Abstract
Glucagon‐like peptide‐1 (GLP‐1) is a product of proglucagon cleavage synthesized in L cells in the intestinal mucosa, α‐cells in the pancreatic islet, and neurons in the nucleus of the solitary tract. GLP‐1 is essential for normal glucose tolerance and acts through a specific GLP‐1 receptor that is expressed by islet β‐cells as well as other cell types. Because plasma concentrations of GLP‐1 increase following meal ingestion it has been generally presumed that GLP‐1 acts as a hormone, communicating information from the intestine to the endocrine pancreas through the circulation. However, there are a number of problems with this model including low circulating concentrations of GLP‐1 in plasma, limited changes after meal ingestion and rapid metabolism in the plasma. Moreover, antagonism of systemic GLP‐1 action impairs insulin secretion in the fasting state, suggesting that the GLP‐1r is active even when plasma GLP‐1 levels are low and unchanging. Consistent with these observations, deletion of the GLP‐1r from islet β‐cells causes intolerance after IP or IV glucose, challenges that do not induce GLP‐1 secretion. Taken together, these data support a model whereby GLP‐1 acts through neural or paracrine mechanisms to regulate physiologic insulin secretion. In contrast, bariatric surgery seems to be a condition in which circulating GLP‐1 could have an endocrine effect. Both gastric bypass and sleeve gastrectomy are associated with substantial increases in postprandial GLP‐1 release and in these conditions interference with GLP‐1r signaling has a significant impact on glucose regulation after eating. Thus, with either bariatric surgery or treatment with long‐acting GLP‐1r agonists, circulating peptide mediates insulinotropic activity. Overall, a case can be made that physiologic actions of GLP‐1 are not hormonal, but that an endocrine mechanism of GLP‐1r activation can be co‐opted for therapeutics.
Keywords: Glucagon‐like peptide‐1, Incretin, Insulin secretion
The incretin effect: regulation of insulin secretion by gastrointestinal peptides
Healthy humans maintain blood glucose within relatively tight limits, a feat of homeostasis that is particularly remarkable considering the wide variations in the amount and timing of carbohydrate intake. An essential feature of the maintenance of glucose tolerance is the ability of the endocrine pancreas to rapidly and accurately secrete insulin in amounts appropriate to the size of the meal. Fundamental to this regulation is the incretin effect, signals initiated in the gastrointestinal tract in response to nutrient ingestion that augment insulin secretion1, 2. First described in the early 1960s, the incretin effect has been ascribed primarily to two peptides produced by endocrine cells of the intestinal mucosa, glucose‐dependent insulinotropic polypeptide (GIP) and glucagon‐like peptide 1 (GLP‐1)1, 2, 3. The two incretins are secreted in response to glucose, lipid or mixed meal ingestion in amounts proportional to meal size, and stimulate insulin secretion in the presence of elevated blood glucose levels1, 2, 3. This feed‐forward system allows for the rapid and appropriate β‐cell response necessary to control postprandial blood glucose.
GLP‐1 and GIP share several characteristics beyond synthesis in the gut. Both signal through family B G‐protein coupled receptors expressed on islet β‐cells, stimulate insulin release only in the presence of supra‐basal glucose concentrations, and have their insulinotropic effect inactivated by the enzyme dipeptidyl peptidase‐4 (DPP‐4). However, there are several major differences between GIP and GLP‐1 with regard to blood glucose control. First, GLP‐1 suppresses glucagon secretion, complementing its effect to stimulate insulin, and coordinating the islet hormones to lower blood glucose1, 2; in contrast, GIP stimulates glucagon release4. Second, GIP shows a large dynamic range of postprandial secretion, approximately 10‐fold, whereas changes in plasma GLP‐1 are modest, 1.5–2‐fold (Figure 1)1, 5, 6. Finally, the effects of GLP‐1 to stimulate insulin secretion and reduce blood glucose are retained in diabetic humans, whereas GIP has a muted effect in the setting of chronic hyperglycemia3, 7.
Figure 1.
