Abstract
Glucagon‐like peptide‐1 (GLP‐1) is derived from both the enteroendocrine L cells and preproglucagon‐expressing neurons in the nucleus tractus solitarius (NTS) of the brain stem. As GLP‐1 is cleaved by dipeptidyl peptidase‐4 yielding a half‐life of less than 2 min, it is plausible that the gut‐derived GLP‐1, released postprandially, exerts its effects on the brain mainly by interacting with vagal afferent neurons located at the intestinal or hepatic portal area. GLP‐1 neurons in the NTS widely project in the central nervous system and act as a neurotransmitter. One of the physiological roles of brain‐derived GLP‐1 is restriction of feeding. GLP‐1 receptor agonists have recently been used to treat type 2 diabetic patients, and have been shown to exhibit pleiotropic effects beyond incretin action, which involve brain functions. GLP‐1 receptor agonist administered in the periphery is stable because of its resistance to dipeptidyl peptidase‐4, and is highly likely to act on the brain by passing through the blood–brain barrier (BBB), as well as interacting with vagal afferent nerves. Central actions of GLP‐1 have various roles including regulation of feeding, weight, glucose and lipid metabolism, cardiovascular functions, cognitive functions, and stress and emotional responses. In the present review, we focus on the source of GLP‐1 and the pathway by which peripheral GLP‐1 informs the brain, and then discuss recent findings on the central effects of GLP‐1 and GLP‐1 receptor agonists.
Keywords: Brain stem, Cardiovascular, Feeding
Introduction
The anti‐obesity effect of incretin‐based medicines is mainly mediated by the central anorectic effect of glucagon‐like peptide‐1 (GLP‐1) and GLP‐1 receptor agonist. They also provoke various effects that involve the central and peripheral nervous systems, which include regulation of glucose and lipid metabolism, cardiovascular functions, cognitive functions, and stress and emotional responses. In the present review, we focus on the properties and functions of gut‐derived vs brain‐derived GLP‐1, distinct roles of humoral vs neural pathways linking peripheral GLP‐1 to the brain, and different modes of action between endogenous GLP‐1 vs GLP‐1 receptor agonists.
Gut‐Derived VS Brain‐Derived GLP‐1
GLP‐1 is released from the enteroendocrine L cells in response to meals, and enhances glucose‐induced insulin secretion from pancreatic islets1, being recognized as an incretin hormone. GLP‐1 is also produced by preproglucagon‐expressing neurons in the nucleus tractus solitarius (NTS) of the brain stem2, and acts as a neurotransmitter. It is important to distinguish the central action of gut‐derived GLP‐1 from that of brain‐derived GLP‐1.
Generally, the peripheral factors send their information to the brain through two distinct pathways, the humoral and neural pathways (Figure 1). In detail, the humoral pathway is composed of the blood–brain barrier (BBB) and the circumventricular organ (CVO) that has a leaky BBB. It is reported that GLP‐1 can enter the brain through the BBB3, and radiolabeled GLP‐1 is bound to its receptors expressed in the CVOs4, 5. However, endogenous GLP‐1 derived from the gut is rapidly cleaved by dipeptidyl peptidase‐4 (DPP‐4), with its half‐life being less than 2 min6. Hence, it is reasonable that gut‐derived GLP‐1 influences the brain mainly through the neural pathway, which is composed of the vagal afferent fibers at the intestinal or hepatic portal area. We previously reported that GLP‐1 evokes action potentials and increases cytosolic Ca2+ concentration ([Ca2+]i) in the neurons of nodose ganglion, where cell bodies of the vagal afferent fibers are located (Figure 2)7. Vagal afferents also directly sense cholecystokinin8, 9, peptide YY3‐36 10, leptin11, oxytocin11 and nesfatin‐112, the hormones regulating feeding and metabolism. Intraperitoneal co‐injection of GLP‐1 and leptin13 or co‐injection of GLP‐1 receptor agonist exendin‐4 and peptide YY3‐36 14 synergistically suppresses food intake. These hormones might act cooperatively on vagal afferents and regulate feeding behavior.
Figure 1.
