Abstract
Glucose‐dependent insulinotropic polypeptide (GIP) and glucagon‐like peptide‐1 (GLP‐1) are the incretin hormones secreted from enteroendocrine K‐cells and L‐cells, respectively, by oral ingestion of various nutrients including glucose. K‐cells, L‐cells and pancreatic β‐cells are glucose‐responsive cells with similar glucose‐sensing machinery including glucokinase and an adenosine triphosphate‐sensitive K+ channel comprising KIR6.2 and sulfonylurea receptor 1. However, the physiological role of the adenosine triphosphate‐sensitive K+ channel in GIP secretion in K‐cells and GLP‐1 secretion in L‐cells is not elucidated. Recently, it was reported that GIP and GLP‐1‐producing cells are present also in pancreatic islets, and islet‐derived GIP and GLP‐1 contribute to glucose‐induced insulin secretion from pancreatic β‐cells. In this short review, we focus on GIP and GLP‐1 secretion by monosaccharides, such as glucose or fructose, and the role of the adenosine triphosphate‐sensitive K+ channel in GIP and GLP‐1 secretion.
Keywords: Carbohydrates, Glucagon‐like peptide‐1, Glucose‐dependent insulinotropic polypeptide
Introduction
Glucose‐dependent insulinotropic polypeptide (GIP) and glucagon‐like peptide‐1 (GLP‐1) are incretin hormones that amplify glucose‐induced insulin secretion from pancreatic β‐cells. Enteral ingestion of nutrition, such as carbohydrate, fat and protein, induces GIP secretion from K‐cells and GLP‐1 secretion from L‐cells. GIP stimulates glucagon secretion from pancreatic α‐cells, and promotes uptake of glucose and fat, and also accumulates triglyceride into adipose tissue. In contrast, GLP‐1 suppresses glucagon secretion and inhibits gastric emptying1, 2. These findings suggest that GLP‐1 is the more suitable target for the improvement of glucose metabolism. However, L‐cells are present mostly in the jejunum, ileum and colon, whereas K‐cells are predominantly present in duodenum. Furthermore, GIP secretion from the duodenum plays an important role in regulating postprandial glycemic levels through its glucose‐dependent potentiation of early‐phase insulin secretion. GLP‐1 secretion by nutrients is biphasic3, and the transient early‐phase GLP‐1 secretion is considered to be mediated by the vagal nerve4. In the present review, we discuss recent advances in understanding the regulatory mechanisms involved in the secretion of GIP and GLP‐1 stimulated by carbohydrates, including artificial sweeteners.
GIP and GLP‐1 Secretion Induced by Glucose
Plasma GIP and GLP‐1 levels are elevated after oral glucose ingestion in both humans and rodents3, 5, 6. However, intravenous or intraperitoneal administration of glucose does not increase plasma GIP or GLP‐1 levels3, 5, 7. In humans, plasma GIP levels peak within 5 min, whereas plasma GLP‐1 levels peak at 30 min after glucose loading3. The mechanism and role of GIP secretion by glucose is therefore distinct from that of GLP‐1 secretion.
Role of Taste Receptors in Secretion of GIP and GLP‐1
It has been reported that artificial sweeteners contribute to the secretion of incretins8.
