Abstract
Liraglutide is a glucagon‐like peptide‐1 receptor (GLP‐1R) agonist marketed for the treatment of type 2 diabetes. Besides lowering blood glucose, liraglutide reduces bodyweight, and has recently also been approved for the obesity indication. Acutely, GLP‐1 markedly reduces gastric emptying, and this effect was previously believed to at least partly explain the effect on bodyweight loss. However, recent studies in both humans and animals have shown that GLP‐1R agonists, such as liraglutide, that lead to pharmacological concentrations for 24 h/day only have a minor effect on gastric emptying; such an effect is unlikely to have lasting effects on appetite reduction. Liraglutide has been shown to have direct effects in the arcuate nucleus of the rodent brain, activating pro‐opiomelanocortin neurons and increasing levels of the cocaine‐ and amphetamine‐stimulated transcript neuropeptide messenger ribonucleic acid, which correlate nicely to clinical studies where liraglutide was shown to increase feelings of satiety. However, despite the lack of a GLP‐1R on agouti‐related peptide/neuropeptide Y neurons, liraglutide also was able to prevent a hunger associated increase in agouti‐related peptide and neuropeptide Y neuropeptide messenger ribonucleic acid, again with a strong correlation to clinical studies that document reduced hunger feelings in patients while taking liraglutide. Studies using fluorescent labeled liraglutide, as well as other GLP‐1R agonists, and analysis using single‐plane illumination microscopy show that such medium‐sized peptide‐based compounds can directly access not only circumventricular organs of the brain, but also directly access discrete regions in the hypothalamus. The direct effects of long‐acting GLP‐1R agonists in the hypothalamus are likely to be an important new pathway in understanding GLP‐1R agonist mediated weight loss.
Keywords: Arcuate nucleus, Liraglutide, Obesity
Introduction
Glucagon‐like peptide‐1 (GLP‐1) was first identified in 1983, cloned by Graham Bell as part of the pre‐proglucagon sequence, with the incretin effect being published in 19871, 2, 3. As the other incretin, glucose‐dependent insulinotropic polypeptide (GIP), has little effect on insulin secretion in patients with type 2 diabetes, drugs giving pharmacological levels of GLP‐1 receptor (GLP‐1R) agonists have become successful therapies in the treatment of type 2 diabetes, providing glucose control as well as weight loss4. GLP‐1 was first shown to lower food intake in rodent animal models in 1996, followed by a study in humans in 19985, 6, 7. The study in humans showed a reduction in energy intake, and also reported that the mechanism involved a reduction in appetite along with an increase in satiety and a reduction in feelings of hunger, as shown by use of a visual analog scale questionnaire7. The most recently approved GLP‐1R agonists for management of type 2 diabetes are dulaglutide and albiglutide, which have been developed for once‐weekly administration. However, as more compounds are approved and results from an increasing number of large, randomized, controlled, double‐blinded trials have been published, treatment‐associated weight loss in patients with type 2 diabetes differs between compounds. Liraglutide is a once‐daily compound acylated with a fatty acid to facilitate non‐covalent binding to albumin in vivo as the protraction mechanism8. Although there were some differences in glycemic control, a consistent finding was that liraglutide resulted in greater weight loss than dulaglutide and albiglutide, which are much larger molecules modified with covalent addition of either a crystallizable fragment or an albumin molecule8, 9, 10. Furthermore, of the five GLP‐1R agonists now approved in treatment of type 2 diabetes in various regions of the world, liraglutide is the only one that has also been investigated and approved for weight management, as adjunct to diet and exercise11. The present mini‐review focuses on a novel pathway in the brain that might mediate the weight‐lowering effect of GLP‐1R agonists, and reviews these data in relation to the rather large existing literature for GLP‐1R action in the brain as well as in the periphery12.