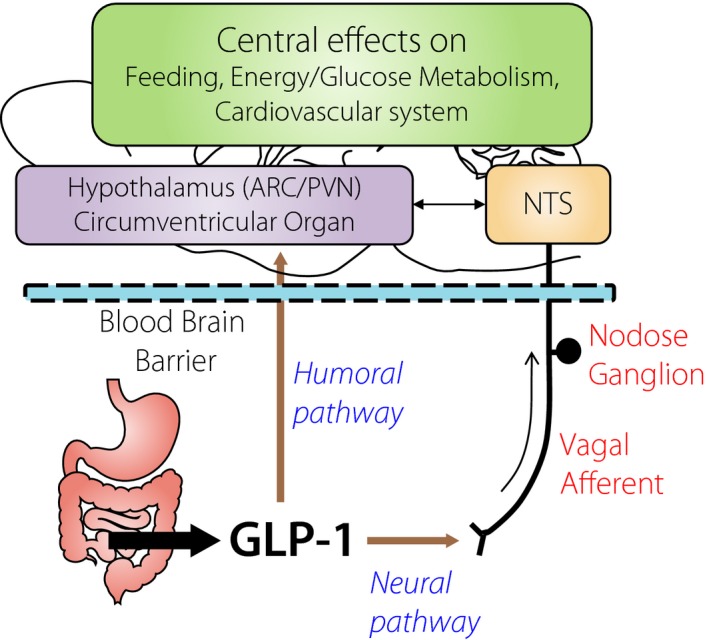
Glucagon‐like peptide‐1 (GLP‐1), released from the intestine, informs the brain by passing through blood–brain barrier (humoral pathway) and by interacting with vagal afferent nerves (neural pathway). Consequent activation of the hypothalamus, circumventricular organ, and brain stem including nucleus tractus solitarius (NTS), regulates feeding, energy/glucose metabolism and the cardiovascular system. ARC, arcuate nucleus; PVN, paraventricular nucleus.
Figure 2.
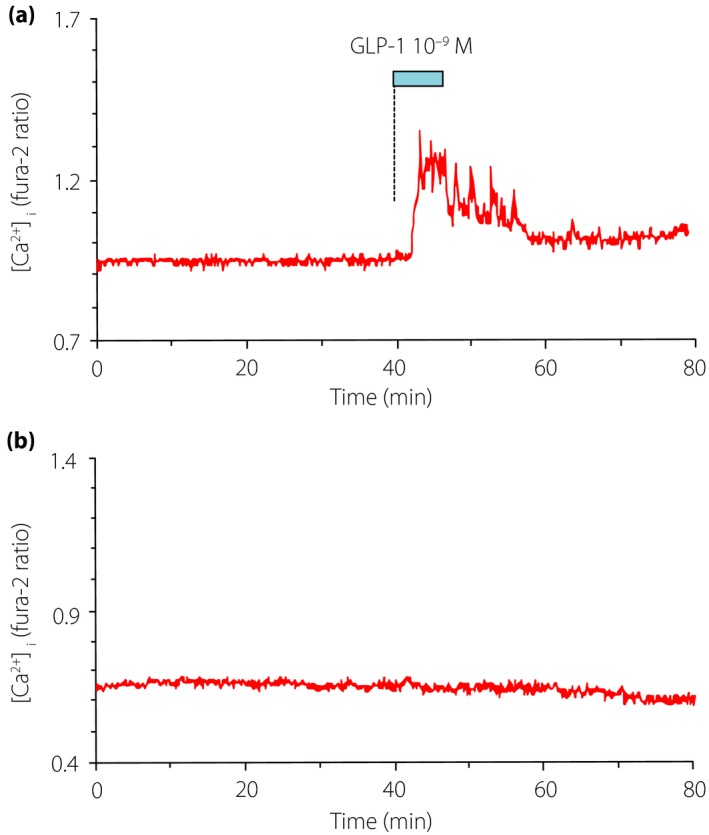
(a) Glucagon‐like peptide‐1 (GLP‐1) at 10−9 mol/L increases cytosolic Ca2+ concentration ([Ca2+]i) in single nodose ganglion neurons isolated from rats. (b) As a control, [Ca2+]i is stable in nodose ganglion neurons without GLP‐1 administration.