Sweeteners bind to sweet taste receptors, which consist of the heterodimers of the G‐protein‐coupled type 1 taste receptors (T1R2 and T1R3) to activate the signal cascade in which α‐gustducin is involved. Artificial sweeteners stimulate two signal transduction pathways: a cyclic adenosine monophosphate‐dependent pathway and a phospholipase C‐Ins(1,4,5)P3‐dependent pathway. The intracellular Ca concentration is increased by these pathways’ activation, which induces membrane depolarization9. Artificial sweeteners do not affect GIP secretion in mice, rats and humans10, 11, 12, and glucose‐induced GIP secretion is maintained in α‐gustducin deficient mice13. Thus, the sweet receptor appears to play a minor role in the regulation of GIP secretion. Indeed, in primary murine K‐cells, the expression of the sweet taste receptor is low, and the artificial sweetener sucralose does not stimulate GIP secretion from primary proximal small intestinal culture14. In contrast, GLP‐1 secretion by glucose is severely impaired in α‐gustducin‐deficient mice13, indicating that GLP‐1 secretion is induced through the sweet taste signal. However, in primary murine L‐cells, the expression of the sweet taste receptor is very low15, and artificial sweetener does not induce GLP‐1 secretion from either primary proximal small intestinal culture or isolated perfused rat small intestine15, 16. Furthermore, in α‐gustducin‐deficient mice, glucose uptake in response to luminal carbohydrate concentrations through sodium‐dependent glucose transporter (SGLT1) in enterocytes, which plays an important role in glucose‐induced GIP and GLP‐1 secretion, is also impaired8. Therefore, the contribution of α‐gustducin to the regulation of secretion of GLP‐1 requires further exploration.
Role of Fructose Transporter in Secretion of GIP and GLP‐1
Fructose is transported by a facilitative glucose transporter 5 (GLUT5) localized in the apical membrane of enterocytes and is metabolized mainly in liver. GLUT5 is highly expressed in pancreatic β‐cells, K‐cells and L‐cells14, 15. In mice, rats and humans, fructose does not significantly stimulate GIP secretion under normal conditions17, 18, 19. However, in insulin resistance ob/ob diabetic mice and streptozotocin (STZ)‐induced insulin‐deficient diabetic mice, fructose significantly induces GIP secretion17, 20. In primary proximal small intestinal culture, fructose stimulates GIP secretion, although the GIP secretory response to fructose is lower than that to glucose14. Therefore, the mechanism of fructose‐induced GIP secretion remains largely to be elucidated. In contrast, fructose significantly stimulates GLP‐1 secretion in mice, rats and humans17, 18. These results accord with an in vitro study using a GLUTag cell line showing that fructose significantly stimulates GLP‐1 secretion21. It is reported that fructose directly induces insulin secretion in a glucose‐dependent manner17, 22. Whether or not fructose is metabolized in K‐cells or L‐cells, and the differential regulatory machinery for the secretion of GIP and GLP‐1 by fructose should be investigated in further study.
SGLT1 Contribution to Glucose‐Induced GIP and GLP‐1 Secretion
SGLT1, a member of the SLC5 family, localizes in the brush border or apical membrane of enterocytes, and plays an important role in the transfer of glucose or galactose from intestinal lumen into intestinal cytosol when accompanied by sodium23. The expression of SGLT1 is highest in the duodenum, and is reduced in the lower intestine24. Thus, the expression pattern of SGLT1 is similar to that of GIP secreting K‐cells25. The mechanism of glucose‐induced GLP‐1 secretion has been investigated by in vitro study using the GLUTag cell line or primary murine L‐cells15, 21, 26. It has been clarified that GLP‐1 secretion is mediated by an influx of sodium through SGLT1, which induces membrane depolarization and voltage dependent‐Ca2+ entry15, 21, 26. In contrast, the mechanism of glucose‐induced GIP secretion has not been characterized, because there have been few cell lines suitable for analysis.
Recently, the contribution of SGLT1 to glucose‐induced GIP and GLP‐1 secretion in vivo has been analyzed by using SGLT1 substrate, SGLT1 inhibitor and mice deficient for SGLT110, 27, 28, 29. Oral administration of a SGLT1 substrate, α‐methyl‐d‐glucopyranoside, which induces sodium influx but is not metabolized, stimulates GIP secretion27. Glucose‐induced GIP secretion is completely blocked in mice administered glucose combined with the SGLT1 inhibitor phlorizin10. Furthermore, glucose‐induced GIP secretion is completely abolished in SGLT1‐deficient mice, and mice treated with a dual SGLT1 and SGLT2 inhibitor28, 29. These findings show that glucose‐induced GIP secretion is SGLT1‐dependent.