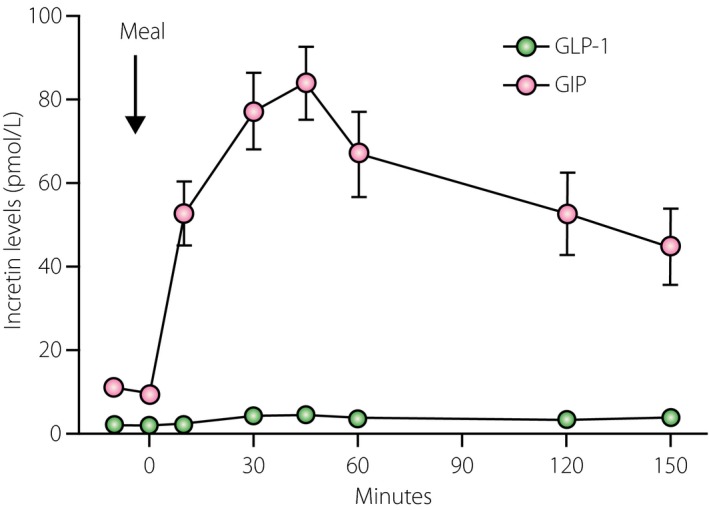
Plasma levels of glucagon‐like peptide‐1 (GLP‐1) and glucose‐dependent insulinotropic polypeptide (GIP) in healthy subjects after a 450‐kcal liquid mixed nutrient meal. Plasma GIP concentrations increased approximately 10‐fold and plasma GLP‐1 increased twofold after the meal. Adapted from Salehi et al.48
[Correction added on 12 April 2016, after first online publication: The colours indicating GLP‐1 and GIP have been swapped.]
GLP‐1 was discovered incidentally after the cloning of proglucagon, and has been one of the most studied regulatory peptides in the field of metabolism over the past three decades2. There is a body of convincing experimental evidence showing that GLP‐1 is essential for normal glucose tolerance8, 9, 10; simply put, interference with GLP‐1 signaling by a variety of interventions causes glucose intolerance. Unlike many previously identified gastrointestinal peptides that were insulinotropic in vitro or in healthy humans, but ineffective in persons with diabetes1, 3, GLP‐1 has potent antidiabetic effects. Infusion of GLP‐1 lowers fasting hyperglycemia11, 12, and corrects abnormal insulin secretion7, 13, 14 in persons with type 2 diabetes. Indeed, it is this remarkable effectiveness in diabetic patients that has led to the rapid development of drugs for diabetes based on GLP‐115, 16. Taken as a whole, basic and clinical investigation of the GLP‐1 system has been one of the most important and rapidly moving areas of diabetes‐related research in the recent past.
Is GLP‐1 a hormone? Problems with the classical model
GLP‐1 is cleaved from proglucagon in specific intestinal enterocytes called L cells, and secreted primarily as an amidated 30‐amino acid peptide GLP‐1(7‐36)NH2 1, 17. It is widely believed that L cells account for almost all of the GLP‐1 in the circulation. On reaching the bloodstream, GLP‐1 has access to a specific GLP‐1 receptor (GLP‐1r) that is expressed in a wide range of target tissues including pancreatic β‐cells, a subset of pulmonary epithelial cells, cells lining the gastric pits and small intestinal mucosa, atrial cardiac myocytes, and neurons in several brain regions and the afferent vagus18, 19. Based on the classical model of the incretin effect, it has been widely assumed that GLP‐1 interacts with these diffuse GLP‐1r through the circulation as a hormone.
There are several problems with a strictly endocrine mechanism to explain the multiple actions of GLP‐1. First, GLP‐1 circulates in relatively low concentrations compared with other gastrointestinal hormones, such as GIP and peptide tyrosine‐tyrosine (PYY)5, 6, 20. Furthermore, the changes in plasma GLP‐1 after eating, the principal stimulus for its release, are very modest5, 6. The narrow range of plasma GLP‐1 concentrations in physiological settings is at odds with experimental data showing a broad dynamic range of action1, 2, 3. Indeed, in healthy humans the insulinotropic effect of GLP‐1 continues to increase in a near exponential profile at plasma levels five‐ to sixfold the upper level of the physiological range21. The discordance between concentrations of circulating peptide and the magnitude of its effects is the first challenge to the conventional view that GLP‐1 acts as a hormone.