Injection of GLP‐1 into the portal vein enhances insulin secretion, and this effect is attenuated by hepatic vagotomy15, suggesting that the insulinotropic effect of GLP‐1 is mediated not only by direct action on the pancreas, but also through vagal afferents, being recognized as a neuroincretin effect. A recent study by Krieger et al.16 used the bilateral nodose ganglion injection technique to deliver a lentiviral vector to knock down the GLP‐1 receptor specifically in the vagal afferent neurons of rats, and found that post‐meal glycemia was elevated and insulin release was blunted in GLP‐1 receptor knockdown rats. These results suggest that the vagal afferent GLP‐1 receptor is a physiological contributor to the neuroincretin effect after meals. Furthermore, they found GLP‐1 receptor knockdown increases meal size and accelerates gastric emptying, whereas it has little effect on the long‐term maintenance of food intake and weight in normal eating conditions. These results highlight a crucial role for the vagal afferent neuron in mediating the effects of endogenous GLP‐1 on glycemia and food intake.
Physiological Role of Brain‐Derived GLP‐1
GLP‐1 neurons in the NTS widely project in the central nervous system including the hypothalamus: the paraventricular nucleus (PVN), supraoptic nucleus (SON), arcuate nucleus (ARC) and hypothalamic dorsomedial nucleus (DMH), and the brainstem – the parabrachial nucleus, reticular formation of medulla and dorsal vagal nucleus, thalamus and intermediolateral cell column2, 17, 18, 19, 20, 21 – where GLP‐1 receptors are expressed22, 23, 24, 25.
We reported that endogenous GLP‐1 in the brain targets PVN to restrict feeding behavior, in which the projection from NTS GLP‐1 neurons and activation of corticotropin‐releasing hormone and nesfatin‐1 neurons might be implicated (Figures 3 and 4)26. NTS GLP‐1 neurons are activated by both central signals and peripheral signals from vagal afferents. As a central signal, oxytocin neurons in PVN project to the NTS GLP‐1 neurons, and central infusion of oxytocin activates c‐Fos expression in NTS GLP‐1 neurons27. It is established that peripheral signals, such as gastric distension28, and intraperitoneal injection of lithium chloride that leads to conditioned taste aversion and nausea29, 30 cause c‐Fos expression in NTS GLP‐1 neurons. In addition, using brain slices of the transgenic mice expressing the yellow fluorescent protein under control of the preproglucagon promoter, the electrical properties of NTS GLP‐1 neurons were reported. That study showed that leptin directly depolarizes NTS GLP‐1 neurons, and cholecystokinin modulates noradrenergic or glutamatergic neurons and thereby secondarily depolarizes NTS GLP‐1 neurons. In contrast, NTS GLP‐1 neurons failed to show electrical responses to peptide YY, GLP‐1 and ghrelin31.
Figure 3.
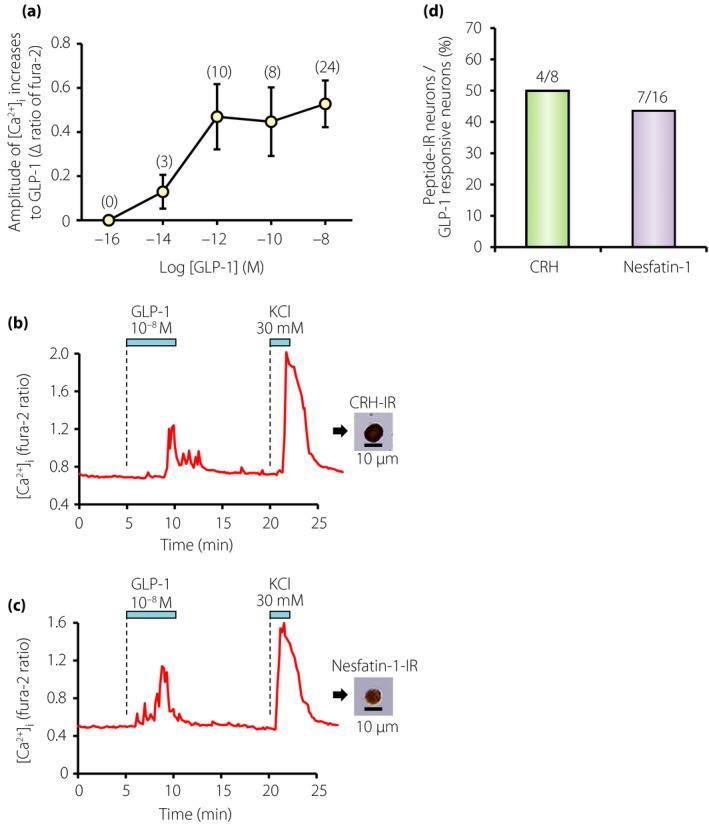
Glucagon‐like peptide‐1 (GLP‐1) concentration‐dependently increases [Ca2+]i in corticotropin‐releasing hormone (CRH) and nesfatin‐1 neurons of paraventricular nucleus (PVN). (a) Amplitude of [Ca2+]i responses to GLP‐1 in GLP‐1 responsive neurons. Numbers around each point indicate number of neurons examined. Values are expressed by mean ± standard error of the mean. (b) GLP‐1 at 10−8 mol/L increased [Ca2+]i in a single neuron isolated from PVN (left panel), and this neuron was subsequently shown to be immunoreactive (IR) to CRH (right panel). This neuron also responded to 30 mmol/L KCl with an increase in [Ca2+]i. (c) GLP‐1 at 10−8 increased [Ca2+]i in a single PVN neuron that was subsequently shown to be IR to nesfatin‐1. Superfusate contained 1 mmol/L glucose and the bars above the tracings indicate the periods of administration of agents in (b) and (c). (d) Incidence of the neurons that express CRH or nesfatin‐1 over those responded to GLP‐1 in the PVN. The numbers above the bars indicate the number of neurons IR to CRH or nesfatin‐1 over that responded to GLP‐1 in the range of 10−14–10−8 mol/L.