In contrast, GLP‐1 secretion is only temporally induced by α‐methyl‐d‐glucopyranoside administration27. Glucose‐induced GLP‐1 secretion is completely blocked at 5 min after glucose load in SGLT1‐deficient mice, and in dual SGLT1 and SGLT2 inhibitor treated‐mice28, 29. However, GLP‐1 oversecretion is observed from 1 to 6 h after glucose administration both in SGLT1‐deficient mice, and in dual SGLT1 and SGLT2 inhibitor treated‐mice29. These findings show that glucose‐induced GLP‐1 secretion includes two phases; an early‐phase, which is SGLT1‐dependent, and a late‐phase, which is SGLT1‐independent. GLP‐1 secretion by nutrients is well known to be biphasic3, and the transient early‐phase GLP‐1 secretion is partially mediated by the vagal nerve4. In addition, it is considered that early‐phase GLP‐1 is secreted from L‐cells in the proximal small intestine, and that late‐phase GLP‐1 is secreted from the distal intestine. Thus, differences in expression of SGLT1 along the intestinal tract and the machinery of GLP‐1 secretion requires detailed examination in future study.
Interestingly, galactose, another monosaccharide transported through SGLT1, induces secretion of both GIP and GLP‐1 in vivo 3, 30, 31.
Adenosine Triposphate‐Sensitive K Channel Contribution to Glucose‐Induced GIP and GLP‐1 Secretion
The adenosine triphosphate‐sensitive K (KATP) channel consists of Kir6.2 and sulfonylurea receptor 1 (SUR1) subunits. In pancreatic β‐cells, the KATP channel plays an essential role in glucose‐induced insulin secretion. Glucose transported through GLUT2 is metabolized, and the resulting increase in the intracellular adenosine triphosphate/adenosine diphosphate ratio leads to closure of the KATP channel and membrane depolarization, influx of Ca through the voltage‐dependent Ca channel, and hormone secretion. It has been reported that a glucose‐sensing molecule, such as GLUT2, glucokinase or KATP channels, are expressed in primary murine K‐ or L‐cells, as in pancreatic ß‐cells14, 15. It also has been reported that tolbutamide, a sulfonylurea, directly stimulates GIP and GLP‐1 secretion from primary small intestine culture or colon culture, respectively14, 15. However, oral sulfonylurea therapy does not potentiate secretion of GIP or GLP‐1 during oral glucose tolerance tests (OGTT) either in healthy subjects or type 2 diabetic patients, or in subjects with KATP channel gain‐of‐function mutations32, 33, 34. In addition, neither GIP nor GLP‐1 secretion during OGTT differ between subjects with heterozygous glucokinase gene mutations and control subjects35. Therefore, the physiological relevance of glucose‐sensing molecules expressed in K‐ or L‐cells remains unclear.
In contrast, the contribution of the KATP channel to glucose‐induced GIP secretion in vivo was recently reported10. Glucose‐induced GIP secretion is enhanced in STZ‐induced diabetic mice and rats10, 36. Although the SGLT1 inhibitor phlorizin completely blocks glucose‐induced GIP secretion in normoglycemic mice, in STZ‐diabetic mice, phlorizin only partially blocks glucose‐induced GIP secretion, but completely blocks it when combined with pretreatment with the KATP channel activator diazoxide10. These results suggest that under normal conditions, glucose‐induced GIP secretion is SGLT1‐dependent, whereas under STZ‐induced hyperglycemic conditions, glucose‐induced GIP secretion is dependent on both SGLT1 and the KATP channel in vivo. This hypothesis is summarized in Figure 1; the metabolic pathway in K‐cells in vitro under normal and hyperglycemic condition requires investigation in future study.
Figure 1.