Liraglutide directly accesses the rodent brain
Using the novel technique of single‐plane illumination microscopy (SPIM), whereby the entire perfused brain is scanned after peripheral administration of fluorescently labeled liraglutide (liraglutide750) in live mice, two‐dimensional digital images of the entire brain were obtained and assembled into a three‐dimensional image for optimal analysis of spatial distribution. The images were analyzed carefully, and the anatomical location of liraglutide750 described. Figure 1 shows a dorsoventral (Figure 1a), or sagittal (Figure 1b), three‐dimensional plane view of liraglutide750 distribution in the mouse brain. As can be seen, liraglutide750 was distributed to discrete regions of the brain. Regions with clear uptake were determined to be the paraventricular nucleus of the hypothalamus (PVN; Figure 1c,d); the arcuate nucleus (ARC) and the median eminence (ME) of the hypothalamus (Figure 1f,g); and the area postrema (AP) in the hindbrain, whereas there was no uptake in the nucleus tractus solitarus in the hindbrain (Figure 1i,j). The signal in all sites of uptake were found to depend entirely on expression of the GLP‐1R, as no uptake was detectable at these sites in GLP‐1R−/− mice (Figure 1e,h,k). Uptake was also found in the organum vasculosum of the lamina terminalis, the subfornical organ, the supraoptic nucleus and the supraoptic decussation, as well as the choriod plexus. Except for the choriod plexus, the signal in these regions also depends on the GLP‐1R. The uptake patterns for liraglutide in the pancreas and the hypothalamus were compared with the patterns of the specific GLP‐1R antagonist exendin(9‐39; Figure 2). A different flourophore was used for these experiments, enabling co‐staining for insulin or cocaine‐ and amphetamine‐stimulated transcript (CART), respectively. In the pancreas, liraglutide594 was found to co‐localize entirely with pancreatic β‐cells, as evidenced by an overlap with insulin staining. Also, the liraglutide594 signal was present within the cells as evidence of internalization with the GLP‐1R (Figure 2i,j). In contrast, exendin(9‐39)594 was retained at the plasma membrane of the β‐cells (Figure 2k,l). In the brain, liraglutide594 also appeared to be present within the neurons (Figure 2m,n), whereas exendin(9‐39)594 appeared to be retained at the membrane. However, in the brain, internalization is more difficult to assess because of the high density of neuronal fibers. To further examine the precise localization of liraglutide594 in the ARC, co‐staining with the CART neuropeptide that labels CART/pro‐opiomelanocortin (POMC) neurons was carried out (Figure 3). Liraglutide594 was detected specifically in the ARC in the cytoplasm of neurons positive for CART (Figure 3). Nearly all CART‐positive cells were positive for liraglutide594. However, a few cells were only positive for liraglutide594, showing that another cell type might be targeted by liraglutide in the hypothalamus.
Figure 1.
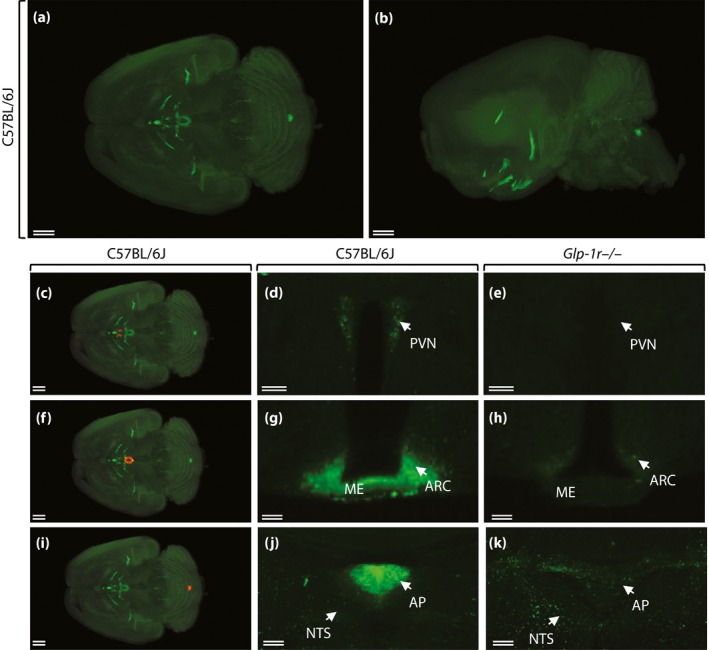
Distribution of fluorescently labeled liraglutide in the mouse brain. Representative whole brain images viewed in the (a) dorsoventral or (b) sagittal plane from C57BL/6J mice given liraglutide750 (unspecific staining has been removed from the left side of the brain). The brain tissue was scanned at 620 nm and 710 nm, representing both autofluorescence from the tissue (gray) and specific signal (green). The (c, f, i) red regions are shown at (d, g, j) higher magnification, respectively. (d, e, g, h, j, k) High‐magnification views of a single section from (d, g, j) C57BL/6J or (e, h, k) Glp1r −/− mice given liraglutide750. Liraglutide750 was detectable in (c, d) paraventricular nucleus of the hypothalamus (PVN), (f, g) the median eminence (ME), the arcuate nucleus (ARC), and (i, j) area postrema (AP). (e, h, k) In mice lacking a functional glucagon‐like peptide‐1 receptor (GLP‐1R), no liraglutide750 signal could be detected in any of these regions. Scale bars, 200 μm (a, b, c, f, i); 50 μm (d, e); 100 μm (g, h, j, k). ©American Society for Clinical Investigation and reproduced from Secher et al.12 with permission.
