in goats. Similarly, the GPX and CAT activities in different bovine muscles on d 1 were similar to those observed on d 8 postmortem [45]. In contrast, the antioxidant enzyme activities in the breast and thigh muscles of Korean chickens decreased over a 10 d postmortem chill storage [54].
Dietary supplementation of oil blend enhanced (P< 0.05) the concentration of α and γ-tocopherol in the GM muscle in goats. This observation presumably reflects the antioxidant contents of the dietary treatments. Tocopherol is a fat-soluble vitamin [43]. Thus, the increase in the dietary fat might have aided the absorption and deposition of tocopherol in the tissue of the oil-fed goats. Similarly, Soler-Velasquez et al. [55] observed that dietary canola oil increased the α-tocopherol content in pork. Diet did not affect the concentration of total carotenoids and δ-tocopherol in goat meat. Regardless of the diet, the concentration of total carotenoids and α, γ, and δ-tocopherol in GM muscle decreased (P< 0.05) over storage. Similar finding was observed during postmortem ageing of beef [56]. The interaction between diet and postmortem ageing was not significant for the concentration of total carotenoids and α, γ, and δ-tocopherol in goat meat.
Diet did not affect (P> 0.05) the TBARS value of GM muscle in goats. Although the meat from the oil-fed goats had greater concentration of n-3 PUFA and consequently presented greater potential for lipid oxidation, the TBARS value was similar to that from the control meat. This observation could be due to the increase in the concentration of α and γ-tocopherol in the meat from the oil-fed goats. Regardless of the dietary treatment, the TBARS value increased as storage progressed. Similarly, postmortem chill storage increased TBARS value in pork [7], mutton [57] and chevon [58].
Free thiol group and carbonyl content are important indicators of protein oxidation in muscle foods [7, 8]. Diet did not affect (P> 0.05) the free thiol and carbonyl contents of GM muscle in goats (Table 4). These observations concur with those of Lund et al. [8] who observed that dietary soybean oil had no effect on the carbonyl content and free thiol group in porcine quadriceps femoris subjected to a 7 d chill storage. Similarly, dietary oxidized linseed oil with or without antioxidants did not affect the carbonyl content and free thiol group in raw and cooked pork refrigerated for 8 d [7]. Irrespective of the diet, the free thiol content decreased (P< 0.05) while carbonyl content (P< 0.05) increased as chill storage progressed. Similar findings were observed during postmortem ageing of pork [7, 8], mutton [59] and beef [45]. There was no significant interaction between diet and postmortem chill storage for carbonyl and free thiol contents in goat meat.
Myofibrillar protein profile
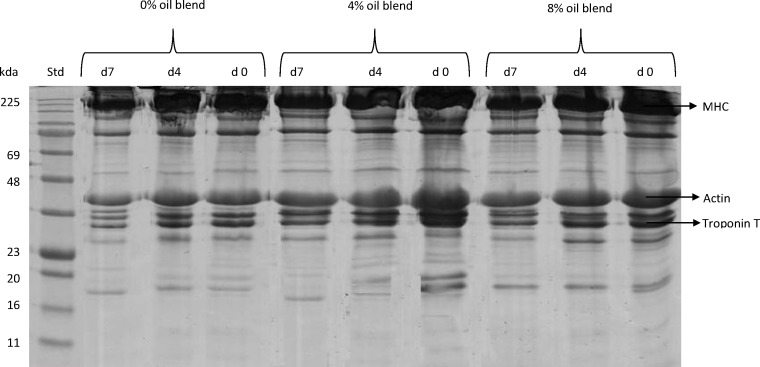
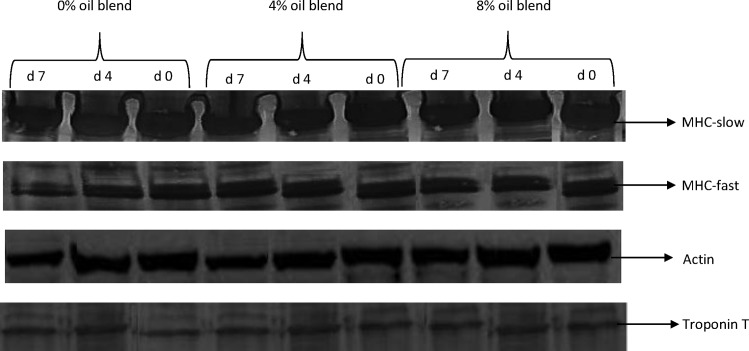
The SDS-PAGE pattern of myofibrillar proteins in GM muscle in goats is shown in Fig 1. The reflective density of myofibrillar proteins in the GM muscle is shown in Table 4. Fig 2 presents the representative Western blots of myofibrillar proteins in GM muscle in goats. Diet did not affect (P> 0.05) the reflective density of myosin heavy chain fast (MHC-f), myosin heavy chain slow (MHC-s) and actin. The GM muscle from the oil-supplemented goats had greater (P< 0.05) reflective density of troponin T than that from the control goats. This observation could be attributed to the greater concentration α and γ-tocopherol in the GM muscle of the oil-fed goats.
