Abstract
Plant defense pathways play a critical role in mediating tritrophic interactions between plants, herbivores, and natural enemies. While the impact of plant defense pathway stimulation on natural enemies has been extensively explored aboveground, belowground ramifications of plant defense pathway stimulation are equally important in regulating subterranean pests and still require more attention. Here we investigate the effect of aboveground stimulation of the salicylic acid pathway through foliar application of the elicitor methyl salicylate on belowground recruitment of the entomopathogenic nematode, Steinernema diaprepesi. Also, we implicate a specific root-derived volatile that attracts S. diaprepesi belowground following aboveground plant stimulation by an elicitor. In four-choice olfactometer assays, citrus plants treated with foliar applications of methyl salicylate recruited S. diaprepesi in the absence of weevil feeding as compared with negative controls. Additionally, analysis of root volatile profiles of citrus plants receiving foliar application of methyl salicylate revealed production of d-limonene, which was absent in negative controls. The entomopathogenic nematode S. diaprepesi was recruited to d-limonene in two-choice olfactometer trials. These results reinforce the critical role of plant defense pathways in mediating tritrophic interactions, suggest a broad role for plant defense pathway signaling belowground, and hint at sophisticated plant responses to pest complexes.
Introduction
Plants adopt constitutive and induced strategies to defend against herbivores and pathogens both aboveground and belowground [1, 2]. These defenses can act directly against the offending herbivore, producing or releasing toxins that deter feeding behavior [3]. Indirectly, these defenses can result in the release of herbivore induced plant volatiles that recruit natural enemies [3]. These tritrophic interactions involving recruitment of natural enemies have been observed aboveground [4, 5] and belowground where feeding by larvae of Diabrotica virgifera virgifera results in release of E-β caryophyllene and recruits the entomopathogenic nematode Heterhorabditis megidis [6]. Similarly, in citrus, feeding belowground by larvae of the weevil Diaprepes abbreviatus results in release of pregeijerene which recruits a wide variety of nematodes, including entomopathogenic nematodes that are natural enemies of D. abbreviatus [7–9].
These tritrophic interactions between plants, herbivores, and their natural enemies above and belowground are mediated by stimulation of defense pathways within plants [3]. Stimulation of these plant defense pathways can occur through herbivory [10], plant-to-plant communication [11], or application of chemicals that elicit plant defense responses [12]. Among a myriad of plant defense pathways, a prominent pathway that has important roles in plant defense against both pathogens and herbivores is the salicylic acid pathway [13, 14]. It is so called because of the prominent role salicylic acid plays in stimulating plant defense and its known role in recruiting natural enemies aboveground [15].
In addition to its role in recruiting natural enemies aboveground, the salicylic acid pathway also mediates interactions between herbivores and pathogens. Stimulation of the salicylic acid pathway through synthetic elicitors can reduce bacterial lesion development [16] and can affect plant resistance to herbivores [17]. In addition, the sequence of induction can have ramifications for plant defense pathway stimulation and herbivore-pathogen resistance [16, 18]. Multiple stimulation of plant defense pathways also has tritrophic effects on natural enemies aboveground [19].
Less is known regarding the role the salicylic acid plant defense pathways play in mediating plant responses belowground. While stimulation of plant defenses aboveground has effects belowground, and vice versa, the dynamic nature of plant defense pathways in mediating this communication between the terrestrial and subterranean environments is less well understood [20–22]. Effects of plant defense stimulation aboveground on interactions belowground are varied and occasionally nonexistent [1, 22, 23]. Similarly, the role of plant defense pathways in stimulating production of herbivore induced plant volatiles for the recruitment of natural enemies belowground is not well understood.
Here, we explore the effect of stimulating the salicylic acid pathway aboveground on recruitment of natural enemies belowground. To do so, we applied an elicitor, methyl salicylate, to the leaves of citrus seedlings while monitoring the response of the entomopathogenic nematode Steinernema diaprepesi belowground both in the presence and absence of the larval weevil herbivore D. abbreviatus, a prominent polyphagous root pest of citrus and many other crops. The entomopathogenic nematode, S. diaprepesi, may be the most effective natural enemy of this polyphagous root herbivore and therefore we focused on this particular nematode as part of our multi-trophic investigation [24, 25].