Figure 2.
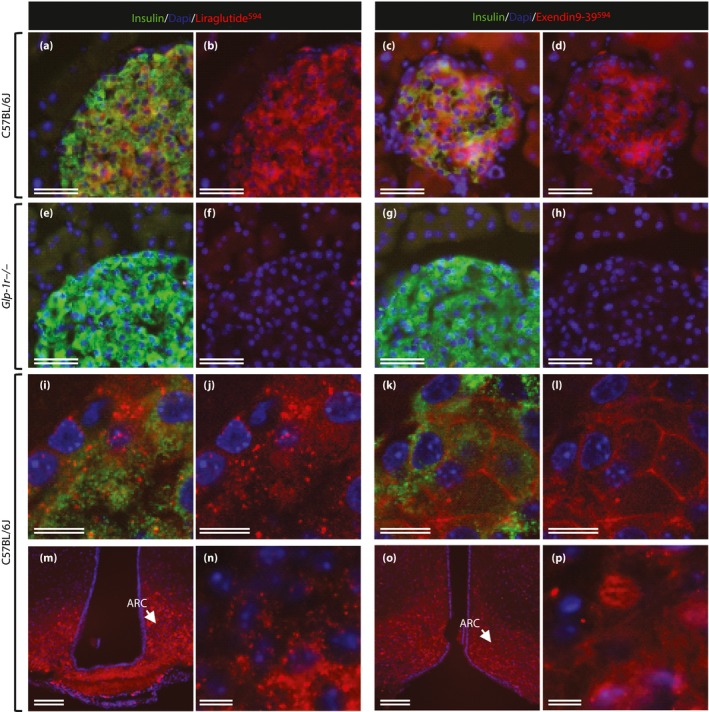
Distribution of liraglutide594 or exendin(9‐39)594 in the pancreas and brain. (a–l) Representative images of mouse islets stained with Hoechst nuclear stain (blue), insulin (green) and liraglutide594/exendin(9‐39)594 (red). (a, c, e, d) Insulin‐ and liraglutide594/exendin(9‐39)594‐positive cells. (b, d, f, h) The same images as in (a, c, e, d) with only Hoechst and liraglutide594/exendin(9‐39)594 signal. (a–d) In C57BL/6J mice, both liraglutide594 and exendin(9‐39)594 were detected in cells expressing insulin; (e–h) however, in mice lacking a functional glucagon‐like peptide‐1 receptor, no liraglutide594 or exendin(9‐39)594 signal could be detected in insulin expressing β‐cells. (i, j, n) High‐magnification images showed that liraglutide594 was internalized and the fluorescent signal was located in the cytoplasm, (k, l, p) while exendin(9‐39)594 remained at the plasma membrane. In the brain, (m, n) liraglutide594 had access to arcuate nucleus (ARC), in which it bound the glucagon‐like peptide‐1 receptor and internalized, (o, p) whereas exendin(9‐39)594 labeled the same population of cells, but without internalization. Scale bars, 100 μm (m, o), 50 μm (a–h), 10 μm (i–l, n, p). ©American Society for Clinical Investigation and reproduced from Secher et al.12 with permission.
Figure 3.