A second, and perhaps more compelling reason to question an endocrine mechanism of GLP‐1 action is its rapid inactivation in the circulation. GLP‐1 is metabolized by the ubiquitous enzyme DPP‐4, which cleaves the N‐terminal dipeptide His‐Ala leaving the circulating congener GLP‐1(9‐36)NH2 22, 23, 24. Metabolism of GLP‐1 by DPP‐4 results in a plasma half‐life of 1–2 min in mammals23, 24, 25, and as GLP‐1(9‐36)NH2 is inactive, this process severely attenuates the overall effect of circulating GLP‐124. It has been estimated that considerable amounts of GLP‐1 are inactivated by the time peptide secreted from the gut reaches the portal vein, and that only a minor fraction of secreted GLP‐1 remains intact by the time it reaches the arterial circulation26. Taken together, these observations raise serious arguments against the traditional endocrine model of the incretin action of GLP‐127, 28, 29. This presents a very fundamental question: How are the diverse actions of GLP‐1 mediated if not by direct actions of circulating peptide on target tissues?
Production of GLP‐1 by the islet α‐cell
After the discovery of GLP‐1 in the mid‐1980s, several groups sought to determine the site of its production30, 31, 32. Early studies focused on differential proglucagon processing by the three cell types that express proglucagon: α‐ and L cells, and neurons. Typical studies made use of extracts from the intestine and pancreas that were fractionated using chromatography, and the products analyzed by radioimmunoassay30. This process identified two patterns of proglucagon processing: an α‐cell profile with the majority of N‐terminal proglucagon processed into glucagon, and the bulk of C‐terminal prohormone left unprocessed as an inactive peptide containing both GLP‐1 and GLP‐2; and an intestinal/brain profile with the majority of N‐terminal proglucagon unprocessed in forms such as glicentin and oxyntomodulin, whereas the C‐terminal portion was cleaved into the bioactive GLPs. The simple explanation for this dichotomization was that α‐cells synthesize proconvertase (PC) 2, and L cells and neurons synthesize PC1/3, giving uniform processing of proglucagon to distinct products33, 34, 35, 36. Although this model is generally accurate, it has been incorrectly extrapolated to mean that α‐cells produce only glucagon and no GLP‐1, and that the brain and intestine make only GLP‐1. New findings suggest that this extrapolation needs to be reconsidered.
Mice with specific deletions of PC1/335 or PC236 show the dominant role of these enzymes in the processing of Proglucagon to GLP‐1 and glucagon, respectively. However, the absence of the dominant proconvertase unmasks a small amount of PC1/3 activity and GLP‐1 production in α‐cells, and PC2 activity and glucagon production in intestinal endocrine cells. Other groups have confirmed these findings by measuring GLP‐1 in α‐ and β‐cell lines, and in isolated islets36, 37. Masur et al.37 showed expression of PC1/3 in α‐cells, release of fully processed, bioactive GLP‐1 in culture, and reduction of insulin secretion and β‐cell growth from cultured islets treated with a GLP‐1r antagonist. These authors concluded that GLP‐1 is produced locally in the islet, where it plays a role in β‐cell function. The potential for a physiological action of islet GLP‐1 was perhaps best shown in the response to β‐cell stress. Nie et al.38 treated rats with streptozotocin for 5 days and analyzed islet constituents. They noted increased expression of PC1/3 throughout the islet, including a 10‐fold increase in α‐cells38. Coincident with this, there was an increase in islet GLP‐1 immunoreactivity, and a doubling of the GLP‐1/glucagon ratio in islet extracts. Although in absolute terms islet GLP‐1 concentrations were only a small fraction of glucagon content, streptozotocin treatment led to a 2.5‐fold increase in plasma GLP‐1(7‐36). These findings support a model whereby β‐cell injury induces increased local production of GLP‐1. This model is supported by the findings of other investigators who showed increased production of PC1/3 and GLP‐1 in the α‐cells of mice with insulin resistance generated by a variety of conditions39.