Fig 1. SDS-PAGE of myofibrillar proteins in gluteus medius muscle in goats.
Equal amounts of protein (30 μg) of each sample was loaded and electrophoresed on a separate 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) at a constant voltage (120 V) for 90 min. The gels were stained with Coomassie blue staining for 60 min and destained with destaining solution for 45 min. d0 = day 0, d4 = day 4, d7 = day 7. Std = standard. MHC = myosin heavy chain.
Fig 2. Western blot analysis of myofibrillar proteins in gluteus medius muscle in goats.
d0 = day 0, d4 = day 4, d7 = day 7. MHC = myosin heavy chain.
Irrespective of the diet, the reflective density of MHC-f and MHC-s decreased (P< 0.05) over storage. Similar observations were observed during postmortem ageing of beef [60] and chevon [61]. In contrast, myosin heavy chain of beef semimembranosus was stable throughout a 28 d chill storage at 4°C [62]. The reflective density of troponin T decreased (P< 0.05) over storage. This observation concurs with that of Sabow et al. [61] who observed a decrease in the reflective density of troponin T of longissimus lumborum muscle in goats during a 14 d postmortem chill storage. Postmortem ageing was not a significant of variation affecting the reflective density of actin. This observation could be due to the masking of oxidation sites caused by the interaction of actin with myosin chain in myofibrillar suspensions [34]. Similar observation was observed during a 14 d chill storage of chevon [61].
Conclusion
The results of this study demonstrate that dietary blend of 80% canola oil and 20% palm oil beneficially altered muscle lipids without hampering the oxidative stability of myoglobin, lipids and myofibrillar proteins and the physicochemical properties of goat meat. Postmortem ageing encouraged oxidative deterioration of lipids, myoglobin and myofibrillar proteins in goat meat.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1.Nantapo CW, Muchenje V, Nkukwana TT, Hugo A, Descalzo A, Grigioni G, et al. (2015) Socio-economic dynamics and innovative technologies affecting health-related lipid content in diets: Implications on global food and nutrition security. Food Res. Int 76: 896–905. [Google Scholar]
- 2.Karami M, Ponnampalam E, Hopkins D (2013) The effect of palm oil or canola oil on feedlot performance, plasma and tissue fatty acid profile and meat quality in goats. Meat Sci 94: 165–169. 10.1016/j.meatsci.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (2015) Carcinogenicity of consumption of red and processed meat. Report of the International Agency for Research on cancer. Press Release no 240, Lyon France, October 26, 2015. [Google Scholar]
- 4.Muíño I, Apeleo E, de la Fuente J, Pérez-Santaescolástica C, Rivas-Cañedo A, Pérez C, et al. (2014) Effect of dietary supplementation with red wine extract or vitamin E, in combination with linseed and fish oil, on lamb meat quality. Meat Sci 98: 116–123. 10.1016/j.meatsci.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 5.Sabow AB, Sazili AQ, Aghwan AA, Zulkifli I, Goh YM, Ab Kadir MZ, et al. (2016) Changes of microbial spoilage, lipid-protein oxidation and physicochemical properties during postmortem refrigerated storage of goat meat. Anim. Sci. J 10.1111/asj.12496 [DOI] [PubMed] [Google Scholar]
- 6.Adeyemi KD, Sabow AB, Shittu RM, Karim R, Karsani SA, Sazili AQ (2015) Impact of chill storage on antioxidant status, lipid and protein oxidation, color, drip loss and fatty acids of semimembranosus muscle in goats. CyTA J. Food 10.1080/19476337.2015.1114974 [DOI] [Google Scholar]