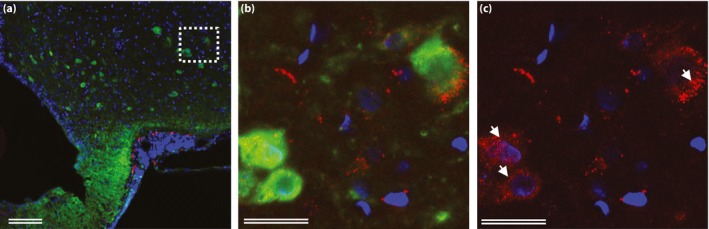
Neuronal accumulation and activity after glucagon‐like peptide‐1 receptor stimulation. (a–c) Hypothalamic sections from rats injected with liraglutide594 (red) and stained with Hoechst nuclear stain (blue) and cocaine‐ and amphetamine‐stimulated transcript (green). (b, c) High‐magnification confocal images showed accumulation of fluoro liraglutide in the cytoplasm of cocaine‐ and amphetamine‐stimulated transcript‐positive cells (arrows). (b) Cocaine‐ and amphetamine‐stimulated transcript and liraglutide594‐positive cells. (c) The same image as in (b) with only liraglutide594 signal. Scale bars, 25 μm (b, c); 100 μm (a). ©American Society for Clinical Investigation and reproduced from Secher et al.12 with permission.
Discussion
It has been suggested that physiologically the inhibitory effect of GLP‐1 on gastric emptying might be more important than the incretin effect13. Delayed gastric emptying leading to prolonged gastric distention might induce short‐term satiety, and could therefore be the relevant mechanism for the postprandial changes in appetite induced by physiological doses of GLP‐1. However, such mechanisms are unlikely to cause the lasting effects on fasting and postprandial appetite seen with treatment with long‐acting GLP‐1R agonists in patients with and without type 2 diabetes14, 15, 16. With continued exposure beyond the duration of a normal postprandial period, the ability of GLP‐1 to delay gastric emptying is much diminished17. Similarly, liraglutide and other long‐acting GLP‐1R agonists have little effect on gastric emptying, making it unlikely that this is the main weight loss mechanism18, 19. The brain is the integrating site for appetite regulation, and numerous studies have documented the expression and importance of GLP‐1Rs in the brain, and its importance in the physiological regulation of appetite20. GLP‐1 is also produced in the hindbrain, and has been proposed as a physiological appetite reduction signal21. Peripherally‐acting GLP‐1R agonists might communicate with the brain through the nodose ganglion where GLP‐1Rs are expressed, either in the form of GLP‐1 released postprandially from the L cells in the gastrointestinal tract or in the form of an exogenously dosed GLP‐1R agonist. Sisley et al.22 described two novel animal models where mice were genetically engineered to be knockdown models for brain or nodose ganglion GLP‐1R expression, respectively. They found that the majority of liraglutide‐induced weight loss required brain GLP‐1R expression, whereas similar weight loss was obtained in normal and nodose ganglion GLP‐1R expression knockdown mice.
Hindbrain GLP‐1Rs expressed in the AP are accessible to peripherally‐dosed liraglutide as shown in Figure 1j. While the exact role of AP GLP‐1Rs have not been determined, numerous studies support the role of hindbrain GLP‐1 and GLP‐1Rs in appetite regulation where they communicate through projections to other sites of the brain, and take part in a complex system integrating homeostatic and likely also hedonic parts of appetite regulation23, 24, 25. Secher et al.12 showed that the AP was not required for the weight loss effect of liraglutide. However, as documented in numerous studies, the hindbrain might still be an important site for integration of physiological effects of GLP‐1; it is just not required for mediating the effects of pharmacological doses of a GLP‐1R agonist on bodyweight loss. Human studies are not available and would be difficult to design.
As also aforementioned, some studies have examined the importance of the vagus nerve in the appetite effect of GLP‐1. Acute dosing studies have shown that the vagus nerve is involved in mediating a reduction in food intake in both rodent models and in humans14, 26. However, studies in animals with chronic exposure have shown that the vagus nerve is not required12, 16. No human studies have used a chronic dosing regime and addressed the importance of the vagus nerve.