More recently, Ellingsgaard et al. showed that the cytokine interleukin (IL)‐6 stimulates Proglucagon expression and GLP‐1 production in murine L‐ and α‐cells40. These effects of IL‐6 increased insulin secretion and improved glucose tolerance, and were dependent on signaling through the GLP‐1r. In cultured α‐cells, IL‐6 increased GLP‐1 secretion in a dose‐dependent manner, but increased glucagon release only in the setting of low glucose. Importantly, the effects shown in vivo and ex vivo in mice were replicated in cultured human islets and isolated primary α‐cells. The authors of that study proposed that IL‐6 served as a link between insulin‐sensitive skeletal muscle and the islet through stimulation of local GLP‐1, mediated by paracrine signaling.
Although the experimental evidence supporting paracrine regulation of β‐cells by GLP‐1 from α‐cells comes from studies in rodents, it is important to note that the architecture of human islets actually provides a superior design for this mechanism41, 42. The distribution of α‐cells in a rim on the outside of the islet is a distinctive aspect of rodent islets. In contradistinction, α‐cells in human islets are mixed into the body of the islet and are thus in a anatomic position to facilitate cell–cell interactions. The proposition that α‐cells regulate β‐cells would seem to fly in the face of another established physiological concept, namely that blood flow is from the core of the islet to the periphery43. However, that model addresses only the regulatory behavior of islet hormones secreted into the circulation, and cannot be applied to intercellular or interstitial communication. Finally, although there is now support for the synthesis and release of GLP‐1 within the islet, it is not clear whether there is DPP‐4 activity in this compartment to inactivate the peptide. Studies have shown considerable DPP‐4 activity in extracts of pancreas and islets22, 44, but it is unclear how much of this resides in the substance of the islet and how much in the endovasculature.
Effects of GLP‐1 on insulin secretion in the fasting state
An essential, and definitional, aspect of incretins is that they stimulate insulin secretion during nutrient absorption, and in fact both GLP‐1 and GIP were initially classified as incretins, because plasma levels rose after food intake. In the case of GIP, evidence from animal studies is consistent with postprandial endocrine actions. For example, mice with a genetic deletion of the GIP receptor are more hyperglycemic than controls after enteral, but not parenteral, glucose challenges45; the strong inference here is that the effect of removing GIP signaling is only evident after a meal when plasma concentrations are elevated, but not during IP glucose when GIP levels are low and unchanging. These observations are in contrast to findings in mice with GLP‐1r knockouts. In these animals, clearance of glycemia is abnormal whether measured after IP or oral administration of glucose8. This point has been extended in studies of mice with a selective deletion of the GLP‐1r in β‐cells46. These mice have normal glucose tolerance after gavage of glucose or ingestion of a liquid meal, but hyperglycemia can be induced by blocking extra‐islet GLP‐1r with exendin‐(9‐39), a peptide antagonist. In contrast to the normal response to oral glucose, the β‐cell GLP‐1r knockouts have elevated fasting glucose levels and impaired clearance of an IP glucose load. These findings show that glucose tolerance is not affected by interference of direct effects of circulating GLP‐1 on β‐cells, but that GLP‐1r signaling is important for a normal insulin response to glucose regulation in the fasting state.
The findings in GLP‐1r knockout mice are supported by studies in humans. Infusion of the GLP‐1r antagonist exendin‐(9‐39) reduces the insulin response to i.v. glucose in fasted humans47, 48, 49. In these studies, exendin‐(9‐39) reduced insulin secretion by 30–40% whether glucose was infused i.v. alone or during oral glucose ingestion (Figure 2)48, 49. Thus, comparable effects were observed with GLP‐1r blockade both when plasma GLP‐1 levels were low and unchanging, and when they were increased by the test meal. Although it is possible that fasting GLP‐1 concentrations are sufficient to have a tonic effect on β‐cell secretion, this seems unlikely, as infusion of exogenous GLP‐1 does not stimulate insulin release until plasma concentrations reach the prandial range21.
Figure 2.