- 7.Haak L, Raes K, Van Dyck S, De Smet S (2008) Effect of dietary rosemary and α-tocopheryl acetate on the oxidative stability of raw and cooked pork following oxidized linseed oil administration. Meat Sci 78: 239–247. 10.1016/j.meatsci.2007.06.005 [DOI] [PubMed] [Google Scholar]
- 8.Lund MN, Hviid MS, Claudi-Magnussen C, Skibsted LH (2008) Effects of dietary soybean oil on lipid and protein oxidation in pork patties during chill storage. Meat Sci 79: 727–33. 10.1016/j.meatsci.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 9.Liu F, Xu Q, Dai R, Ni Y (2015) Effects of natural antioxidants on colour stability, lipid oxidation and metmyoglobin reducing activity in raw beef patties. Acta. Sci. Pol. Technol. Aliment 1: 37–44. [DOI] [PubMed] [Google Scholar]
- 10.Adeyemi KD, Olorunsanya AO(2012) Comparative analysis of phenolic composition and antioxidant effect of seed coat extracts of four cowpea (Vigna unguiculata) varieties on broiler meat. Iranian J. App. Anim. Sci 2:343–349. [Google Scholar]
- 11.Kahl R, Kappus H (1993) Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Zeitschrift fu¨ r Lebensmittel Untersuchung und Forschung 196: 329–338 [DOI] [PubMed] [Google Scholar]
- 12.Falowo AB, Fayemi PO, Muchenje V (2014) Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: A review. Food Res Int 64: 171–81. [DOI] [PubMed] [Google Scholar]
- 13.Olorunsanya AO, Adeyemi KD, Babatunde IA (2011) Effect of Bamboo (Bambusa valgaris) and Elephant grass (Pennisetum purpureum) leaf extracts on oxidative stability of cooked and raw broiler meat. J. Agr. Res.Dev 10: 1–10. [Google Scholar]
- 14.Adeyemi KD, Olorunsanya OA, Abe OT (2013) Effect of citrus seed extracts on oxidative stability of raw and cooked chicken meat. Iranian J. App. Anim. Sci 3: 195–199. [Google Scholar]
- 15.Nicole Loor J, Herbein J, Jenkins T (2002) Nutrient digestion, biohydrogenation, and fatty acid profiles in blood plasma and milk fat from lactating Holstein cows fed canola oil or canolamide. Anim Feed Sci Technol 97: 65–82. [Google Scholar]
- 16.Oguntibeju O, Esterhuyse A, Truter E (2009) Red palm oil: Nutritional, physiological and therapeutic roles in improving human wellbeing and quality of life. British J. Biomed. Sci 66, 216–222. [DOI] [PubMed] [Google Scholar]
- 17.Ghazani SM, García‐Llatas G, Marangoni AG (2014) Micronutrient content of cold‐pressed, hot‐pressed, solvent extracted and RBD canola oil: Implications for nutrition and quality. Eur. J. Lipid Sci. Tech 116: 380–387. [Google Scholar]
- 18.NRC (2007) Nutrient Requirements of Small Ruminants National Academic Press, Washington, DC. [Google Scholar]
- 19.Adeyemi KD, Sazili AQ, Ebrahimi M, Samsudin AA, Alimon AR, Karim R, et al. (2015) Effects of blend of canola oil and palm oil on nutrient digestibility, growth performance, rumen fermentation and fatty acids in goats. Anim. Sci. J 10.1111/asj.12549 [DOI] [PubMed] [Google Scholar]
- 20.AOAC (1990). Official methods of analysis, 15th ed Arlington, VA: Association of Official Analytical Chemists; p. 931–932. [Google Scholar]
- 21.Van Soest PJ, Robertson J, Lewis B (1991) Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci 74: 3583–3597. [DOI] [PubMed] [Google Scholar]
- 22.Department of standards, Malaysia (2009) MS1500:2009: Halal food production, preparation, handling and storage-General guidelines (second revision) Malaysia.