Figure 4.
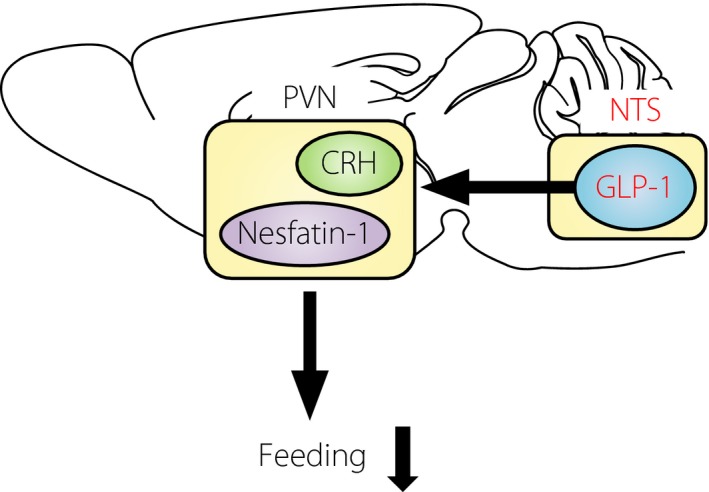
Model for feeding suppression by endogenous brain‐derived glucagon‐like peptide‐1 (GLP‐1). Nucleus tractus solitarius (NTS) GLP‐1 neurons project to paraventricular nucleus (PVN), and activate corticotropin‐releasing hormone (CRH) and nesfatin‐1 neurons to suppress feeding, and possibly regulate glucose metabolism.
The role of brain‐derived GLP‐1 in glucose metabolism has been reported32. Intragastric glucose infusion, compared with intragastric water infusion, increased muscle glycogen synthesis and c‐Fos expression in the NTS, and decreased c‐Fos expression in the neuropeptide Y‐positive neurons in the ARC. These effects were diminished with simultaneous central infusion of the GLP‐1 receptor antagonist exendin(9‐39), and abolished in GLP‐1 receptor knockout mice. Because intragastric glucose infusion does not affect the blood levels of glucose, insulin and GLP‐1, enteric glucose might send signals to the brain through vagal afferents and activate a GLP‐1 receptor‐dependent signal in the brain to control peripheral glucose metabolism (Figure 4).
Central Effects of Endogenous GLP‐1 vs GLP‐1 Receptor Agonists
Several GLP‐1 receptor agonists have recently been used to treat type 2 diabetes patients. GLP‐1 receptor agonists have DPP‐4‐resistance and a longer half‐life of several hours or more because of their structural modification. Unlike the gut‐derived endogenous GLP‐1 that is inactivated within 2 min by DPP‐4, GLP‐1 receptor agonists administered in the periphery are stable and highly likely to act on the brain through the humoral pathway in addition to the neural pathway. In addition, GLP‐1 receptor agonist could remain in the brain for several hours, and hence exert effects similar to those induced by the brain‐derived endogenous GLP‐1.