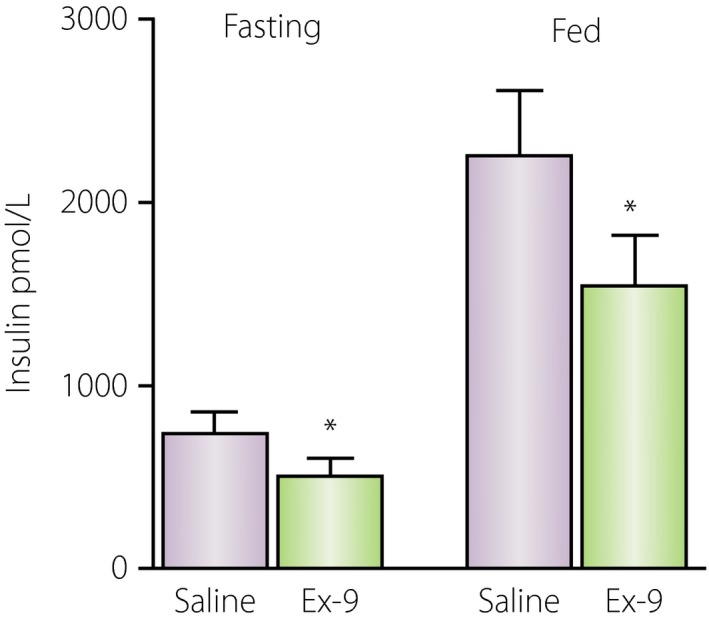
Effect of exendin‐(9‐39) to suppress insulin secretion at equivalent levels of glycemia in fed and fasted subjects. In either setting, exendin‐(9‐39) reduced glucose‐stimulated insulin secretion by approximately 30%. Ex‐9, exendin‐(9‐39). Adapted from48 with permission.
Taken together, findings from studies of transgenic mice and humans show that interference with GLP‐1r signaling impairs insulin secretion independent of changes in plasma GLP‐1 concentration. In the context of recent observations that GLP‐1 is made in the islet, these results raise the possibility that a paracrine, α‐cell to β‐cell system of communication exists to regulate insulin release. In this model, islet GLP‐1 could provide tonic support for β‐cell function as a mechanism of chronic adaptation to environmental perturbation. In fact, the fasting GLP‐1 effect; that is, the degree to which exendin‐(9‐39) reduces insulin secretion in the absence of elevated circulating GLP‐1, is greater in diabetic and obese patients than in lean subjects (Figure 3)48, 49, 50.
Figure 3.
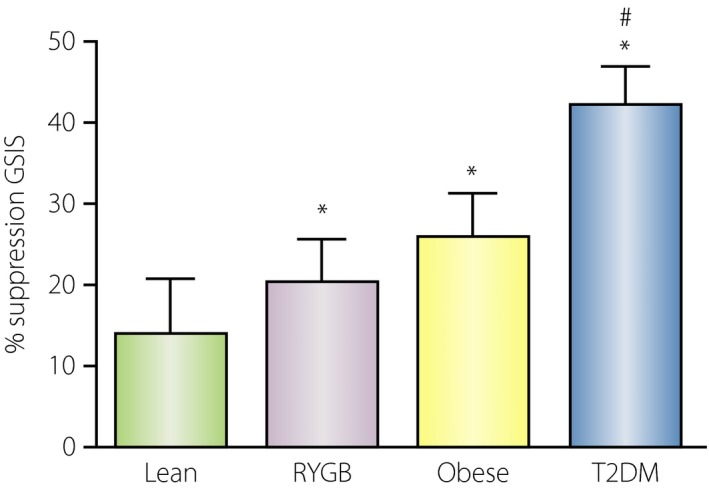
Effect of glucagon‐like peptide‐1 (GLP‐1) receptor blockade during hyperglycemic clamps in fasted subjects. The relative effect was largest in subjects with type 2 diabetes (T2DM), followed by obese subjects, individuals with Roux‐en‐Y gastric bypass (RYGB) and lean healthy subjects. *Significant differences (P < 0.05) between control and exendin‐(9‐39) study; #significant difference from other groups (P < 0.05). Glucose‐stimulated insulin secretion (GSIS). Adapted from Salehi et al.48, 49, 50 with permission.