Materials and Methods
To evaluate the effect of plant defense pathway stimulation on recruitment of natural enemies belowground, particularly in the case of the salicylic acid pathway, 30mL of 130μl/L methyl salicylate was applied to the aboveground portion of citrus seedlings while nematode response was monitored in olfactometer bioassays belowground. Based on the nematode response, volatiles were collected from the roots of treated and control plants. Volatiles unique to treated plants were then evaluated for activity in two-choice bioassays.
Organisms
Response of the infective juvenile stage of the entomopathogenic nematode Steinernema diaprepesi to 20 cm citrus Swingle Citrumelo (Citrus paradisi Macf. × Poncirus trifoliata L. Raf.) seedlings was evaluated in four-choice olfactometers. S. diaprepesi infective juveniles were originally collected from sentinel D. abbreviatus larvae in Florida citrus groves and then reared on Galleria mellonela larvae and collected on White traps [26, 27]. S. diaprepesi infective juveniles were maintained in shallow tissue culture flasks at 14°C and were used within two weeks after emergence. Fifth instar D. abbreviatus larvae used in methyl salicylate bioassay trials were reared on artificial diet from eggs laid by adults collected from Florida citrus groves [28, 29].
Methyl Salicylate Bioassays
The attraction of the entomopathogenic nematode S. diaprepesi to citrus seedlings treated with foliar applications of elicitors in the presence and absence of belowground herbivory by D. abbreviatus larvae was evaluated in four-choice olfactometers (similar to six-choice olfactometers used for evaluating nematode behavior [6]) filled with clean autoclaved sand adjusted to 12% moisture by volume. Four-choice olfactometers were constructed from 4×4×4 inch (10.16 × 10.16 × 10.16cm) containers (Tupperware Corporation, Orlando, FL) perforated on each of the four sides to accomodate 2 inch (5.08cm) PVC pipe elbows. Connections were sealed with insulation and one citrus seedling was placed in each of the elbows. After allowing 48 hours for acclimatization, plants were treated with elicitor sprays. In each four-choice olfactometer, two opposing seedlings received treatment with methyl salicylate (MeSA) and two opposing seedlings were left as untreated, negative controls. Methyl salicylate treated seedlings each received 30mL of 130μl/L methyl salicylate (Sigma; CAS:119-36-8) by foliar spray in a Tween 20 and ethanol solution at 0.1 and 2.5mL/L respectively. Control seedlings did not receive the elicitor, only the Tween 20 and ethanol solution. For experiments involving D. abbreviatus herbivory, five approximately five week old D. abbreviatus larvae were placed directly on the roots of methyl salicylate treated and control seedlings. Forty-eight hours after application of the elicitors, approximately 2500 S. diaprepesi infective juveniles were released into the center of the olfactometer. After an additional 24 hours, nematodes were extracted from the responding arms using sugar centrifugation, then counted [30].
Volatile Collection and Analysis
To investigate the potential role of volatile-mediated nematode attraction in the four arm olfactometers, volatiles were collected from the root systems of untreated citrus seedlings and seedlings treated with methyl salicylate. Volatiles were collected 48 hours after application of elicitors for one hour onto 30mg HayesepQ adsorbent filters (Volatile Assay Systems; VAS) at a flow rate of 160ml/min. Extracted volatiles were eluted off of the collection filters with two aliquots of 75μl methylene chloride. Five microliters of 1.5μg/μl nonyl acetate was added as an internal standard. A one microliter aliquot of each sample was then injected onto a Clarus 500 gas chromatograph—mass spectrometer (PerkinElmer, Waltham, MA) containing a 30m × 0.25mm−ID DB-5 capillary column. The column was held at 35°C for 3 minutes after injection and then increased 10°C per minute until reaching 260°C where it remained for an additional five minutes. Helium was used as a carrier gas at a flow rate of 2 ml per minute. Electron ionization spectra were compared with references found in the NIST Mass Spectral Library (2008) and then confirmed with available standards. Differences in volatile profiles between treated and control plants were examined and quantified by comparison to the nonyl-acetate internal standard.
Volatile Bioassays
To investigate whether d-limonene, primarily responsible for the differences between volatile profiles of methyl salicylate treated and untreated control plants (see Results), may attract S. diaprepesi, two-choice sand-filled assays consisting of inverted 1.5 inch (3.81 cm) diameter PVC T-Tubes, capped on each end, were used. Individual assay tubes were filled with clean autoclaved sand adjusted to 12% moisture by volume after placing filter paper treated with either a blank control, 10μl of water, or 10μl aliquots of doses of d-limonene in water for a total of 17ng, 170ng, 1.7μg, or17μg at opposing ends of the olfactometer. Approximately 2000 S. diaprepesi infective juveniles were applied to the central orifice of each olfactometer. After 24 hours, responding nematodes were extracted from the sand in each PVC cap using Baermann funnels and counted [31].