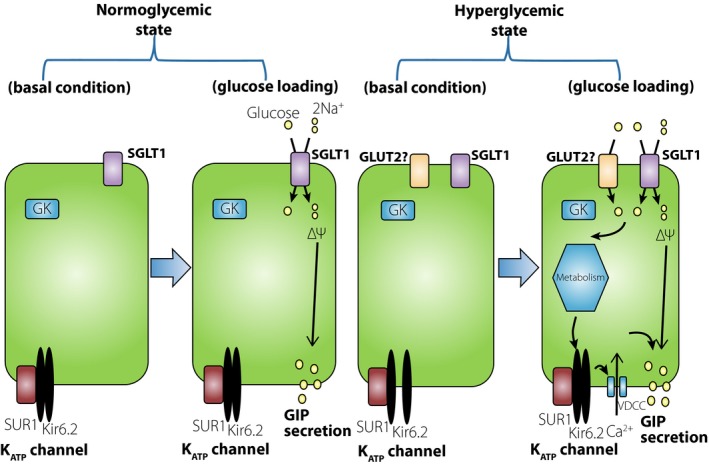
Glucose‐dependent insulinotropic polypeptide (GIP) secretory mechanism induced by glucose in K‐cells. Under the normoglycemic state, adenosine triphosphate‐sensitive K (KATP) channels in K‐cells are closed in the basal condition. On glucose loading, glucose transported through sodium‐dependent glucose transporter (SGLT1) induces membrane depolarization and GIP secretion from K‐cells. Under the hyperglycemic condition, KATP channels in K‐cells are open in basal condition. On glucose loading, incremental increase in ATP closes the KATP channel and depolarizes the membrane, generating an increase in GIP secretion in addition to the SGLT1‐dependent GIP secretion. GK, glucokinase; GLUT5, glucose transporter 5.
The GLP‐1 secretion in response to high‐dose sulfonylurea has been shown in perfused rat small intestine experiments16, 37. The authors suggest that a KATP channel‐dependent pathway plays an important role in glucose‐induced GLP‐1 secretion when glucose passes through GLUT2 in L‐cells. This accords with the fact that glucose‐induced GLP‐1 secretion, but not GIP secretion, is reduced in GLUT2‐deficient mice38. Another group reported that GLP‐1 is secreted in response to sulfonylurea from colon explants, but not from ileum explants in mice39. The differential mechanism of GLP‐1 secretion between the small intestine and large intestine should also be investigated in future study.
The contribution of KATP channels to glucose‐induced GIP or GLP‐1 secretion has been investigated using KATP channel‐deficient mice40. In these mice, glucose‐induced GIP secretion is enhanced, although glucose‐induced GLP‐1 secretion is not changed compared with those in wild‐type mice10, 41, 42. Sglt1 messenger ribonucleic acid expression in duodenum and glucose uptake is increased in KATP channel‐deficient mice compared with those in wild‐type mice10. These results suggest that in the absence of KATP channels, SGLT1 in the duodenum might play some role in the compensatory mechanism for glucose uptake and/or in GIP secretion in vivo.
The issues below require clarification regarding the contribution of KATP channels in GIP and GLP‐1 secretion.
Localization of GLUT2: can GLUT2 localize in apical membrane in K‐cells or L‐cells to transport glucose from intestinal lumen? Can glucose be transported into cytosol of intestinal endocrine cells through basolateral GLUT2?
The interaction between intestinal absorptive epithelial cells and K‐cells or L‐cells regarding glucose flow.
The functional relationship between SGLT1 and the KATP channel in intestinal endocrine cells.
GIP and GLP‐1 Secretion in Islets
It was recently reported that GIP and GLP‐1 are also expressed in pancreatic islets.