Indeed, it was reported that the GLP‐1 receptor agonist, liraglutide, administered in the periphery can reach the brain and exert central effects33. That study investigated the distribution of fluorescence‐labeled liraglutide in the mouse brain after peripheral administration. Fluorescence‐labeled liraglutide was observed in all CVOs, the zona interna of the median eminence (ME), the area postrema (AP), the sobfornical organ (SFO) and the organum vasculosum of the lamina terminalis (OVLT), and also in hypothalamic regions, the ARC, PVN and SON. These signals of fluorescence‐labeled liraglutide in specific brain areas were blunted in GLP‐1 receptor knockout mice. Interestingly, no fluorescence‐labeled liraglutide was observed in the NTS, suggesting that peripheral liraglutide could not directly interact with the NTS GLP‐1 neurons. This study also showed that peripheral liraglutide‐induced feeding suppression was abolished by infusion of GLP‐1 receptor antagonist exendin(9‐39) into the ARC, but not the PVN. Neither the ablation of the AP nor deafferentation of the subdiaphragmatic vagal afferent affected the peripheral liraglutide‐induced feeding suppression. Furthermore, electrophysiological measurements of murine brain slices showed that GLP‐1 directly stimulates pro‐opiomelanocortin/cocaine‐ and amphetamine‐stimulated transcript neurons, and indirectly inhibits neurotransmission to neuropeptide Y/Agouti‐related peptide neurons through gamma‐aminobutyric acid‐dependent signals. Taken together, it was concluded that peripheral liraglutide directly acts on the ARC, most likely pro‐opiomelanocortin/cocaine‐ and amphetamine‐stimulated transcript neurons, through the humoral pathway to suppress feeding.
Differences between GLP‐1 and GLP‐1 receptor agonist exendin‐4 in their central anorectic effects have been reported. This is partly explained by their different sensitivity to DPP‐434, but might also involve other factors. The anorectic effect of intracerebroventricular (i.c.v.) injection of GLP‐1 was attenuated by i.c.v. injection of GLP‐1 receptor antagonist before GLP‐1. By contrast, the anorectic effect of i.c.v. injection of exendin‐4 was not attenuated by i.c.v. injection of GLP‐1 receptor antagonist before exendin‐4, but was abolished in GLP‐1 receptor knockout mice. In contrast, the anorectic effect of intraperitoneal (i.p.) injection of exendin‐4 was completely blocked by i.p. injection of GLP‐1 receptor antagonist before exendin‐4. These data suggest a key difference between GLP‐1 and exendin‐4 in their interaction with the brain GLP‐1 receptor. There might be a difference in the GLP‐1 receptor properties between the brain and the periphery, which could include post‐translational processing, binding to GLP‐1 receptor antagonist, interaction with other peptides and signal transduction.
A recent report by Beiroa et al.35 investigated the effects of specific injection of liraglutide in the various hypothalamic sites that regulate food intake and brown adipose tissue thermogenesis. Injection of liraglutide specifically into the hypothalamic ARC, PVN and lateral hypothalamic area (LHA), but not ventromedial nucleus (VMH), reduced food intake. Conversely, injection of liraglutide specifically into the VMH, but not ARC, PVN and LHA, stimulated brown adipose tissue thermogenesis. This effect depends on AMPK in the VMH, and involves expression of uncoupling protein 1 in the brown adipose tissue to higher levels.
Cardiovascular Effects of Endogenous GLP‐1 vs GLP‐1 Receptor Agonists
Central GLP‐1 and GLP‐1 receptor agonists have cardiovascular actions. The i.c.v. infusion of GLP‐1 and GLP‐1 receptor agonist transiently increases blood pressure and heart rate in normal rodents. The possible underlying mechanisms involve central cholinergic system36, neurohypophysial hormones36, 37 and medullary catecholamine neurons38. Regarding heart rate regulation, it was reported that exendin‐4 inhibits neurotransmission to cardiac vagal neurons in the medullary nucleus ambiguus, leading to suppression of cardiac parasympathetic activity thereby increasing heart rate39. Studies in hypertensive patients with type 2 diabetes or obesity showed that long‐term treatment with GLP‐1 receptor agonists reduced blood pressure40. In animal studies, chronic peripheral injection of GLP‐1 receptor agonists reduced blood pressure. The i.p. injection of exendin‐4 twice daily for 12 weeks reduced blood pressure in db/db mice41. Furthermore, twice‐daily exendin‐4 attenuated the increase in blood pressure by angiotensin II infusion for 2 weeks in mice41. Underlying mechanisms for the chronic antihypertensive effect of GLP‐1 receptor agonists has not yet been fully elucidated. It is postulated as possible mechanisms that GLP‐1 receptor agonist directly acts on the kidney to induce natriuresis41, 42, and on the endothelial cells to induce vasodilatation43. Additionally or alternatively, chronic peripheral injection of GLP‐1 receptor agonists would possibly inform the brain through humoral and/or neural pathways to activate central GLP‐1 receptors and/or neural circuits, thereby evoking these chronic antihypertensive effects.