Statistical Analysis
S. diaprepesi infective juvenile response to salicylate-treated citrus plants in four-choice olfactometers was summed within each replicate for each treatment to avoid aggregation effects then examined for normality by visual inspection with quantile-quantile plots and Shapiro-Wilk’s test. Wilcoxon signed rank tests were then used to evaluate preference. Differences in volatile profiles between treated and control plants were quantified through comparison to internal standards. Mean quantities of collected volatiles were calculated and bootstrapped to determine 95 percent confidence intervals. S. diaprepesi infective juvenile preference for doses of d-limonene in two-choice olfactometers was evaluated by determining the percentage of infective juveniles responding to d-limonene in each replicate for each dose. Preference percentages were examined for normality through visual inspection with quantile-quantile plots and interrogation with Shapiro-Wilk’s test and subsequently evaluated for differences from a 50% response of no preference through one-sided t-tests with Bonferroni correction (reported as padj). Data were collated in Microsoft Excel 2011 and analyzed using R version 3.2.2 [32] in the R Studio version 0.99.484 development environment [33]. Analysis was facilitated using the packages xlsx [34] for interface with Microsoft Excel, tidyr [35] and dplyr [36] for data arrangement and summary statistics, ggplot2 [37] for graphics capabilities, and scales for visual representation of scaling [38].
Results
Methyl Salicylate Bioassays
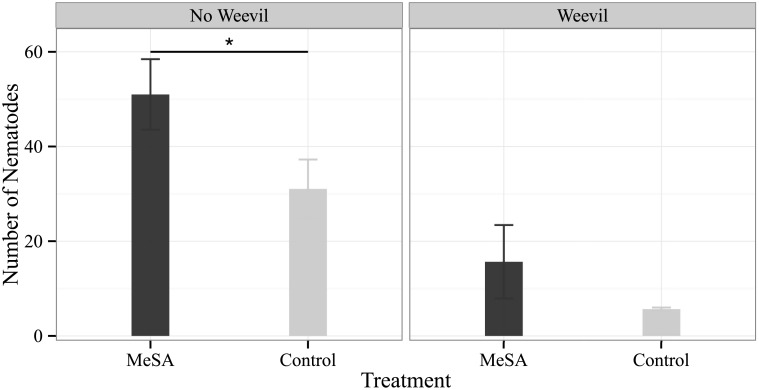
The infective juveniles of the entomopathogenic nematode S. diaprepesi significantly (p = 0.01) preferred (27.7%; 95% Confidence Interval: 16.4%, 38.9% difference) plants treated with methyl salicylate (MeSA) over control plants in the absence of a weevil pest (Fig 1). Data were non-normal by visual inspection and interrogation with the Shapiro-Wilk normality test (W = 0.83, p = 0.004). In the presence of belowground feeding by the insect herbivore D. abbreviatus on both the control and treated plants, methyl salicylate treated plants were not significantly (p = 0.25) more attractive than controls (Fig 1).
Fig 1. S. diaprepesi attraction to methyl salicylate (MeSA) treated citrus seedlings.
Entomopathogenic nematode S. diaprepesi infective juvenile response to citrus seedlings treated aboveground with methyl salicylate in four-choice sand filled olfactometers both in the presence and absence of belowground herbivory by D. abbreviatus weevil larvae (n = 21). Bars and error bars denote mean number of respondents and standard error respectively. S. diaprepesi infective juveniles significantly preferred plants treated with methyl salicylate (MeSA) over control plants in the absence of weevil feeding damage.