- 23.AMSA (2012) AMSA Meat Color Measurement Guidelines American Meat Science Association, Champaign, IL, USA. [Google Scholar]
- 24.Sabow AB, Sazili AQ, Zulkifli I, Goh YM, Ab Kadir MZA, Adeyemi KD (2015) Physico-chemical characteristics of longissimus lumborum muscle in goats subjected to halal slaughter and anesthesia (halothane) pre-slaughter. Anim. Sci. J 86: 981–991 10.1111/asj.12385 [DOI] [PubMed] [Google Scholar]
- 25.Warriss PD (1979) The extraction of haem pigments from fresh meat. J. Food Tech 14: 75–80. [Google Scholar]
- 26.Krzywicki K (1982) The determination of haem pigments in meat. Meat Sci 7: 29–36. 10.1016/0309-1740(82)90095-X [DOI] [PubMed] [Google Scholar]
- 27.Mikkelsen A, Juncher D, Skibsted LH (1999) Metmyoglobin reductase activity in porcine m. longissimus dorsi muscle. Meat Sci 51: 155–161. [DOI] [PubMed] [Google Scholar]
- 28.Folch J, Lees M, Sloane-Stanley G (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226: 497–509. [PubMed] [Google Scholar]
- 29.Rajion M, McLean J, Cahill RN (1985) Essential fatty acids in the fetal and newborn lamb. Aust. J. Biol. Sci 38: 33–40. [PubMed] [Google Scholar]
- 30.Okonkwo J (2009) Effects of breed and storage duration on the beta-carotene content of egg yolk. Pakistan J. Nutr 8: 1629–1630. [Google Scholar]
- 31.Kamal-Eldin A, Frank J, Razdan A, Tengblad S, Basu S, Vessby B (2000) Effects of dietary phenolic compounds on tocopherol, cholesterol, and fatty acids in rats. Lipid 35: 427–435. [DOI] [PubMed] [Google Scholar]
- 32.Pegg RB, Amarowicz R (2009) Content of tocopherol isomers in oilseed radish cultivars–a short report. Polish J. Food. Nutr Sci 59: 129–133. [Google Scholar]
- 33.Winterbourn CC (1990) Oxidative reactions of hemoglobin. Method Enzymol. 186: 265. [DOI] [PubMed] [Google Scholar]
- 34.Morzel M, Gatellier P, Sayd T, Renerre M, Laville E (2006) Chemical oxidation decreases proteolytic susceptibility of skeletal muscle myofibrillar proteins. Meat Sci 73: 536–543. 10.1016/j.meatsci.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 35.Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt. Biochem 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 36.Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- 37.SAS (2003) Statistical Analysis System package (SAS) Version 9.2 software. SAS Institute Inc., Cary, NC, USA.
- 38.Adeyemi KD, Sazili AQ (2014) Efficacy of carcass electrical stimulation in meat quality enhancement: A review. Asian-Aust. J. Anim. Sci 27: 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawrie R, Ledward D (2006) Lawrie’s meat science Woodhead Publishing Ltd., Cambridge. [Google Scholar]
- 40.Salwani MS, Adeyemi KD, Sarah SA, Vejayan J, Zulkifli I, Sazili AQ (2015) Skeletal muscle proteome and meat quality of broiler chickens subjected to gas stunning prior slaughter or slaughtered without stunning. CyTA J Food. 10.1080/19476337.2015.1112838 [DOI] [Google Scholar]
- 41.Mir P, McAllister T, Zaman S, Morgan Jones S, He M, et al. (2003) Effect of dietary sunflower oil and vitamin E on beef cattle performance, carcass characteristics and meat quality. Can J. Anim. Sci 83: 53–66. [Google Scholar]
- 42.Offer G, Trinick J (1983) On the mechanism of water holding in meat: the swelling and shrinking of myofibrils. Meat Sci 8: 245–81. 10.1016/0309-1740(83)90013-X [DOI] [PubMed] [Google Scholar]
- 43.Jensen C, Flensted-Jensen M, Skibsted L, Bertelsen G (1998) Effects of dietary rapeseed oil, copper (II) sulphate and vitamin E on drip loss, color and lipid oxidation of chilled pork chops packed in atmospheric air or in a high oxygen atmosphere. Meat Sci 50: 211–221. [DOI] [PubMed] [Google Scholar]
- 44.Bekhit A, Geesink G, Morton J, Bickerstaffe R (2001) Metmyoglobin reducing activity and colour stability of ovine longissimus muscle. Meat Sci 57:427–435. [DOI] [PubMed] [Google Scholar]
- 45.Renerre M, Dumont F, Gatellier P (1996) Antioxidant enzyme activities in beef in relation to oxidation of lipid and myoglobin. Meat Sci 43: 111–121. [DOI] [PubMed] [Google Scholar]