These studies suggest that central GLP‐1 or GLP‐1 receptor agonists exert different actions depending on acute vs chronic paradigms, and on physiological vs pathological subjects. The result from a clinical trial of GLP‐1 receptor agonist have provided cardiovascular safety for clinical use, although risk reduction of cardiovascular events has not been observed44. The follow‐up period so far is approximately 3 years, and further investigations will be required to evaluate longer‐term efficacy and safety.
Conclusion
In the past several years of clinical experience, incretin‐based medicines have been shown to exhibit pleiotropic effects beyond glycemic control, which include the effects on feeding, weight, lipid metabolism, cardiovascular functions and cognitive functions. Central effects of GLP‐1 contribute to these extrapancreatic effects, regulating systemic homeostasis in physiological states, and providing beneficial responses to pathological states. This review has shown that gut‐derived GLP‐1 and GLP‐1 receptor agonist send their information to the brain through neural and humoral pathways. However, how their information is relayed to efferent nervous systems to control peripheral tissues and organs remains to be clarified. Further investigations in basic and clinical studies will provide new insights about central actions and the underlying mechanisms of incretin‐based medicines.
Disclosure
The authors declare no conflict of interest.
Acknowledgements
This work was supported by a Grant‐in‐Aid for Challenging Exploratory Research (26670453) from JSPS, Programs for Strategic Research Foundation at Private Universities 2011–2015 and 2013–2017, supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan, and a Lilly‐Incretin Basic Research Grant from Japan Diabetes Foundation to TY. This study was subsidized by JKA through its promotion funds from KEIRIN RACE to TY.
J Diabetes Investig, 2016; 7: 64–69
This article is based on the presentations given by the authors at a symposium, Incretin 2015, July 29–31, 2015, Vancouver, BC Canada.
References
- 1. Varndell IM, Bishop AE, Sikri KL, et al Localization of glucagon‐like peptide (GLP) immunoreactants in human gut and pancreas using light and electron microscopic immunocytochemistry. J Histochem Cytochem 1985; 33: 1080–1086. [DOI] [PubMed] [Google Scholar]
- 2. Larsen PJ, Tang‐Christensen M, Holst JJ, et al Distribution of glucagon‐like peptide‐1 and other preproglucagon‐derived peptides in the rat hypothalamus and brainstem. Neuroscience 1997; 77: 257–270. [DOI] [PubMed] [Google Scholar]
- 3. Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon‐like peptide‐1 (GLP‐1) with the blood‐brain barrier. J Mol Neurosci 2002; 18: 7–14. [DOI] [PubMed] [Google Scholar]
- 4. Tang‐Christensen M, Vrang N, Larsen PJ. Glucagon‐like peptide 1(7‐36) amide's central inhibition of feeding and peripheral inhibition of drinking are abolished by neonatal monosodium glutamate treatment. Diabetes 1998; 47: 530–537. [DOI] [PubMed] [Google Scholar]
- 5. Orskov C, Poulsen SS, Møller M, et al Glucagon‐like peptide I receptors in the subfornical organ and the area postrema are accessible to circulating glucagon‐like peptide I. Diabetes 1996; 45: 832–835. [DOI] [PubMed] [Google Scholar]
- 6. Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose‐dependent insulinotropic polypeptide and truncated glucagon‐like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 1995; 136: 3585–3596. [DOI] [PubMed] [Google Scholar]
- 7. Kakei M, Yada T, Nakagawa A, et al Glucagon‐like peptide‐1 evokes action potentials and increases cytosolic Ca2+ in rat nodose ganglion neurons. Auton Neurosci 2002; 102: 39–44. [DOI] [PubMed] [Google Scholar]
- 8. Simasko SM, Wiens J, Karpiel A, et al Cholecystokinin increases cytosolic calcium in a subpopulation of cultured vagal afferent neurons. Am J Physiol Regul Integr Comp Physiol 2002; 283: R1303–R1313. [DOI] [PubMed] [Google Scholar]