GIP is expressed in the embryonic pancreas or pancreatic α‐cells in mice, pythons and humans43, 44. Fujita et al.44 reported that GIP secreted from α‐cells is a shorter isoform, GIP1–30, which is different from GIP1–42 secreted from intestinal K‐cells. They also found that GIP1–30 is secreted from human and mice islets by arginine stimulation, and that GIP1–30 contributes to glucose‐induced insulin secretion from isolated islets in vitro 44. In mice deficient in proglucagon‐derived peptides45, the insulin secretory response is enhanced during OGTT or intraperitoneal glucose tolerance test although GLP‐1 is absent7. In these mice, immunohistochemical study shows GIP expression in pancreatic β‐cells, and enhanced glucose‐induced insulin secretion from isolated islets is observed. Furthermore, the insulin secretory response during OGTT or intraperitoneal glucose tolerance test and glucose‐induced insulin secretion from isolated islets is remarkably reduced in mice deficient in both the GIP receptor and proglucagon‐derived peptides. These results show that islet‐derived GIP contributes to glucose‐induced insulin secretion. Whether or not islet‐derived GIP participates in glucose‐induced insulin secretion under hyperglycemic condition or β‐cell protection should be characterized in future study.
Glucagon and GLP‐1 are produced from the same precursor, proglucagon. In pancreatic α‐cells, glucagon is produced through cleavage by the enzyme prohormone convertase 1/2; in intestinal L‐cells, GLP‐1 and GLP‐2 are produced through cleavage by enzyme prohormone convertase 1/3. It is reported that GLP‐1 production in islets or the pancreas is increased in mice deficient in glucagon action, mice fed with a high‐fat diet and STZ‐treated rats, mainly as a result of increased prohormone convertase 1/3 expression in pancreatic α‐cells46, 47, 48. In addition, GLP‐1 secreted from isolated non‐diabetic mice or human islets potentiates glucose‐induced insulin secretion, showing that islet‐derived GLP‐1 contributes to glucose‐induced insulin secretion under normal conditions49. Furthermore, GLP‐1 secreted from islets is considered to play an important role in protection from β‐cell damage.
The machinery of glucose‐induced GIP or GLP‐1 secretion from islets under physiological condition or in diabetic states is not well known; it has recently been reported that SGLT1 and SGLT2 are found in pancreatic α‐cells in addition to KATP channels50. Whether or not SGLT1 or the KATP channel contributes to glucose‐induced GIP or GLP‐1 secretion from pancreatic α‐cells should be investigated in future study.
Conclusions
There are several well‐known distinct characteristics in the secretory responses to carbohydrates between GIP and GLP‐1: the localization of secreting cells in the gastrointestinal tract, the response to fructose, and the contribution of SGLT1 and the KATP channel. In vitro analysis using primary K‐cells or L‐cells obtained from various parts of the gastrointestinal tract will further clarify novel physiological and pathological aspects of GIP and GLP‐1.
Disclosure
YS received speaker fees from MSD and Eli Lilly. YH received speaker fees from Ono, Sumitomo Dainippon Pharma, Kissei, Mitsubishi Tanabe Pharma and Astellas Pharma. YS received a research grant from Sanwa Kagaku Kenkyusho co., LTD.
Acknowledgments
This work was supported by grants for young researchers from the Japan Association for Diabetes Education and Care, and Grants‐in‐Aid for Scientific Research from Japan Society for the Promotion of Science to YS (25461338).
J Diabetes Investig 2016; 7: 27–32
This article is based on the presentations given by the authors at a symposium, Incretin 2015, July 29‐31, 2015, Vancouver, BC Canada.