Volatile Collection and Analysis
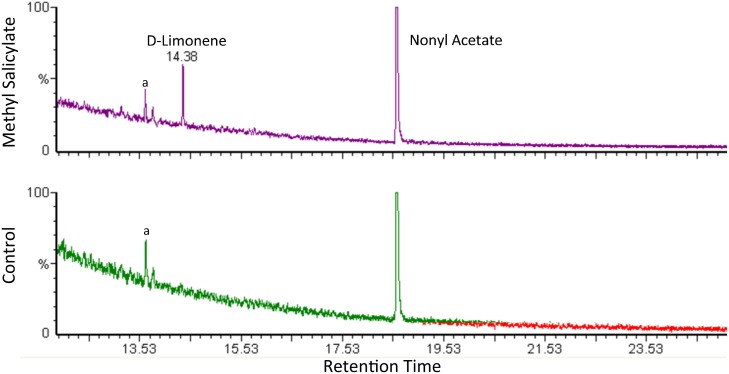
d-Limonene (retention time 14.38) was present in root volatile profiles of methyl salicylate treated plants but not detectable in the controls (Fig 2). An average of 0.61ng/μl (from 0.04 to 2.22ng/μl) d-limonene was detected in eluted samples from methyl salicylate treated plants; total amount of volatile d-limonene collected averaged 91.5ng.
Fig 2. Volatile Profiles of Methyl Salicylate Treated and Control Plants.
Sample chromatograms with volatile profiles of methyl salicylate treated (above) and control (below) plants. d-limonene (retention time 14.38; from 0.04 to 2.22ng) was present in treated plants, but not in controls (n = 10). Nonyl acetate was used as an internal standard. Decane (a) was also recovered in both standards and controls.
Volatile Bioassays
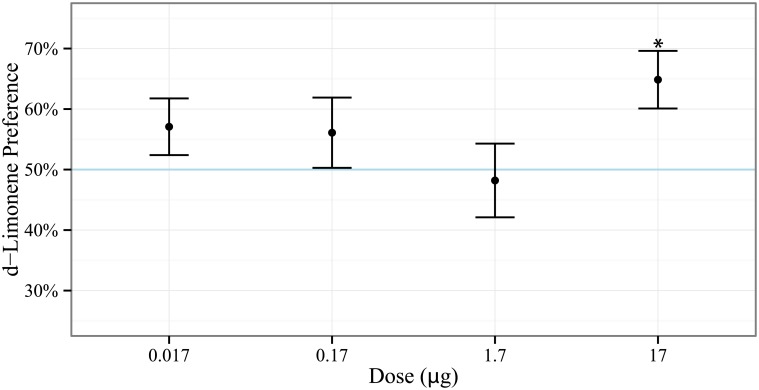
Entomopathogenic nematode S. diaprepesi infective juveniles significantly (padj = 0.02) preferred d-limonene at doses of 17μg in two-choice olfactometer assays as compared with negative controls (Fig 3). Data were not significantly different from normal by visual inspection with quantile-quantile plots and interrogation with the Shapiro-Wilk test (p>0.28). Preferences for d-limonene at other doses were not significantly different from 50% (padj > 0.32).
Fig 3. S. diaprepesi preference for d-limonene.
Entomopathogenic nematode S. diaprepesi infective juvenile preference for doses of d-limonene as evaluated in two-choice sand filled olfactometers (n = 48). 50% response (horizontal blue line) indicates no preference. Points and error bars denote mean and standard error respectively. S. diaprepesi significantly preferred d-limonene at doses of 17μg.
Discussion
Stimulation of the salicylic acid pathway through aboveground application of methyl salicylate resulted in recruitment of the entomopathogenic nematode S. diaprepesi. Herbivory by larvae of the weevil D. abbreviatus attenuates this response. Attraction in the absence of the weevil herbivore is likely mediated by belowground root release of the volatile d-limonene. This result suggests that insect larval feeding may induce a competitive plant defense response belowground.
These results highlight, for what we believe to be the first time, the direct role of the salicylic acid pathway in releasing induced plant volatiles for the recruitment of entomopathogenic nematode natural enemies belowground. While previous work has shown that herbivory belowground by the weevil D. abbreviatus can induce production of pregeijerene and attract entomopathogenic nematodes [8], the effects of stimulating the salicylic acid pathway on recruitment of subterranean natural enemies suggests a broader role for plant defense signaling for belowground natural enemies of herbivores.
This signaling serves little purpose if no receiver perceives the stimulus. The response of entomopathogenic nematodes to the d-limonene cue suggests that the entomopathogenic nematodes in this system are highly attuned to the volatiles in their environment. Entomopathogenic nematodes have been shown to respond to herbivory in connection to a variety of plant and herbivore species and to a variety of induced host plant volatiles belowground (e.g., E-β caryophyllene and pregeijerene) [6, 8, 39]. In previous work, however, such induced host plant volatiles were produced through herbivory or mechanical damage of a potential host. In our case, the d-limonene cue was released after stimulation of the salicylic acid pathway aboveground and in the absence of weevil herbivory. Interestingly, d-limonene is a terpene related to belowground signals indentified in earlier work [6, 8]. This may provide a different and complementary information pathway for plant defense belowground and does not simply signal presence of a host herbivore feeding on the roots.