- 9. Lankisch TO, Tsunoda Y, Lu Y, et al Characterization of CCKA receptor affinity states and Ca2+ signal transduction in vagal nodose ganglia. Am J Physiol Gastrointest Liver Physiol 2002; 282: G1002–G1008. [DOI] [PubMed] [Google Scholar]
- 10. Iwasaki Y, Kakei M, Nakabayashi H, et al Pancreatic polypeptide and peptide YY3‐36 induce Ca2+ signaling in nodose ganglion neurons. Neuropeptides 2013; 47: 19–23. [DOI] [PubMed] [Google Scholar]
- 11. Iwasaki Y, Maejima Y, Suyama S, et al Peripheral oxytocin activates vagal afferent neurons to suppress feeding in normal and leptin‐resistant mice: a route for ameliorating hyperphagia and obesity. Am J Physiol Regul Integr Comp Physiol 2015; 308: R360–R369. [DOI] [PubMed] [Google Scholar]
- 12. Iwasaki Y, Nakabayashi H, Kakei M, et al Nesfatin‐1 evokes Ca2+ signaling in isolated vagal afferent neurons via Ca2+ influx through N‐type channels. Biochem Biophys Res Commun 2009; 390: 958–962. [DOI] [PubMed] [Google Scholar]
- 13. Poleni PE, Akieda‐Asai S, Koda S, et al Possible involvement of melanocortin‐4‐receptor and AMP‐activated protein kinase in the interaction of glucagon‐like peptide‐1 and leptin on feeding in rats. Biochem Biophys Res Commun 2012; 420: 36–41. [DOI] [PubMed] [Google Scholar]
- 14. Talsania T, Anini Y, Siu S, et al Peripheral exendin‐4 and peptide YY3‐36 synergistically reduce food intake through different mechanisms in mice. Endocrinology 2005; 146: 3748–3756. [DOI] [PubMed] [Google Scholar]
- 15. Nishizawa M, Nakabayashi H, Uehara K, et al Intraportal GLP‐1 stimulates insulin secretion predominantly through the hepatoportal‐pancreatic vagal reflex pathways. Am J Physiol Endocrinol Metab 2013; 305: E376–E387. [DOI] [PubMed] [Google Scholar]
- 16. Krieger JP, Arnold M, Pettersen KG, et al Knockdown of GLP‐1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes 2015; 65: 34–33. [DOI] [PubMed] [Google Scholar]
- 17. Vrang N, Hansen M, Larsen PJ, et al Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain Res 2007; 1149: 118–126. [DOI] [PubMed] [Google Scholar]
- 18. Tauchi M, Zhang R, D'Alessio DA, et al Distribution of glucagon‐like peptide‐1 immunoreactivity in the hypothalamic paraventricular and supraoptic nuclei. J Chem Neuroanat 2008; 36: 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Llewellyn‐Smith IJ, Reimann F, Gribble FM, et al Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience 2011; 180: 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alhadeff AL, Rupprecht LE, Hayes MR. GLP‐1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 2012; 153: 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Llewellyn‐Smith IJ, Gnanamanickam GJ, Reimann F, et al Preproglucagon (PPG) neurons innervate neurochemically identified autonomic neurons in the mouse brainstem. Neuroscience 2013; 229: 130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Göke R, Larsen PJ, Mikkelsen JD, et al Distribution of GLP‐1 binding sites in the rat brain: evidence that exendin‐4 is a ligand of brain GLP‐1 binding sites. Eur J Neurosci 1995; 7: 2294–2300. [DOI] [PubMed] [Google Scholar]
- 23. Merchenthaler I, Lane M, Shughrue P. Distribution of pre‐pro‐glucagon and glucagon‐like peptide‐1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 1999; 403: 261–280. [DOI] [PubMed] [Google Scholar]
- 24. Richards P, Parker HE, Adriaenssens AE, et al Identification and characterization of GLP‐1 receptor‐expressing cells using a new transgenic mouse model. Diabetes 2014; 63: 1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cork SC, Richards JE, Holt MK, et al Distribution and characterisation of Glucagon‐like peptide‐1 receptor expressing cells in the mouse brain. Mol Metab 2015; 4: 718–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Katsurada K, Maejima Y, Nakata M, et al Endogenous GLP‐1 acts on paraventricular nucleus to suppress feeding: projection from nucleus tractus solitarius and activation of corticotropin‐releasing hormone, nesfatin‐1 and oxytocin neurons. Biochem Biophys Res Commun 2014; 451: 276–281. [DOI] [PubMed] [Google Scholar]