References
- 1. Baggio LL, Drucker DJ. Biology of incretins: GLP‐1 and GIP. Gastroenterology 2007; 132: 2131–2157. [DOI] [PubMed] [Google Scholar]
- 2. Seino Y, Yabe D. Glucose‐dependent insulinotropic polypeptide and glucagon‐like peptide‐1: incretin actions beyond the pancreas. J Diabetes Investig 2013; 4: 108–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herrmann C, Göke R, Richter G, et al Glucagon‐like peptide‐1 and glucose‐dependent insulin‐releasing polypeptide plasma levels in response to nutrients. Digestion 1995; 56: 117–126. [DOI] [PubMed] [Google Scholar]
- 4. Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient‐induced glucagon‐like peptide‐1 secretion. Endocrinology 1999; 140: 1687–1694. [DOI] [PubMed] [Google Scholar]
- 5. Cataland S, Crockett SE, Brown JC, et al Gastric inhibitory polypeptide (GIP) stimulation by oral glucose in man. J Clin Endocrinol Metab 1974; 39: 223–228. [DOI] [PubMed] [Google Scholar]
- 6. Sakamoto E, Seino Y, Fukami A, et al Ingestion of a moderate high‐sucrose diet results in glucose intolerance with reduced liver glucokinase activity and impaired glucagon‐like peptide‐1 secretion. J Diabetes Investig 2012; 3: 432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fukami A, Seino Y, Ozaki N, et al Ectopic expression of GIP in pancreatic β‐cells maintains enhanced insulin secretion in mice with complete absence of proglucagon‐derived peptides. Diabetes 2013; 62: 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Margolskee RF, Dyer J, Kokrashvili Z, et al T1R3 and gustducin in gut sense sugars to regulate expression of Na+‐glucose cotransporter 1. Proc Natl Acad Sci U S A 2007; 104: 15075–15080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lindemann B. Receptors and transduction in taste. Nature 2001; 413: 219–225. [DOI] [PubMed] [Google Scholar]
- 10. Ogata H, Seino Y, Harada N, et al KATP channel as well as SGLT1 participates in GIP secretion in the diabetic state. J Endocrinol 2014; 222: 191–200. [DOI] [PubMed] [Google Scholar]
- 11. Fujita Y, Wideman RD, Speck M, et al Incretin release from gut is acutely enhanced by sugar but not by sweeteners in vivo. Am J Physiol Endocrinol Metab 2009; 296: E473–E479. [DOI] [PubMed] [Google Scholar]
- 12. Ma J, Bellon M, Wishart JM, et al Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am J Physiol Gastrointest Liver Physiol 2009; 296: G735–G739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jang H, Kokrashvili Z, Theodorakis MJ, et al Gut‐expressed gustducin and taste receptors regulate secretion of glucagon‐like peptide‐1. Proc Natl Acad Sci U S A 2007; 104: 15069–15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parker HE, Habib AM, Rogers GJ, et al Nutrient‐dependent secretion of glucose‐dependent insulinotropic polypeptide from primary murine K cells. Diabetologia 2009; 52: 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reimann F, Habib AM, Tolhurst G, et al Glucose sensing in L cells: a primary cell study. Cell Metab 2008; 8: 532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuhre RE, Frost CR, Svendsen B, et al Molecular mechanisms of glucose‐stimulated GLP‐1 secretion from perfused rat small intestine. Diabetes 2015; 64: 370–382. [DOI] [PubMed] [Google Scholar]
- 17. Seino Y, Ogata H, Maekawa R, et al Fructose induces glucose‐dependent insulinotropic polypeptide, glucagon‐like peptide‐1 and insulin secretion: role of adenosine triphosphate‐sensitive K + channels. J Diabetes Investig 2015; 6: 522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuhre RE, Gribble FM, Hartmann B, et al Fructose stimulates GLP‐1 but not GIP secretion in mice, rats, and humans. Am J Physiol Gastrointest Liver Physiol 2014; 306: G622–G630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sirinek KR, Levine BA, O'Dorisio TM, et al Gastric inhibitory polypeptide (GIP) release by actively transported, structurally similar carbohydrates. Proc Soc Exp Biol Med 1983; 173: 379–385. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6867011. [DOI] [PubMed] [Google Scholar]