The study by Secher et al.12 is the first to show that a peripherally‐dosed GLP‐1R agonist (liraglutide) can directly access and affect the hypothalamus in the rodent brain. Data in that study showed that liraglutide might have important effects on the CART/POMC neurons that it is shown to access directly. Using the native ligand, GLP‐1, GLP‐1Rs in CART/POMC neurons are shown to be activated. Using electrophysiological methods, GLP‐1 was shown to cause dose‐dependent membrane depolarization and an increased firing rate of spontaneous action potentials. Furthermore, the effect was shown to be post‐synaptic, in agreement with a potential direct effect of peripherally‐dosed liraglutide. Importantly, liraglutide was shown to increase the messenger ribonucleic acid levels of CART in the ARC, and to keep levels of neuropeptide Y (NPY) and agouti‐related peptide (AgRP) at the level of normal control animals, whereas reduced‐feed ‘control animals’ weight‐matched to those on liraglutide had increased levels of both NPY and AgRP. The effect on AgRP/NPY is suggested to be indirect inhibition of the AgRP/NPY neurons through a local inhibitory gamma‐aminobutyric acid neuron, as AgRP/NPY neurons do not have GLP‐1Rs. The hypothalamus is a key region in the brain for integration of appetite signals. The main primary neurons in the ARC are the CART/POMC neurons and the AgRP/NPY neurons. Appetite‐inhibiting neurons contain POMC peptides, such as α‐melanocyte‐stimulating hormone and CART. α‐Melanocyte‐stimulating hormone acts on melanocortin anoxigenic MC3 and MC4 receptors. Appetite‐stimulating neurons in the ARC contain NPY, which acts on the orexigenic Y1 and Y5 receptors; and AgRP, which is an antagonist on MC3/4 receptors. NPY and AgRP are the most important regulatory peptides made in the same neurons in the ARC that both act to increase food intake; similarly important are neurons in ARC that co‐express POMC and CART signals, which reduce food intake27, 28, 29, 30, 31. The direct access of liraglutide to this part of the brain, along with the uptake in CART/POMC neurons and the effects of increasing levels of CART, and maintenance of the low levels of NPY and AgRP, highlight a likely important new pathway of how peripherally‐dosed GLP‐1R agonists can regulate appetite. Previously, mainly insulin and leptin have been shown to be transported into the brain, and to have direct effects in the hypothalamus; recent evidence proposes that specialized tanycytes are mediating the uptake through the ME28, 32, 33. Schaeffer et al.34 recently showed circulating acylated ghrelin to be transported to the ARC and to have directs effects there, much like what is now proposed for GLP‐1R agonists, such as liraglutide.
It is likely that the vast distribution of GLP‐1Rs in the brain, along with integration of peripheral signals to the hindbrain, makes up a complicated interplay between the hypothalamus and hindbrain structures, including the nucleus tractus solitarus where GLP‐1 is produced and which receives vagal afferent inputs. Inputs from higher‐order areas of the brain involved in reward and cognition are integrated as well, with resultant effects on meal size and frequency, gut handling of ingested food, and energy expenditure. However, the way native GLP‐1 affects postprandial appetite under physiological conditions is different from the way pharmacological exogenous doses of long‐acting GLP‐1R agonists affect appetite. As such, it is important to discriminate between those studies where GLP‐1 or GLP‐1R agonists are dosed directly into localized places in the brain, and those where pharmacological doses are injected peripherally. With so many different GLP‐1R sites of expression in the brain, it will be very complicated to evaluate the exact importance of each individual site in the total resulting appetite regulation. Use of genetically‐engineered mice might be able to show some further details, but then the precise relationship to humans will need to be investigated.
The hedonic pathways of appetite regulation are likely important for GLP‐1R agonists. In fact, the only striking difference in GLP‐1R expression between rodents and primates in the brain is that there seems to be much more GLP‐1R expression in the primate brain in areas involved in hedonic aspects of food intake, such as the amygdala and the bed nucleus of the stria terminalis35. Although it is unclear how all these signals integrate, GLP‐1 has been shown to reduce both basal and induced reward, and to change food preference in rodent animal models36, 37, 38. Raun et al.36 showed that when rats dosed with liraglutide for 3 months were given a choice between different kinds of candy and chocolate vs normal chow, they selectively chose to eat less chocolate and candy, and more chow. GLP‐1R agonists have been shown to lead to taste aversion in rodents, and to nausea in humans; however, in rodents these effects are very short‐lived (typically only 1–2 days), so the effect shown by Raun et al.36 is unlikely to be a taste aversion effect. Hansen et al.37 compared liraglutide with sibutramine in a model where rats were offered a choice between a chocolate and hazelnut spread (Nutella®; Ferrero SpA, Pino Torinese, Italy)/peanut butter and chow paste/regular chow; both compounds led to a reduction in Nutella/peanut butter paste, but only liraglutide led to an increase in chow intake, as in Raun et al. Nausea is a relatively common side‐effect with GLP‐1R agonist treatment in humans with and without type 2 diabetes, but also transient and dose‐dependent – although it does last longer than in rodents, it is not believed to be the mediator of chronic weight loss39. No human studies have yet investigated whether long‐term GLP‐1R agonist treatment in humans leads to changes in food preferences and/or eating behavior, but studies using functional magnetic resonance imaging scanning have shown an involvement of parts of the brain known for involvement in hedonic aspects of food intake40, 41.