Indeed, feeding by the weevil herbivore seemed to attenuate the response of belowground entomopathogenic nematodes. In the absence of salicylic acid pathway stimulation, herbivory by D. abbreviatus on Swingle Citrumelo citrus seedlings recruits entomopathogenic nematodes through release of the herbivore-induced volatile pregeijerene within twenty-four hours [8]. In the absence of herbivory, salicylic acid pathway stimulation recruited entomopathogenic nematodes through release of d-limonene. In the case where herbivory by larvae of the weevil D. abbreviatus was coincident with stimulation of the salicylic acid pathway, entomopathogenic nematode response was attenuated in this investigation. This interaction suggests a possible case of crosstalk between plant defense pathways. Insect herbivory has been shown in many instances to stimulate the jasmonic acid pathway [2, 14]. The jasmonic acid pathway, when stimulated, can antagonistically interact with the salicylic acid pathway, in some cases shutting down plant defense response [14].
While the jasmonic acid pathway is traditionally associated with plant responses to herbivory, stimulation of the salicylic acid pathway is often associated with defense against biotrophic pathogens [14]. In this case, its role in recruiting natural enemies may seem counter intuitive. Indeed the evolution and advantages of such attraction remain to be explored. One possible explanation is that the citrus-D. abbreviatus-entomopathogenic nematode interaction is not a simple closed system. There is a fourth, and prominent, player. The oomycete Phytophthora is frequently found in association with D. abbreviatus herbivory. Wounding of plant roots by D. abbreviatus opens a passage for infection by Phytophthora causing much greater damage to citrus trees and other plants than weevil herbivory alone [40]. The Phytophthora-Diaprepes weevil system is a complex that must be considered when developing management strategies for commercial citrus and plant production [41]. Because Phytophthora infections frequently accompany belowground weevil herbivory, recruitment of entomopathogenic nematodes by stimulation of the salicylic acid pathway may be an effective response for defense against attack by both an insect herbivore and a phytopathogen. We are currently exploring this hypothesis.
Acknowledgments
We thank Bo H. Holladay, and Joshua Fluty for assistance with bioassays. Maria Eduarda C.F.R Silva assisted and provided invaluable support for all aspects of the project. The Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) program and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) provided financial support for the project through studentships and grants to CCF. MP was supported by a FAEPEX-PAPDIC grant from UNICAMP, and CNPq project 474449/2012-2.
Data Availability
The data are available on Dryad via DOI: doi:10.5061/dryad.2b2b5.
Funding Statement
The Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) program and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) provided financial support for the project through studentships and grants to CCF. MP was supported by a FAEPEX-PAPDIC grant from UNICAMP, and CNPq project 474449/2012-2.