- 27. Rinaman L, Rothe EE. GLP‐1 receptor signaling contributes to anorexigenic effect of centrally administered oxytocin in rats. Am J Physiol Regul Integr Comp Physiol 2002; 283: R99–R106. [DOI] [PubMed] [Google Scholar]
- 28. Vrang N, Phifer CB, Corkern MM, et al Gastric distension induces c‐Fos in medullary GLP‐1/2‐containing neurons. Am J Physiol Regul Integr Comp Physiol 2003; 285: R470–R478. [DOI] [PubMed] [Google Scholar]
- 29. Rinaman L. A functional role for central glucagon‐like peptide‐1 receptors in lithium chloride‐induced anorexia. Am J Physiol 1999; 277: R1537–R1540. [DOI] [PubMed] [Google Scholar]
- 30. Rinaman L. Interoceptive stress activates glucagon‐like peptide‐1 neurons that project to the hypothalamus. Am J Physiol 1999; 277: R582–R590. [DOI] [PubMed] [Google Scholar]
- 31. Hisadome K, Reimann F, Gribble FM, et al Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: electrical properties of glucagon‐like Peptide 1 neurons. Diabetes 2010; 59: 1890–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Knauf C, Cani PD, Kim DH, et al Role of central nervous system glucagon‐like Peptide‐1 receptors in enteric glucose sensing. Diabetes 2008; 57: 2603–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Secher A, Jelsing J, Baquero AF, et al The arcuate nucleus mediates GLP‐1 receptor agonist liraglutide‐dependent weight loss. J Clin Invest 2014; 124: 4473–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barrera JG, D'Alessio DA, Drucker DJ, et al Differences in the central anorectic effects of glucagon‐like peptide‐1 and exendin‐4 in rats. Diabetes 2009; 58: 2820–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beiroa D, Imbernon M, Gallego R, et al GLP‐1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 2014; 63: 3346–3358. [DOI] [PubMed] [Google Scholar]
- 36. Isbil‐Buyukcoskun N, Gulec G. Effects of intracerebroventricularly injected glucagon‐like peptide‐1 on cardiovascular parameters; role of central cholinergic system and vasopressin. Regul Pept 2004; 118: 33–38. [DOI] [PubMed] [Google Scholar]
- 37. Bojanowska E, Stempniak B. Effects of centrally or systemically injected glucagon‐like peptide‐1 (7‐36) amide on release of neurohypophysial hormones and blood pressure in the rat. Regul Pept 2000; 91: 75–81. [DOI] [PubMed] [Google Scholar]
- 38. Yamamoto H, Lee CE, Marcus JN, et al Glucagon‐like peptide‐1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest 2002; 110: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Griffioen KJ, Wan R, Okun E, et al GLP‐1 receptor stimulation depresses heart rate variability and inhibits neurotransmission to cardiac vagal neurons. Cardiovasc Res 2011; 89: 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ussher JR, Drucker DJ. Cardiovascular actions of incretin‐based therapies. Circ Res 2014; 114: 1788–1803. [DOI] [PubMed] [Google Scholar]
- 41. Hirata K, Kume S, Araki S, et al Exendin‐4 has an anti‐hypertensive effect in salt‐sensitive mice model. Biochem Biophys Res Commun 2009; 380: 44–49. [DOI] [PubMed] [Google Scholar]
- 42. Yu M, Moreno C, Hoagland KM, et al Antihypertensive effect of glucagon‐like peptide 1 in Dahl salt‐sensitive rats. J Hypertens 2003; 21: 1125–1135. [DOI] [PubMed] [Google Scholar]
- 43. Erdogdu O, Nathanson D, Sjöholm A, et al Exendin‐4 stimulates proliferation of human coronary artery endothelial cells through eNOS‐, PKA‐ and PI3K/Akt‐dependent pathways and requires GLP‐1 receptor. Mol Cell Endocrinol 2010; 325: 26–35. [DOI] [PubMed] [Google Scholar]
- 44. Bentley‐Lewis R, Aguilar D, Riddle MC, et al Rationale, design, and baseline characteristics in Evaluation of LIXisenatide in Acute Coronary Syndrome, a long‐term cardiovascular end point trial of lixisenatide versus placebo. Am Heart J 2015; 169: 631–638. [DOI] [PubMed] [Google Scholar]