Case for GLP‐1 as a hormone: bariatric surgery
While the notion that gut‐derived GLP‐1 acts on distant pancreatic β‐cells is open to skepticism, there are settings where an endocrine action is more plausible. Patients undergoing bariatric surgical procedures, such as gastric bypass or sleeve gastrectomy, have massive elevations of plasma GLP‐1 after meals. Indeed, levels can be increased 10–20‐fold those of unoperated control subjects. In these individuals, it is more realistic to conceive of sufficient unmetabolized, plasma GLP‐1 reaching the islet to directly stimulate β‐cells. In fact, patients with gastric bypass given exendin‐(9‐39) have a two‐ to threefold greater reduction of meal‐induced insulin secretion compared with controls, suggesting a greater GLP‐1 effect that coincides with higher plasma concentrations49. Furthermore, GLP‐1r blockade can correct the syndrome of hyperinsulinemic hypoglycemia that affects a discrete population of gastric bypass patients50; this finding also implicates augmented GLP‐1 action after weight loss surgery.
Summary
The discovery of GLP‐1 and subsequent rediscovery of the incretin effect have been major themes in diabetes research over the past two decades. It seems clear that GLP‐1 is essential for normal glucose tolerance, and in experimental and clinical settings has marked effects on glucose regulation in persons with type 2 diabetes. However, the principle mechanism of action of GLP‐1 has recently been questioned, with a solid body of evidence that can be arrayed against a primary endocrine effect. Coincident with these findings are new observations that suggest significant α‐cell production of GLP‐1 and a physiological role of local islet peptide through paracrine signaling. The major implication of these findings is that there are important effects of GLP‐1r signaling that occur independently of plasma levels of peptide. Understanding the full range of physiological GLP‐1 actions is important in that this pathway has been successfully targeted to treat diabetes, but the full potential of this mechanism has not yet been realized.
Disclosure
The authors declare no conflict of interest.
Acknowledgment
This work was supported in part by PHS grant R01DK101991.
J Diabetes Investig 2016; 7: 50–55
This article is based on the presentations given by the authors at a symposium, Incretin 2015, July 29–31, 2015, Vancouver, BC Canada.
References
- 1. Kieffer TJ, Habener JF. The glucagon‐like peptides. Endocr Rev 1999; 20: 876–913. [DOI] [PubMed] [Google Scholar]
- 2. Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab 2013; 17: 819–837. [DOI] [PubMed] [Google Scholar]
- 3. Creutzfeldt W, Nauck M. Gut hormones and diabetes mellitus. Diabetes Metab Rev 1992; 8: 149–177. [DOI] [PubMed] [Google Scholar]
- 4. Holst JJ, Christensen M, Lund A, et al Regulation of glucagon secretion by incretins. Diabetes Obes Metab 2011; 13(Suppl 1): 89–94. [DOI] [PubMed] [Google Scholar]
- 5. Vilsboll T, Krarup T, Deacon CF, et al Reduced postprandial concentrations of intact biologically active glucagon‐like peptide 1 in type 2 diabetic patients. Diabetes 2001; 50: 609–613. [DOI] [PubMed] [Google Scholar]
- 6. Vilsboll T, Krarup T, Sonne J, et al Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes. JCEM 2003; 88: 2706–2713. [DOI] [PubMed] [Google Scholar]
- 7. Nauck MA, Heimesaat MM, Orskov C, et al Preserved incretin activity of GLP‐1[7‐36 amide] but not of synthetic human GIP in patients with type‐2 diabetes mellitus. J Clin Invest 1993; 91: 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scrocchi LA, Brown TJ, MaClusky N, et al Glucose intolerance but normal satiety in mice with a null mutation in the GLP‐1 receptor gene. Nat Med 1996; 2: 1254–1258. [DOI] [PubMed] [Google Scholar]
- 9. D'Alessio DA, Vogel R, Prigeon R, et al Elimination of the action of GLP‐1 causes an impairment of glucose tolerance after nutrient ingestion by healthy baboons. J Clin Invest 1996; 97: 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Edwards CM, Todd JF, Mahmoudi M, et al Glucagon‐like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9‐39. Diabetes 1999; 48: 86–93. [DOI] [PubMed] [Google Scholar]