References
- 1. Kaplan I, Halitschke R, Kessler A, Sardanelli S, Denno RF. Constitutive and induced defenses to herbivory in above-and belowground plant tissues. Ecology. 2008;89(2):392–406. 10.1890/07-0471.1 [DOI] [PubMed] [Google Scholar]
- 2. Howe GA, Jander G. Plant immunity to insect herbivores. Annu Rev Plant Biol. 2008;59:41–66. 10.1146/annurev.arplant.59.032607.092825 [DOI] [PubMed] [Google Scholar]
- 3. Bezemer TM, van Dam NM. Linking aboveground and belowground interactions via induced plant defenses. Trends in Ecology & Evolution. 2005;20(11):617–624. 10.1016/j.tree.2005.08.006 [DOI] [PubMed] [Google Scholar]
- 4. Turlings TC, Tumlinson JH, Lewis WJ. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;250(4985):1251–1253. 10.1126/science.250.4985.1251 [DOI] [PubMed] [Google Scholar]
- 5. De Moraes C, Lewis W, Pare P, Alborn H, Tumlinson J. Herbivore-infested plants selectively attract parasitoids. Nature. 1998;393(6685):570–573. 10.1038/31219 [DOI] [Google Scholar]
- 6. Rasmann S, Köllner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, et al. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature. 2005;434(7034):732–737. 10.1038/nature03451 [DOI] [PubMed] [Google Scholar]
- 7. Ali JG, Campos-Herrera R, Alborn HT, Duncan LW, Stelinski LL. Sending mixed messages: a trophic cascade produced by a belowground herbivore-induced cue. Journal of chemical ecology. 2013;39(8):1140–1147. 10.1007/s10886-013-0332-x [DOI] [PubMed] [Google Scholar]
- 8. Ali JG, Alborn HT, Stelinski LL. Subterranean herbivore-induced volatiles released by citrus roots upon feeding by Diaprepes abbreviatus recruit entomopathogenic nematodes. Journal of chemical ecology. 2010;36(4):361–368. 10.1007/s10886-010-9773-7 [DOI] [PubMed] [Google Scholar]
- 9. Ali JG, Alborn HT, Stelinski LL. Constitutive and induced subterranean plant volatiles attract both entomopathogenic and plant parasitic nematodes. Journal of Ecology. 2011;99(1):26–35. 10.1111/j.1365-2745.2010.01758.x [DOI] [Google Scholar]
- 10. Bari R, Jones JD. Role of plant hormones in plant defence responses. Plant molecular biology. 2009;69(4):473–488. 10.1007/s11103-008-9435-0 [DOI] [PubMed] [Google Scholar]
- 11. Heil M, Karban R. Explaining evolution of plant communication by airborne signals. Trends in ecology & evolution. 2010;25(3):137–144. 10.1016/j.tree.2009.09.010 [DOI] [PubMed] [Google Scholar]
- 12. Bi HH, Zeng RS, Su LM, An M, Luo SM. Rice allelopathy induced by methyl jasmonate and methyl salicylate. Journal of chemical ecology. 2007;33(5):1089–1103. 10.1007/s10886-007-9286-1 [DOI] [PubMed] [Google Scholar]
- 13. Raskin I. Role of salicylic acid in plants. Annual review of plant biology. 1992;43(1):439–463. 10.1146/annurev.pp.43.060192.002255 [DOI] [Google Scholar]
- 14. Thaler JS, Humphrey PT, Whiteman NK. Evolution of jasmonate and salicylate signal crosstalk. Trends in plant science. 2012;17(5):260–270. 10.1016/j.tplants.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 15. van Poecke RM, Dicke M. Induced parasitoid attraction by Arabidopsis thaliana: involvement of the octadecanoid and the salicylic acid pathway. Journal of Experimental Botany. 2002;53(375):1793–1799. 10.1093/jxb/erf022 [DOI] [PubMed] [Google Scholar]
- 16. Thaler JS, Fidantsef AL, Bostock RM. Antagonism between jasmonate-and salicylate-mediated induced plant resistance: effects of concentration and timing of elicitation on defense-related proteins, herbivore, and pathogen performance in tomato. Journal of chemical ecology. 2002;28(6):1131–1159. 10.1023/A:1016225515936 [DOI] [PubMed] [Google Scholar]
- 17. Thaler JS, Agrawal AA, Halitschke R. Salicylate-mediated interactions between pathogens and herbivores. Ecology. 2010;91(4):1075–1082. 10.1890/08-2347.1 [DOI] [PubMed] [Google Scholar]
- 18. Viswanathan D, Lifchits O, Thaler J. Consequences of sequential attack for resistance to herbivores when plants have specific induced responses. Oikos. 2007;116(8):1389–1399. 10.1111/j.0030-1299.2007.15882.x [DOI] [Google Scholar]
- 19. Rodriguez-Saona C, Chalmers JA, Raj S, Thaler JS. Induced plant responses to multiple damagers: differential effects on an herbivore and its parasitoid. Oecologia. 2005;143(4):566–577. 10.1007/s00442-005-0006-7 [DOI] [PubMed] [Google Scholar]