- 46.Madhavi D, Carpenter CE (1993) Aging and processing affect color, metmyoglobin reductase and oxygen consumption of beef muscles. J. Food Sci 58: 939–942. [Google Scholar]
- 47.Bekhit A, Geesink G, Ilian M, Morton J, Bickerstaffe R (2003) The effects of natural antioxidants on oxidative processes and metmyoglobin reducing activity in beef patties. Food Chem 81: 175–187. [Google Scholar]
- 48.Maleki E, Kafilzadeh F, Goh YM, Cheghamirza K, Rajion MA, Khamisabadi H, et al. (2015) Breed effects on muscle fatty acid composition in fat-tailed sheep. J. Food Agr. Environ 13: 18–23. [Google Scholar]
- 49.Bessa RJ, Alves SP, Jeronimo E, Alfaia CM, Prates JA, Santos-Silva J (2007) Effect of lipid supplements on ruminal biohydrogenation intermediates and muscle fatty acids in lambs. Eur J Lipid Sci Technol 109: 868–878. [Google Scholar]
- 50.Otto JR, Malau-Aduli BS, Nichols P, Malau-Aduli AE (2014) Influence of supplementing pasture-based primiparous Holstein-Friesian dairy cows with crude degummed canola oil on milk fatty acid composition. J Nutr Therapeutics 3: 55–66. [Google Scholar]
- 51.Adeyemi KD, Ebrahimi M, Samsudin AA, Alimo AR, Karim R, Karsani SA, et al. (2015) Influence of Carotino oil on in vitro rumen fermentation, metabolism and apparent biohydrogenation of fatty acids. Anim. Sci. J 86: 270–278 10.1111/asj.12279 [DOI] [PubMed] [Google Scholar]
- 52.Renerre M, Poncet K, Mercier Y, Gatellier P, Métro B (1999) Influence of dietary fat and vitamin E on antioxidant status of muscles of turkey. J Agr Food Chem 47:237–44. [DOI] [PubMed] [Google Scholar]
- 53.Maraschiello C, Sárraga C, Garcia Regueiro J (1999) Glutathione peroxidase activity, TBARS, and α-tocopherol in meat from chickens fed different diets. J Agr Food Chem 47: 867–872. [DOI] [PubMed] [Google Scholar]
- 54.Utama DT, Lee SG, Baek KH, Kim HK, Cho CY, Lee CK, et al. (2016) Correlation between antioxidant enzyme activity, free iron content and lipid oxidation in four lines of Korean native chicken meat. Korean J Food Sci Anim Res 36: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soler-Velasquez MP, Brendemuhl JH, McDowell LR, Sheppard KA, Johnson DD, Williams SN (1998) Effects of supplemental vitamin E and canola oil on tissue tocopherol and liver fatty acid profile of finishing swine. J. Anim. Sci 76: 110–117. [DOI] [PubMed] [Google Scholar]
- 56.Clausen I, Jakobsen M, Ertbjerg P, Madsen NT (2009) Modified atmosphere packaging affects lipid oxidation, myofibrillar fragmentation index and eating quality of beef. Pack Technol Sci 22: 85–96. 37. [Google Scholar]
- 57.Popova T, Marinova P (2013) Lipid oxidation in M. longissimus dorsi and M. semimembranosus in lambs reared indoors and on pasture. Iranian J Appl Anim Sci 3: 533–537 [Google Scholar]
- 58.Sabow AQ, Sazili AQ, Zulkifli I, Goh YM, Ab Kadir MZA, Abdulla N, et al. (2015) A comparison of bleeding efficiency, microbiological quality and lipid oxidation in goats subjected to conscious halal slaughter and slaughter following minimal anesthesia. Meat Sci 104: 78–84. 10.1016/j.meatsci.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 59.Popova T, Marinova P (2013) Protein oxidation in M. longissimus dorsi and M. semimembranosus in lambs reared indoors and on pasture. Iranian J Appl Anim Sci 3: 673–677. [Google Scholar]
- 60.Martinaud A, Mercier Y, Marinova P, Tassy C, Gatellier P, Renerre M (1997) Comparison of oxidative processes on myofibrillar proteins from beef during maturation and by different model oxidation systems. J. Agr. Food Chem 45: 2481–2487. [Google Scholar]
- 61.Sabow AB, Zulkifli I, Goh YM, Ab Kadir MZA, Kaka U, Imlan JC, et al. (2016) Bleeding efficiency, microbiological quality and oxidative stability of meat from goats subjected to slaughter without stunning in comparison with different methods of pre-slaughter electrical stunning. Plos one 10.1371/journal.pone.0152661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bandman E, Zdanis D (1988) An immunological method to assess protein degradation in post-mortem muscle. Meat Sci 22: 1–19. 10.1016/0309-1740(88)90023-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.