- 11. Nauck MA, Kleine N, Orskov C, et al Normalization of fasting hyperglycaemia by exogenous glucagon‐like peptide 1 (7‐36 amide) in type 2 (non‐insulin‐dependent) diabetic patients. Diabetologia 1993; 36: 741–744. [DOI] [PubMed] [Google Scholar]
- 12. Rachman J, Barrow B, Levy JC, et al Near‐normalisation of diurnal glucose concentrations by continuous administration of glucagon‐like peptide‐1 (GLP‐1) in subjects with NIDDM. Diabetologia 1997; 40: 205–211. [DOI] [PubMed] [Google Scholar]
- 13. Quddusi S, Vahl TP, Hanson K, et al Differential effects of acute and extended infusions of GLP‐1 on first‐ and second‐phase insulin secretion in diabetic and nondiabetic humans. Diabetes Care 2003; 26: 791–798. [DOI] [PubMed] [Google Scholar]
- 14. Schirra J, Nicolaus M, Roggel R, et al Endogenous glucagon‐like peptide 1 controls endocrine pancreatic secretion and antro‐pyloro‐duodenal motility in humans. Gut 2006; 55: 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahren B. Islet G protein‐coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov 2009; 8: 369–385. [DOI] [PubMed] [Google Scholar]
- 16. Drucker DJ, Nauck MA. The incretin system: glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696–1705. [DOI] [PubMed] [Google Scholar]
- 17. Orskov C, Rabenhøj L, Wettergren A, et al Tissue and plasma concentrations of amidated and glycine‐extended glucagon‐like peptide I in humans. Diabetes 1994; 43: 535–539. [DOI] [PubMed] [Google Scholar]
- 18. Pyke C, Heller RS, Kirk RK, et al GLP‐1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 2014; 155: 1280–1290. [DOI] [PubMed] [Google Scholar]
- 19. Richards P, Parker HE, Adriaenssens AE, et al Identification and characterization of GLP‐1 receptor‐expressing cells using a new transgenic mouse model. Diabetes 2014; 63: 1224–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adrian TE, Ferri GL, Bacarese‐Hamilton AJ, et al Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 1985; 89: 1070–1077. [DOI] [PubMed] [Google Scholar]
- 21. Aulinger BA, Vahl TP, Wilson‐Pérez HE, et al β‐cell sensitivity to GLP‐1 in healthy humans is variable and proportional to insulin sensitivity. JCEM 2015; 100: 2489–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mentlein R, Dipeptidyl‐peptidase IV. (CD26)–role in the inactivation of regulatory peptides. Regul Pept 1999; 85: 9–24. [DOI] [PubMed] [Google Scholar]
- 23. Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon‐like peptide‐1 by human plasma in vitro yields an N‐terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab 1995; 80: 952–957. [DOI] [PubMed] [Google Scholar]
- 24. Vahl TP, Paty BW, Fuller BD, et al Effects of GLP‐1‐(7‐36)NH2, GLP‐1‐(7‐37), and GLP‐1‐ (9‐36)NH2 on intravenous glucose tolerance and glucose‐induced insulin secretion in healthy humans. J Clin Endocrinol Metab 2003; 88: 1772–1779. [DOI] [PubMed] [Google Scholar]
- 25. Jessen L, Aulinger BA, Hassel JL, et al Suppression of food intake by glucagon‐like peptide‐1 receptor agonists: relative potencies and role of dipeptidyl peptidase‐4. Endocrinology 2012; 153: 5735–5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hansen L, Deacon CF, Orskov C, et al Glucagon‐like peptide‐1‐(7‐36)amide is transformed to glucagon‐like peptide‐1‐(9‐36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 1999; 140: 5356–5363. [DOI] [PubMed] [Google Scholar]
- 27. Holst JJ, Deacon CF. Glucagon‐like peptide‐1 mediates the therapeutic actions of DPP‐IV inhibitors. Diabetologia 2005; 48: 612–615. [DOI] [PubMed] [Google Scholar]
- 28. D'Alessio DA. What if gut hormones aren't really hormones. DPP‐4 inhibition and local action of GLP‐1 in the gastrointestinal tract. Endocrinology 2011; 152: 2925–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Donath MY, Burcelin R. GLP‐1 effects on islets: hormonal, neuronal, or paracrine? Diabetes Care 2013; 36 (Suppl 2): S145–S148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holst JJ, Bersani M, Johnsen AH, et al Proglucagon processing in porcine and human pancreas. J Biol Chem 1994; 269: 18827–18833. [PubMed] [Google Scholar]