Most of the aforementioned areas in the brain, to which liraglutide has direct access, and further areas described in Secher et al.12 , are so‐called circumventricular organs; that is, areas of the brain that have no classical blood–brain barrier. The AP, ME, the subfornical organ and the organum vasculosum of the lamina terminalis are circumventricular organs. Although it is logical that a peptide, such as GLP‐1, and other drug‐like GLP‐1R agonists can bind to GLP‐1Rs in circumventricular organs, it was perhaps less expected that pharmacological doses of a GLP‐1R agonist, such as liraglutide, could reach areas in the brain that are protected by the blood–brain barrier. The PVN and the ARC are well‐described areas for GLP‐1R action in the brain, but direct access from the periphery has not been described before. GLP‐1 and liraglutide have been described to cross the blood–brain barrier, but in light of the very specific binding in select regions as shown here, perhaps those older kinds of studies need to be re‐thought as a methodology where the entire brain parenchyma is extracted seems less well suited for a very specific pattern of uptake42, 43. How then does liraglutide get into the hypothalamus? Two hypotheses are worth mentioning. One is that certain parts of the hypothalamus, like the ventromedial ARC and the PVN, might be supplied directly by fenestrated capillaries: this could be consistent with the pattern of uptake seen with liraglutide44, 45. The other hypothesis is based on leptin and ghrelin studies, where specialized ependymal/glia cells called tanycytes, expressed in the ME, form part of an access gate for specific signals whose receptors are also expressed in the ME32, 46. After having passed the ME, the compounds end up in the cerebrospinal fluid (CSF) where another specialized glia cell type, ependymocytes, has cilia that facilitate fluid movement in the bottom of the third ventricle; thereafter, compounds such as liraglutide could access the ARC from the CSF. Interestingly, a human study has investigated the presence of liraglutide in the CSF and found measurable amounts of approximately 30 pmol/L47. Although the plasma concentration of liraglutide is much higher than that, because >99% of liraglutide is bound to albumin as part of the protraction mechanism, a CSF average concentration of 30 pmol/L might be relevant for a pharmacological effect.
In conclusion, new evidence from animal models has highlighted that pharmacological levels of peripherally‐dosed GLP‐1R agonists can access the hypothalamus directly and have local effects on key primary neurons in ARC, leading to an increase in satiety signals and a decrease in hunger signals. These data highlight a potential new important pathway that likely integrates with several other brain pathways where GLP‐1Rs are expressed. Human mechanistic data showed that liraglutide increased feelings of satiety and decreased hunger, leading to an overall reduction of appetite and energy intake. More studies are required to further understand the differences in weight loss efficacy between structurally different GLP‐1R agonists.
Disclosure
All authors are full‐time employees of, and minor employee‐based shareholders in, Novo Nordisk, the company that developed the liraglutide compound.
J Diabetes Investig 2016; 7: 56–63
This article is based on the presentations given by the authors at a symposium, Incretin 2015, July 29–31, 2015, Vancouver, BC Canada.