- 20. Flatt PR, Kwasowski P, Bailey CJ. Stimulation of gastric inhibitory polypeptide release in ob/ob mice by oral administration of sugars and their analogues. J Nutr 1989; 119: 1300–1303. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2795243. [DOI] [PubMed] [Google Scholar]
- 21. Gribble FM, Williams L, Simpson AK, et al A novel glucose‐sensing mechanism contributing to glucagon‐like peptide‐1 secretion from the GLUTag cell line. Diabetes 2003; 52: 1147–1154. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12716745. [DOI] [PubMed] [Google Scholar]
- 22. Kyriazis GA, Soundarapandian MM, Tyrberg B. Sweet taste receptor signaling in beta cells mediates fructose‐induced potentiation of glucose‐stimulated insulin secretion. Proc Natl Acad Sci U S A 2012; 109: E524–E532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferraris RP. Dietary and developmental regulation of intestinal sugar transport. Biochem J 2001(Pt 2); 360: 265–276. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1222226&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yoshikawa T, Inoue R, Matsumoto M, et al Comparative expression of hexose transporters (SGLT1, GLUT1, GLUT2 and GLUT5) throughout the mouse gastrointestinal tract. Histochem Cell Biol 2011; 135: 183–194. [DOI] [PubMed] [Google Scholar]
- 25. Damholt AB, Kofod H, Buchan AM. Immunocytochemical evidence for a paracrine interaction between GIP and GLP‐1‐producing cells in canine small intestine. Cell Tissue Res 1999; 298: 287–293. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10571117. [DOI] [PubMed] [Google Scholar]
- 26. Parker HE, Adriaenssens A, Rogers G, et al Predominant role of active versus facilitative glucose transport for glucagon‐like peptide‐1 secretion. Diabetologia 2012; 55(9): 2445–55. doi: 10.1007/s00125-012-2585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moriya R, Shirakura T, Ito J, et al Activation of sodium‐glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am J Physiol Endocrinol Metab 2009; 297: E1358–E1365. [DOI] [PubMed] [Google Scholar]
- 28. Gorboulev V, Schürmann A, Vallon V, et al Na(+)‐d‐glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose‐dependent incretin secretion. Diabetes 2012; 61: 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Powell DR, Smith M, Greer J, et al LX4211 increases serum glucagon‐like peptide 1 and peptide YY levels by reducing sodium/glucose cotransporter 1 (SGLT1)‐mediated absorption of intestinal glucose. J Pharmacol Exp Ther 2013; 345: 250–259. [DOI] [PubMed] [Google Scholar]
- 30. Ganda OP, Soeldner JS, Gleason RE, et al Metabolic effects of glucose, mannose, galactose, and fructose in man. J Clin Endocrinol Metab 1979; 49: 616–622. Available from: http://www.ncbi.nlm.nih.gov/pubmed/479351. [DOI] [PubMed] [Google Scholar]
- 31. Ritzel U, Fromme A, Ottleben M, et al Release of glucagon‐like peptide‐1 (GLP‐1) by carbohydrates in the perfused rat ileum. Acta Diabetol 1997; 34: 18–21. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9134052. [DOI] [PubMed] [Google Scholar]
- 32. El‐Ouaghlidi A, Rehring E, Holst JJ, et al The dipeptidyl peptidase 4 inhibitor vildagliptin does not accentuate glibenclamide‐induced hypoglycemia but reduces glucose‐induced glucagon‐like peptide 1 and gastric inhibitory polypeptide secretion. J Clin Endocrinol Metab 2007; 92: 4165–4171. [DOI] [PubMed] [Google Scholar]
- 33. Stephens JW, Bodvarsdottir TB, Wareham K, et al Effects of short‐term therapy with glibenclamide and repaglinide on incretin hormones and oxidative damage associated with postprandial hyperglycaemia in people with type 2 diabetes mellitus. Diabetes Res Clin Pract 2011; 94: 199–206. [DOI] [PubMed] [Google Scholar]
