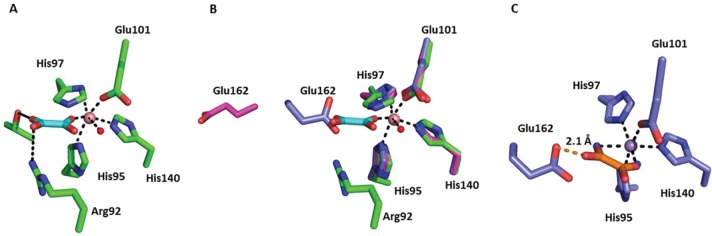
Figure 3.
(A) N-Terminal active site in the X-ray crystal structure of the ΔE162 OxDC/oxalate complex. Carbon atoms in the protein and bound oxalate are colored green and cyan, respectively. The Co(II) ion is rendered as a pink sphere. (B) Overlay of the active sites of the Co(II)-substituted ΔE162 OxDC variant (C, green), WT OxDC (C, purple) with the Glu162 side chain in the “closed” orientation (PDB entry 1UW8), and WT OxDC (C, magenta) with the Glu162 side chain in the “open” orientation (PDB entry 1J58). Only the metal from the Co(II)-substituted ΔE162 OxDC variant is shown for the sake of clarity. (C) N-Terminal active site of WT OxDC (C and Mn, purple) with oxalate (C, orange) modeled in a “side on” conformation by placing the carboxylate oxygens in the two positions occupied by waters (blue spheres) in the wild-type structure (PDB entry 1UW8). The steric clash with Glu162 is denoted by an orange line. The oxygen atoms of metal-bound water molecules are rendered as red spheres, and metal–ligand coordinate bonds are shown as black dashed lines in all three images.