- 31. Mojsov S, Heinrich G, Wilson IB, et al Preproglucagon gene expression in pancreas and intestine diversifies at the level of post‐translational processing. J Biol Chem 1986; 261: 11880–11889. [PubMed] [Google Scholar]
- 32. Orskov C, Holst JJ, Poulsen SS, et al Pancreatic and intestinal processing of proglucagon in man. Diabetologia 1987; 30: 874–881. [DOI] [PubMed] [Google Scholar]
- 33. Rouille Y, Martin S, Steiner DF. Differential processing of proglucagon by the subtilisin‐like prohormone convertases PC2 and PC3 to generate either glucagon or glucagon‐like peptide. J Biol Chem 1995; 270: 26488–26496. [DOI] [PubMed] [Google Scholar]
- 34. Furuta M, Yano H, Zhou A, et al Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proc Natl Acad Sci 1997; 94: 6646–6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ugleholdt R, Zhu X, Deacon CF, et al Impaired intestinal proglucagon processing in mice lacking prohormone convertase 1. Endocrinology 2004; 145: 1349–1355. [DOI] [PubMed] [Google Scholar]
- 36. Webb GC, Dey A, Wang J, et al Altered proglucagon processing in an alpha‐cell line derived from prohormone convertase 2 null mouse islets. J Biol Chem 2004; 279: 31068–31075. [DOI] [PubMed] [Google Scholar]
- 37. Masur K, Tibaduiza EC, Chen C, et al Basal receptor activation by locally produced glucagon‐like peptide‐1 contributes to maintaining beta‐cell function. Mol Endocrinol 2005; 19: 1373–1382. [DOI] [PubMed] [Google Scholar]
- 38. Nie Y, Nakashima M, Brubaker PL, et al Regulation of pancreatic PC1 and PC2 associated with increased glucagon‐like peptide 1 in diabetic rats. J Clin Invest 2000; 105: 955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kilimnik G, Kim A, Steiner D, et al Intraislet production of GLP‐1 by activation of prohormone convertase 1/3 in pancreatic α‐cells in mouse models of & #x03B2;‐cell regeneration. Islets 2010; 2: 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ellingsgaard H1, Hauselmann I, Schuler B, et al Interleukin‐6 enhances insulin secretion by increasing glucagon‐like peptide‐1 secretion from L cells and alpha cells. Nat Med 2011; 17: 1481–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cabrera O, Berman DM, Kenyon NS, et al The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA 2006; 103: 2334–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marchetti P, Lupi R, Bugliani M, et al A local glucagon‐like peptide 1 (GLP‐1) system in human pancreatic islets. Diabetologia 2012; 55: 3262–3272 [DOI] [PubMed] [Google Scholar]
- 43. Menger MD, Vajkoczy P, Beger C, et al Orientation of microvascular blood flow in pancreatic islet isografts. J Clin Invest 1994; 93: 2280–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mentzel S, Dijkman HB, Van Son JP, et al Organ distribution of aminopeptidase A and dipeptidyl peptidase IV in normal mice. J Histochem Cytochem 1996; 44: 445–461. [DOI] [PubMed] [Google Scholar]
- 45. Miyawaki K, Yamada Y, Yano H, et al Glucose intolerance caused by a defect in the entero‐insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci U S A. 1999; 96: 14843–14847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smith EP, An Z, Wagner C, et al The role of β cell glucagon‐like peptide‐1 signaling in glucose regulation and response to diabetes drugs. Cell Metab 2014; 19: 1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schirra J, Sturm K, Leicht P, et al Exendin(9‐39)amide is an antagonist of glucagon‐like peptide‐1(7‐36)amide in humans. J Clin Invest 1998; 101: 1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Salehi M, Aulinger B, Prigeon RL, et al Effect of endogenous GLP‐1 on insulin secretion in type 2 diabetes. Diabetes 2010; 59: 1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Salehi M, Prigeon RL, D'Alessio DA. Gastric bypass surgery enhances glucagon‐like peptide 1 stimulated postprandial insulin secretion in humans. Diabetes 2011; 60: 2308–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Salehi M, Gastaldelli A, D'Alessio DA. Blockade of glucagon‐like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology 2014; 146: 669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]