- 34. Pearson ER, Flechtner I, Njølstad PR, et al Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med 2006; 355: 467–477. [DOI] [PubMed] [Google Scholar]
- 35. Murphy R, Tura A, Clark PM, et al Glucokinase, the pancreatic glucose sensor, is not the gut glucose sensor. Diabetologia 2009; 52: 154–159. [DOI] [PubMed] [Google Scholar]
- 36. Irwin N, Francis JM, Flatt PR. Insulin modulates glucose‐dependent insulinotropic polypeptide (GIP) secretion from enteroendocrine K cells in rats. Biol Chem 2011; 392: 909–918. [DOI] [PubMed] [Google Scholar]
- 37. Mace OJ, Schindler M, Patel S. The regulation of K‐ and L‐cell activity by GLUT2 and the calcium‐sensing receptor CasR in rat small intestine. J Physiol 2012; 590(Pt 12): 2917–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cani PD, Holst JJ, Drucker DJ, et al GLUT2 and the incretin receptors are involved in glucose‐induced incretin secretion. Mol Cell Endocrinol 2007; 276: 18–23. [DOI] [PubMed] [Google Scholar]
- 39. Geraedts MC, Takahashi T, Vigues S, et al Transformation of postingestive glucose responses after deletion of sweet taste receptor subunits or gastric bypass surgery. Am J Physiol Endocrinol Metab 2012; 303: E464–E474. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40. Miki T, Nagashima K, Tashiro F, et al Defective insulin secretion and enhanced insulin action in KATP channel‐deficient mice. Proc Natl Acad Sci U S A 1998; 95: 10402–10406. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=27906&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miki T, Minami K, Shinozaki H, et al Distinct effects of glucose‐dependent insulinotropic polypeptide and glucagon‐like peptide‐1 on insulin secretion and gut motility. Diabetes 2005; 54: 1056–1063. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15793244. [DOI] [PubMed] [Google Scholar]
- 42. Lee EY, Kaneko S, Jutabha P, et al Distinct action of the ‐glucosidase inhibitor miglitol on SGLT3, enteroendocrine cells, and GLP1 secretion. J Endocrinol 2014; 224: 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Prasadan K, Koizumi M, Tulachan S, et al The expression and function of glucose‐dependent insulinotropic polypeptide in the embryonic mouse pancreas. Diabetes 2011; 60: 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fujita Y, Wideman RD, Asadi A, et al Glucose‐dependent insulinotropic polypeptide is expressed in pancreatic islet alpha‐cells and promotes insulin secretion. Gastroenterology 2010; 138: 1966–1975. [DOI] [PubMed] [Google Scholar]
- 45. Hayashi Y, Yamamoto M, Mizoguchi H, et al Mice deficient for glucagon gene‐derived peptides display normoglycemia and hyperplasia of islet {alpha}‐cells but not of intestinal L‐cells. Mol Endocrinol 2009; 23: 1990–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hayashi Y. Metabolic impact of glucagon deficiency. Diabetes Obes Metab 2011; 13(Suppl. 1): 151–157. [DOI] [PubMed] [Google Scholar]
- 47. Ellingsgaard H, Hauselmann I, Schuler B, et al Interleukin‐6 enhances insulin secretion by increasing glucagon‐like peptide‐1 secretion from L cells and alpha cells. Nat Med 2011; 17: 1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nie Y, Nakashima M, Brubaker PL, et al Regulation of pancreatic PC1 and PC2 associated with increased glucagon‐like peptide 1 in diabetic rats. J Clin Invest 2000; 105: 955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Omar BA, Liehua L, Yamada Y, et al Dipeptidyl peptidase 4 (DPP‐4) is expressed in mouse and human islets and its activity is decreased in human islets from individuals with type 2 diabetes. Diabetologia 2014; 57: 1876–1883. [DOI] [PubMed] [Google Scholar]
- 50. Bonner C, Kerr‐Conte J, Gmyr V, et al Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med 2015; 21: 512–517. [DOI] [PubMed] [Google Scholar]