References
- 1. Bell GI, Santerre RF, Mullenbach GT. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature 1983; 302: 716–718. [DOI] [PubMed] [Google Scholar]
- 2. Mojsov S, Weir GC, Habener JF. Insulinotropin: glucagon‐like peptide I (7‐37) co‐encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest 1987; 79: 616–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holst JJ, Orskov C, Nielsen OV, et al Truncated glucagon‐like peptide I, an insulin‐releasing hormone from the distal gut. FEBS Lett 1987; 211: 169–174. [DOI] [PubMed] [Google Scholar]
- 4. Vilsboll T, Krarup T, Madsbad S, et al Defective amplification of the late phase insulin response to glucose by GIP in obese type II diabetic patients. Diabetologia 2002; 45: 1111–1119. [DOI] [PubMed] [Google Scholar]
- 5. Turton MD, O'Shea D, Gunn I, et al A role for glucagon‐like peptide‐1 in the central regulation of feeding. Nature 1996; 379: 69–72. [DOI] [PubMed] [Google Scholar]
- 6. Tang‐Christensen M, Larsen PJ, Göke R, et al Central administration of GLP‐1‐(7‐36) amide inhibits food and water intake in rats. Am J Physiol 1996; 271: R848–R856. [DOI] [PubMed] [Google Scholar]
- 7. Flint A, Raben A, Astrup A, et al Glucagon‐like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 1998; 101: 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Knudsen LB, Nielsen PF, Huusfeldt PO, et al Potent derivatives of glucagon‐like peptide‐1 with pharmacokinetic properties suitable for once daily administration. J Med Chem 2000; 43: 1664–1669. [DOI] [PubMed] [Google Scholar]
- 9. Pratley RE, Nauck MA, Barnett AH, et al Once‐weekly albiglutide versus once‐daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open‐label, multicentre, non‐inferiority phase 3 study. Lancet Diabetes Endocrinol 2014; 4: 289–297. [DOI] [PubMed] [Google Scholar]
- 10. Dungan KM, Povedano ST, Forst T, et al Once‐weekly dulaglutide versus once‐daily liraglutide in metformin‐treated patients with type 2 diabetes (AWARD‐6): a randomised, open‐label, phase 3, non‐inferiority trial. Lancet 2014; 384: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 11. Pi‐Sunyer X, Astrup A, Fujioka K, et al A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015; 373: 11–22. [DOI] [PubMed] [Google Scholar]
- 12. Secher A, Jelsing J, Baquero AF, et al The arcuate nucleus mediates GLP‐1 receptor agonist liraglutide‐dependent weight loss. J Clin Invest 2014; 124: 4473–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nauck MA, Niedereichholz U, Ettler R, et al Glucagon‐like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol 1997; 273: E981–E988. [DOI] [PubMed] [Google Scholar]
- 14. Ruttimann EB, Arnold M, Hillebrand JJ, et al Intrameal hepatic portal and intraperitoneal infusions of glucagon‐like peptide‐1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology 2009; 150: 1174–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon‐like peptide‐1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 2009; 150: 2654–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Labouesse MA, Stadlbauer U, Weber E, et al Vagal afferents mediate early satiation and prevent flavour avoidance learning in response to intraperitoneally infused exendin‐4. J Neuroendocrinol 2012; 24: 1505–1516. [DOI] [PubMed] [Google Scholar]
- 17. Nauck MA, Kemmeries G, Holst JJ, et al Rapid tachyphylaxis of the glucagon‐like peptide 1‐induced deceleration of gastric emptying in humans. Diabetes 2011; 60: 1561–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jelsing J, Vrang N, Hansen G, et al Liraglutide: short‐lived effect on gastric emptying/long lasting effects on body weight. Diabetes Obes Metab 2012; 14: 531–538. [DOI] [PubMed] [Google Scholar]
- 19. van Can J, Sloth B, Jensen CB, et al Effects of the once‐daily GLP‐1 analog liraglutide on gastric emptying, glycemic parameters, appetite, and energy metabolism in obese, non‐diabetic adults. Int J Obes (Lond) 2014; 38: 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vrang N, Larsen PJ. Preproglucagon derived peptides GLP‐1, GLP‐2 and oxyntomodulin in the CNS: role of peripherally secreted and centrally produced peptides. Prog Neurobiol 2010; 92: 442–462. [DOI] [PubMed] [Google Scholar]