- 20. van Dam NM, Heil M. Multitrophic interactions below and above ground: en route to the next level. Journal of Ecology. 2011;99(1):77–88. 10.1111/j.1365-2745.2010.01761.x [DOI] [Google Scholar]
- 21. Van der Putten WH, Vet LE, Harvey JA, Wäckers FL. Linking above-and belowground multitrophic interactions of plants, herbivores, pathogens, and their antagonists. Trends in Ecology & Evolution. 2001;16(10):547–554. 10.1016/S0169-5347(01)02265-0 [DOI] [Google Scholar]
- 22. van Dam NM. Belowground herbivory and plant defenses. Annu Rev Ecol Evol Syst. 2009;40:373–391. 10.1146/annurev.ecolsys.110308.120314 [DOI] [Google Scholar]
- 23. Bezemer T, Wagenaar R, Van Dam N, Wäckers F. Interactions between above-and belowground insect herbivores as mediated by the plant defense system. Oikos. 2003;101(3):555–562. 10.1034/j.1600-0706.2003.12424.x [DOI] [Google Scholar]
- 24. Nguyen KB, Duncan LW. Steinernema diaprepesi n. sp.(Rhabditida: Steinernematidae), a parasite of the citrus root weevil Diaprepes abbreviatus (L)(Coleoptera: Curculionidae). Journal of Nematology. 2002;34(2):159 [PMC free article] [PubMed] [Google Scholar]
- 25. Simpson S, Nigg H, Coile N, Adair R. Diaprepes abbreviatus (Coleoptera: Curculionidae): host plant associations. Environmental Entomology. 1996;25(2):333–349. 10.1093/ee/25.2.333 [DOI] [Google Scholar]
- 26. Kaya HK, Stock S. Techniques in insect nematology. Manual of techniques in insect pathology. 1997;1:281–324. 10.1016/B978-012432555-5/50016-6 [DOI] [Google Scholar]
- 27. White G. A method for obtaining infective nematode larvae from cultures. Science. 1927;66(1709):302–303. 10.1126/science.66.1709.302-a [DOI] [PubMed] [Google Scholar]
- 28. Lapointe SL, Evens TJ, Niedz RP. Insect diets as mixtures: optimization for a polyphagous weevil. Journal of insect physiology. 2008;54(7):1157–1167. 10.1016/j.jinsphys.2008.04.020 [DOI] [PubMed] [Google Scholar]
- 29. Lapointe SL, Shapiro JP. Effect of soil moisture on development of Diaprepes abbreviatus (Coleoptera: Curculionidae). Florida Entomologist. 1999; p. 291–299. 10.2307/3496582 [DOI] [Google Scholar]
- 30. JENKINS W, et al. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant disease reporter. 1964;48(9). [Google Scholar]
- 31. Viglierchio D, Schmitt RV. On the methodology of nematode extraction from field samples: Baermann funnel modifications. Journal of Nematology. 1983;15(3):438 [PMC free article] [PubMed] [Google Scholar]
- 32.R Core Team. R: A Language and Environment for Statistical Computing; 2015. Available from: https://www.R-project.org/.
- 33.RStudio Team. RStudio: Integrated Development Environment for R; 2015. Available from: http://www.rstudio.com/.
- 34.Dragulescu AA. xlsx: Read, write, format Excel 2007 and Excel 97/2000/XP/2003 files; 2014. Available from: http://CRAN.R-project.org/package=xlsx.
- 35.Wickham H. tidyr: Easily Tidy Data with ‘spread()‘ and ‘gather()‘ Functions; 2015. Available from: http://CRAN.R-project.org/package=tidyr.
- 36.Wickham H, Francois R. dplyr: A Grammar of Data Manipulation; 2015. Available from: http://CRAN.R-project.org/package=dplyr.
- 37.Wickham H. ggplot2: elegant graphics for data analysis. Springer New York; 2009. Available from: http://had.co.nz/ggplot2/book.
- 38.Wickham H. scales: Scale Functions for Visualization; 2015. Available from: http://CRAN.R-project.org/package=scales.
- 39. Van Tol RW, Van Der Sommen AT, Boff MI, Van Bezooijen J, Sabelis MW, Smits PH. Plants protect their roots by alerting the enemies of grubs. Ecology Letters. 2001;4(4):292–294. 10.1046/j.1461-0248.2001.00227.x [DOI] [Google Scholar]
- 40. Rogers S, Graham J, McCoy C. Insect-plant pathogen interactions: Preliminary studies of Diaprepes root weevil injuries and Phytophthora infections. Science. 1996;5:472–473. [Google Scholar]
- 41. Graham J, Bright D, McCoy C. Phytophthora-Diaprepes weevil complex:Phytophthora spp. relationship with citrus rootstocks. Plant Disease. 2003;87(1):85–90. 10.1094/PDIS.2003.87.1.85 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available on Dryad via DOI: doi:10.5061/dryad.2b2b5.