- 21. Barrera JG, Jones KR, Herman JP, et al Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon‐like peptide‐1 loss of function. J Neurosci 2011; 31: 3904–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sisley S, Gutierrez‐Aguilar R, Scott M, et al Neuronal GLP1R mediates liraglutide's anorectic but not glucose‐lowering effect. J Clin Invest 2014; 124: 2456–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alhadeff AL, Rupprecht LE, Hayes MR. GLP‐1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 2012; 153: 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mietlicki‐Baase EG, Ortinski PI, Reiner DJ, et al Glucagon‐like peptide‐1 receptor activation in the nucleus accumbens core suppresses feeding by increasing glutamatergic AMPA/kainate signaling. J Neurosci 2014; 34: 6985–6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alhadeff AL, Baird JP, Swick JC, et al Glucagon‐like peptide‐1 receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake and motivation to feed. Neuropsychopharmacology 2014; 39: 2233–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Plamboeck A, Veedfald S, Deacon CF, et al The effect of exogenous GLP‐1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am J Physiol Gastrointest Liver Physiol 2013; 304: G1117–G1127. [DOI] [PubMed] [Google Scholar]
- 27. Kristensen P, Judge ME, Thim L, et al Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 1998; 393: 72–76. [DOI] [PubMed] [Google Scholar]
- 28. Schwartz MW, Woods SC, Porte D, et al Central nervous system control of food intake. Nature 2000; 404: 661–671. [DOI] [PubMed] [Google Scholar]
- 29. Woods SC, Schwartz MW, Baskin DG, et al Food intake and the regulation of body weight. Annu Rev Psychol 2000; 51: 255–277. [DOI] [PubMed] [Google Scholar]
- 30. Luquet S, Perez FA, Hnasko TS, et al NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 2005; 310: 683–685. [DOI] [PubMed] [Google Scholar]
- 31. Cansell C, Denis RG, Joly‐Amado A, et al Arcuate AgRP neurons and the regulation of energy balance. Front Endocrinol (Lausanne) 2012; 3: 169 doi: 10.3389/fendo.2012.00169. eCollection 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Balland E, Dam J, Langlet F, et al Hypothalamic tanycytes are an ERK‐gated conduit for leptin into the brain. Cell Metab 2014; 19: 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hileman SM, Pierroz DD, Masuzaki H, et al Characterizaton of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology 2002; 143: 775–783. [DOI] [PubMed] [Google Scholar]
- 34. Schaeffer M, Langlet F, Lafont C, et al Rapid sensing of circulating ghrelin by hypothalamic appetite‐modifying neurons. Proc Natl Acad Sci USA 2013; 110: 1512–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heppner KM, Kirigiti M, Secher A, et al Expression and distribution of glucagon‐like peptide‐1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology 2015; 156: 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raun K, von Voss P, Gotfredsen CF, et al Liraglutide, a long‐acting glucagon‐like peptide‐1 analog, reduces body weight and food intake in obese candy‐fed rats, whereas a dipeptidyl peptidase‐IV inhibitor, vildagliptin, does not. Diabetes 2007; 56: 8–15. [DOI] [PubMed] [Google Scholar]
- 37. Hansen G, Jelsing J, Vrang N. Effects of liraglutide and sibutramine on food intake, palatability, body weight and glucose tolerance in the gubra DIO‐rats. Acta Pharmacol Sin 2012; 33: 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Erreger K, Davis AR, Poe AM, et al Exendin‐4 decreases amphetamine‐induced locomotor activity. Physiol Behav 2012; 106: 574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lean ME, Carraro R, Finer N, et al Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non‐diabetic adults. Int J Obes (Lond) 2014; 38: 689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Van Bloemendaal L, IJzerman RG, Ten Kulve JS, et al GLP‐1 receptor activation modulates appetite‐ and reward‐related brain areas in humans. Diabetes 2014; 63: 4186–4196. [DOI] [PubMed] [Google Scholar]
- 41. van Bloemendaal L, Veltman DJ, Ten Kulve JS, et al Brain reward‐system activation in response to anticipation and consumption of palatable food is altered by GLP‐1 receptor activation in humans. Diabetes Obes Metab 2015; 17: 878–886. [DOI] [PubMed] [Google Scholar]
- 42. Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon‐like peptide‐1 (GLP‐1) with the blood‐brain barrier. J Mol Neurosci 2002; 18: 7–14. [DOI] [PubMed] [Google Scholar]
- 43. Hunter K, Holscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci 2012; 13: 33. doi: 10.1186/1471-2202-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abernethy WB, Bell MA, Morris M, et al Microvascular density of the human paraventricular nucleus decreases with aging but not hypertension. Exp Neurol 1993; 121: 270–274. [DOI] [PubMed] [Google Scholar]
- 45. Ciofi P. The arcuate nucleus as a circumventricular organ in the mouse. Neurosci Lett 2011; 487: 187–190. [DOI] [PubMed] [Google Scholar]
- 46. Langlet F, Mullier A, Bouret SG, et al Tanycyte‐like cells form a blood‐cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. J Comp Neurol 2013; 521: 3389–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Christensen M, Sparre‐Ulrich AH, Hartmann B, et al Transfer of liraglutide from blood to cerebrospinal fluid is minimal in patients with type 2 diabetes. Int J Obes (Lond) 2015; 39: 1651–1654. [DOI] [PubMed] [Google Scholar]


